Abstract
Graft-versus-host disease (GVHD) remains the major complication after allogeneic bone marrow transplantation (BMT). The process whereby acute GVHD mediated by alloreactive donor T cells transitions into chronic GVHD, which is characterized by prominent features of auto-immunity, has long been unresolved. In this study, we demonstrate that GVHD-associated autoimmunity and, by extension, chronic GVHD is attributable to the progressive loss of CD4+CD25+Foxp3+ regulatory T cells during the course of acute GVHD. This leads to the expansion of donor-derived CD4+ T cells with TH1 and TH17 cytokine phenotypes that release proinflammatory cytokines and cause autoimmune-mediated pathological damage. These T cells are present early after transplantation, indicating that the pathophysiological events that lead to chronic GVHD are set in motion during the acute phase of GVHD. We conclude that the absence of CD4+CD25+ regulatory T cells coupled with unregulated TH1 and TH17 cells leads to the development of autoimmunity and that donor-derived TH1 and TH17 cells serve as the nexus between acute and chronic GVHD.
Introduction
Graft-versus-host disease (GVHD) remains the major complication of allogeneic bone marrow transplantation (BMT). Clinically, GVHD has been divided into acute and chronic phases that have historically been distinguished primarily by their temporal onset. In contrast to acute GVHD, where pathology is generally restricted to the skin, liver, and intestinal tract,1 chronic GVHD has unique clinical features in that organ involvement tends to be more extensive and more frequently involves the lung, eyes, and mucous membranes, tissue sites that are less involved in acute GVHD.2 Moreover, chronic GVHD often presents with clinical manifestations that resemble those seen in autoimmune diseases such as systemic lupus erythrematosus, Sjogren's syndrome, scleroderma, and rheumatoid arthritis.3–5 In these patients, autoantibody formation is a common feature, and some patients actually develop autoimmune diseases such as myasthenia gravis,6 autoimmune thyroiditis,5 and immune hemolytic anemia.7 These distinguishing clinical features of chronic GVHD have been the impetus for the recent re-classification of this disease with diagnostic criteria that, in part, highlight the similarities that this syndrome has with other autoimmune disorders.8 These new criteria are meant to emphasize that chronic GVHD is not merely an extension of antecedent acute GVHD, but is a distinct clinical and pathophysiological entity.
Although there is now consensus regarding the clinical manifestations that define the syndrome of chronic GVHD, the pathophysiological link between acute and chronic GVHD remains poorly understood, particularly with respect to how an alloreactive donor T-cell response ultimately evolves into a syndrome with prominent autoimmune features. Recently, in an effort to enhance our understanding of GVHD pathophysiology, we performed studies that demonstrated that, during acute GVHD, there is breaking of tolerance to self antigens.9 These experiments showed that spleen cells from mice undergoing acute GVHD were capable of inducing autoimmune-mediated pathological damage after adoptive transfer into secondary immunodeficient animals of donor major histocompatibility complex (MHC) type. The observation that GVHD can result in loss of self tolerance provides a potential explanation for how autoimmunity might emerge in GVHD recipients and become a dominant aspect of this syndrome. In this study, we now provide a pathophysiological basis for chronic GVHD-associated autoimmunity and for its evolution from antecedent acute GVHD.
Materials and methods
Mice
C57BL/6 (B6) (H-2b), Balb/cJ (H-2d), B10.BR (H-2k), B6.PL (Thy 1.1+), B6.129S7-Rag 1 (B6 Rag), and C.129S7-Rag 1 (Balb Rag) mice were bred in the Animal Resource Center at the Medical College of Wisconsin (MCW) or purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed in the American Association for Laboratory Animal Care (AALAC)–accredited Animal Resource Center of the Medical College of Wisconsin. Experiments all were carried out under protocols approved by the MCW Institutional Animal Care and Use Committee. Mice received regular mouse chow and acidified tap water ad libitum.
Cell isolation
B cells, CD4+, and CD8+ T cells were isolated from the spleens of mice by positive selection using the MACS magnetic bead cell separation system (Miltenyi Biotech, Auburn, CA). CD4+CD25+ T cells were isolated using the MACS magnetic bead regulatory T-cell isolation kit according to the manufacturer's instructions. To isolate intraepithelial lymphocytes, colon tissue was incubated with EDTA (ethylenediaminetetraacetic acid) for 30 minutes at 37°C, and cells were then passed through a 100-μm strainer to remove cellular debris. The resulting cell suspension was layered on a 44/67% Percoll gradient. Intraepithelial lymphocytes were removed from the interface after centrifugation. For the isolation of lamina propria lymphocytes (LPLs), the remaining colon tissue was cut into smaller pieces and incubated in phosphate buffered saline (PBS) containing 2% fetal bovine serum and 0.15 mg/mL collagenase D (Sigma, St Louis, MO) for 75 minutes at 37°C. The cell suspension was then filtered through a strainer and isolated on a Percoll separation gradient. Lung and liver lymphocytes were isolated by collagenase D digestion followed by layering on a Percoll gradient as previously described in this paragraph. CD11c+ dendritic cells were isolated from lung or liver by collagenase D digestion followed by layering on NYcoPrep Axis-Shield, Oslo, Norway).
Bone marrow transplantation
Bone marrow (BM) was flushed from donor femurs and tibias with Dulbecco modified media (DMEM) and passed through sterile mesh filters to obtain single cell suspensions. BM was T-cell depleted (TCD) in vitro with anti-Thy1.2 monoclonal antibody plus low toxicity rabbit complement (C-6 Diagnostics, Mequon, WI). The hybridoma for 30-H12 (anti-Thy1.2, rat IgG2b) antibody was purchased from the American Type Culture Collection (Rockville, MD). BM cells were washed and resuspended in DMEM prior to injection. Red cells were removed from spleen cell suspensions by hypotonic lysis using distilled water. Host mice were conditioned with total body irradiation (TBI) administered as a single exposure at a dose rate of 86 cGy using a Shepherd Mark I Cesium Irradiator (JL Shepherd and Associates, San Fernando, CA). Irradiated recipients received a single intravenous injection in the lateral tail vein (0.4 mL) of TCD BM and/or added spleen, T, or B cells.
Generation of bone marrow chimeras
Balb/c Rag mice were lethally irradiated (900 cGy) and received transplants of 10 × 106 B6 Rag BM cells on day 0. Mice were bled 2 months after transplantation to confirm complete donor cell engraftment prior to use in experiments.
Flow cytometry
Cells were washed in PBS containing 2% fetal bovine serum (FACS buffer) and then incubated for 15 minutes at 4°C with antibodies. In some instances, cells were first incubated with Fc block (Caltag, San Francisco, CA) for 15 minutes at 4°C prior to staining with antibody. Monoclonal antibodies (mAbs) conjugated to either fluorescein isothiocyanate (FITC), phycoerythrin (PE), or Tricolor (TC) were used to assess cell populations in mice. PE-anti-CD8 (clone CT-CD8a, rat IgG2a) and TC-anti-CD4 (clone CT-CD4, rat IgG2a) were obtained from Caltag. PE-anti-Mac1 (clone M1/70 rat IgG2b), PE-anti-CD4 (clone GK1.5, rat IgG2b), PE–anti-TCR αβ (clone H57-597, hamster IgG), FITC–anti-Thy1.2 (clone 30-H12, rat IgG2b), FITC–anti-Thy 1.1 (clone OX-7, mouse IgG1), PE-CD45R (clone RA3-6B2, rat IgG2a), FITC–anti-Gr-1 (clone RB6-8C5, rat IgG2b), FITC–anti-CD11c (clone HL3, hamster IgG), and FITC–anti-H-2Kb (clone AF6-88.5, mouse IgG2a) all were purchased from BD Biosciences Pharmingen (San Diego, CA). Intracellular staining for foxp3 was performed using the Foxp3 kit (EBiosciences, San Diego, CA) according to the manufacturer's instructions. Cells were analyzed on a FACScan flow cytometer with Cellquest software (Becton Dickinson, Mountain View, CA). Data were analyzed using FlowJo (Treestar, Ashland, OR).
Histologic analysis
Representative samples of liver and colon were obtained from mice and fixed in 10% neutral-buffered formalin. Samples were then embedded in paraffin, cut into 5-micron thick sections, and stained with hematoxylin and eosin. A semiquantitative scoring system was employed to account for histological changes in the colon. Changes compatible with GVHD were deemed to be goblet cell depletion, apoptotic enterocytes, crypt cell abscesses, lamina propria inflammation, and ulceration of the colonic mucosa. Histological tissue damage was graded as 0 for normal, 1 for mild, 2 for moderate, and 3 for severe for each of these criteria. To assess the severity of liver pathology, we employed a GVHD grading system previously published by Cooke and colleagues.10 This scoring system evaluates apoptosis, portal infiltrates, lobular infiltrates, bile duct damage, and vascular endothelialitis. The scoring system for each of these parameters denoted 0 as normal, 1 as mild, 2 as moderate, and 3 as severe. All slides were coded and read in a blinded fashion. Images were visualized using a Nikon Eclipse E400 microscope and a Nikon Plan APO 10×/0.45 NA objective lens (Nikon, Tokyo, Japan). Image acquisition was performed with a Zeiss Axiom camera and Axiovision 3.0.6 SP2 software (Zeiss, Berlin, Germany).
Immunofluorescence
Frozen colonic tissue samples were sectioned on a cryostat and fixed in ice-cold acetone for 10 minutes. Slides were allowed to dry, washed in PBS, and then quenched with 0.3% hydrogen peroxide. To minimize nonspecific staining, tissue samples were treated with Fc block and then incubated with PE-anti-CD8 (1:200 dilution) or APC-anti-CD4 (1:500 dilution) antibodies for one hour at 4°C. Images were then acquired using a Leica TCS SP2 laser scanning confocal microscopic imaging system (Wetzlar, Germany).
Serum cytokine analysis
Serum was collected from mice by retroorbital bleeds and analyzed on a Bioplex System (BioRad Laboratories, Hercules, CA) according to the manufacturer's instructions. All samples were run in duplicate.
Intracellular cytokine staining
Spleen cells or purified CD4+ T cells were stimulated with 50 ng/mL phorbol myristate acetate (PMA) (Sigma) and 750 ng/mL ionomycin (Calbiochem, La Jolla, CA) for 2.5 hours and then incubated with GolgiStop (BD Pharmingen, San Jose, CA) for an additional 2.5 hours. Cells were then surface stained with TC-CD4 and then intracellularly stained with PE-labeled antibodies to interleukin-17 (IL-17) or IL-4, or FITC-labeled antibody to interferon (IFN)-γ (BD Pharmingen). PE-labeled IgG2a and FITC-labeled IgG1 were used as isotype controls. Cells were analyzed on a FACScan flow cytometer with Cellquest software.
Statistics
Data in text are given as means plus or minus SEM. Group comparisons of T-cell and granulocyte numbers, serum cytokine levels, and overall pathology scores were performed using the Mann Whitney U test. Comparative analysis of cell ratios was performed by log transformation to stabilize the variance followed by the Student t test. A P value less than or equal to .05 was deemed to be significant in all experiments.
Results
CD4+ T cells are necessary and sufficient for the induction of GVHD-associated autoimmunity
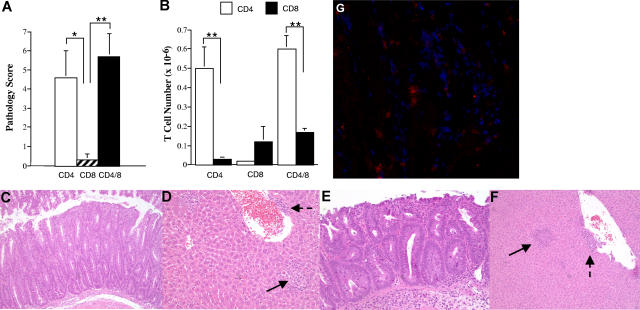
We have previously shown that splenic T cells obtained from mice undergoing acute GVHD can induce autoimmunity after adoptive transfer into secondary immunodeficient hosts of donor MHC type.9 To further understand this phenomenon, the role of specific T-cell populations in the induction of autoimmunity was determined. Lethally irradiated Balb/c mice received transplants of TCD B6 BM plus 3 × 105 B6 spleen cells to induce acute GVHD. CD4+, CD8+, or both CD4+ and CD8+ T cells were purified from the spleens of primary (B6→ Balb) GVHD mice 19 to 21 days after BMT and then transplanted into nonirradiated B6 Rag animals. Animals were killed 8 to 10 weeks after transfer, unless progressive weight loss necessitated earlier termination, and examined for histological evidence of target organ damage in the colon or liver and expansion of CD4+ and CD8+ T cells in the spleen. Recipients of either CD4+ T cells alone or CD4+ and CD8+ T cells had significantly higher pathological scores when compared with mice that received CD8+ T cells only, where overall pathology was negligible (Figure 1A). There was no statistically significant difference in the pathological score between mice that received a combination of both CD4+ and CD8+ T cells versus recipients of purified CD4+ T cells alone (P = .43), demonstrating that the addition of CD8+ T cells did not significantly exacerbate disease. Coincident with the observed pathology, there was expansion of CD4+ T cells in the spleens of mice that received either purified CD4+ T cells alone or the combination of CD4+ and CD8+ T cells (Figure 1B, see figure legend for T-cell input numbers). In animals that received both CD4+ and CD8+ T cells, the absolute number of CD4+ T cells was significantly greater than the number of CD8+ T cells (P < .001). In contrast, no splenic CD8+ T-cell expansion was observed in mice reconstituted with CD8+ T cells alone. Histological analysis demonstrated similar pathological abnormalities in the colons and livers of mice that received either CD4+ T cells alone or CD4+ and CD8+ T cells (Figure 1C-F). Immunofluorescence staining of colonic tissue from mice that received both CD4+ and CD8+ GVHD T cells revealed that both T-cell populations were detectable in inflamed colons (Figure 1G). Thus, the failure of CD8+ T cells to induced autoimmune-mediated pathology was not due to the inability of these cells to traffic to target organs.
Figure 1.
CD4+ T cells are necessary for the induction of GVHD-associated autoimmunity. Lethally irradiated (900 cGy) Balb/c mice received transplants of TCD B6 BM plus 3 × 105 B6 spleen cells. At 19 to 21 days after BMT, animals were killed and cohorts of nonirradiated B6 Rag mice received transplants of either purified CD4+ (n = 18), CD8+ (n = 8), or the combination of CD4+ and CD8+ T cells (n = 24) from pooled spleen cell suspensions using the MACS isolation system. B6 Rag animals were then killed 35 to 74 days after transfer. (A) Overall pathological score, as defined in “Histologic analysis,” of mice that received transplants of either CD4+ (□), CD8+ (▨), or CD4+ and CD8+ T cells (■). The CD4+ T-cell dose ranged from 0.3 to 0.5 × 106 (mean, 0.43 × 106), while the CD8+ T-cell dose ranged from 0.35 to 0.5 × 106 (mean, 0.43 × 106). Animals that received transplants of the combination of CD4+ and CD8+ T cells received 0.5 × 106 cells (mean, 3 × 105 CD4+ and 2 × 105 CD8+ T cells). Results are derived from 3 to 5 experiments per T-cell group. Data are presented as the mean plus or minus SEM. (B) Absolute number of CD4+ (□) or CD8+ (■) T cells in the spleens of mice that received transplants of purified CD4+, CD8+, or CD4+ and CD8+ T cells from B6→ Balb chimeras. Data are presented as the mean plus or minus SEM. (C-F) Histology of colon (C,E) and liver (D,F) from representative B6 Rag recipients that received either purified CD4+ T cells (C,D) or both CD4+ and CD8+ T cells (E,F) from primary (B6→ Balb) GVHD animals. Colons show marked infiltration of mononuclear and granulocytic cells into the lamina propria along with extensive goblet cell depletion. Livers reveal portal (dashed arrows) and lobular (solid arrows) inflammation with mononuclear and granulocytic infiltrates. (G) Immunofluorescence staining from the colon of a representative B6 Rag mouse that received purified CD4+ and CD8+ T cells from primary GVHD animals showing infiltration of the lamina propria with both CD4+ (blue) and CD8+ (red) T cells. Statistics: *P ≤ .05; **P < .01.
GVHD-associated autoimmunity is due to the absence of CD4+CD25+ regulatory T cells
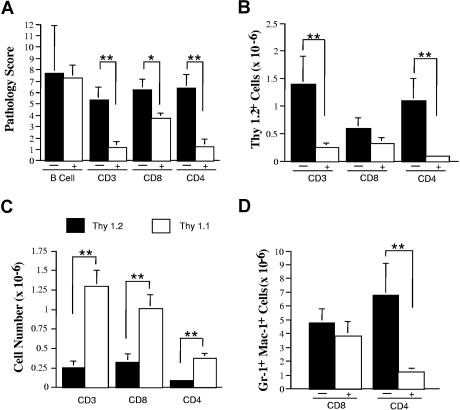
The prominent histological manifestation of colitis that we observed in mice with autoimmunity has typically been ascribed in other murine models of inflammatory bowel disease to an inadequate regulatory response.11,12 This led us to postulate that lack of effective regulation was a possible mechanism for the development of disease. To address this question in a systematic fashion, we examined the ability of B and T cells obtained from normal mice to rescue animals from autoimmune-mediated pathological damage. Spleen cells from GVHD animals 19 to 23 days after BMT were transferred alone or together with B or T cells from normal B6 or B6.PL (Thy 1.1+) mice into B6 Rag recipients. The administration of purified B cells failed to prevent autoimmunity, as the pathological score in mice given GVHD spleen cells alone (7.7 ± 4.1, n = 3) was not significantly different than that observed in animals administered adjunctive B cells (7.3 ± 1.1, n = 9) (P = .84) (Figure 2A). Conversely, co-transfer of normal αβ+ T cells from B6.PL mice significantly reduced the pathological score (Figure 2A) and resulted in a 6-fold reduction in the absolute number of Thy 1.2+ effector T cells in the spleen (Figure 2B). In fact, the majority of T cells were Thy 1.1+, indicative of the preferential survival of these cells (Figure 2C). CD8+ T cells alone had a partially protective effect with respect to pathological score, but these cells did not affect the degree of myeloid expansion in the spleen, which is a hallmark of autoimmunity in this model9 (P = .47) (Figure 2D), or the absolute number of Thy 1.2+ T cells (P = .12) (Figure 2B) when compared with control animals. This was in contrast to CD4+ T cells that resulted in statistically significant reductions in overall pathologic score, splenic Thy 1.2+ cells, and myeloid expansion (Figure 2A,B,D).
Figure 2.
GVHD-associated autoimmunity is due to the absence of appropriate T-cell regulation. Lethally irradiated (900 cGy) B10.BR mice received transplants of TCD B6 BM (10 × 106) plus 2 × 106 B6 spleen cells. Mice were killed 21 days after transplantation, and spleen cells (adjusted to yield a T-cell dose of 106 T cells per animal) were transferred into nonirradiated B6 Rag mice alone (n = 3) or together with 19 × 106 purified B cells (n = 9) from normal B6 animals. In subsequent experiments, lethally irradiated (900 cGy) Balb/c mice received transplants of TCD B6 BM (10 × 106) plus 3-4 × 105 B6 spleen cells. At 20 to 23 days after BMT, mice were killed, and spleen cells (adjusted to yield 0.5-1 × 106 T cells) were transferred alone (n = 8-21 mice/group) or together with an equivalent number of CD4+ plus CD8+ (n = 11), CD8+ (n = 21), or CD4+ T cells (n = 8) from B6.PL (Thy1.1+) animals into nonirradiated B6 Rag animals. Data are presented as means plus or minus SEM. (A) Overall pathology score of mice that received transplants of GVHD spleen cells alone (■) or together with the specified population of purified B or T cells from normal B6 or B6.PL mice (□). (B) Absolute number of Thy 1.2+ T cells in the spleens of mice that received transplants of GVHD spleen cells alone (■) or together with either CD3+, CD8+, or CD4+ T cells from normal B6.PL animals (□). (C) Absolute number of Thy 1.2+ (■) and Thy1.1+ (□) T cells in the spleens of mice that received transplants of GVHD spleen cells and either CD3+, CD8+, or CD4+ T cells from normal B6.PL animals. (D) Absolute number of Gr-1+ Mac-1+ cells in the spleens of mice that received transplants of GVHD spleen cells alone (■) or together with either purified CD8+ or CD4+ T cells (□) from normal B6.PL mice. Statistics: *P < .05; **P < .01.
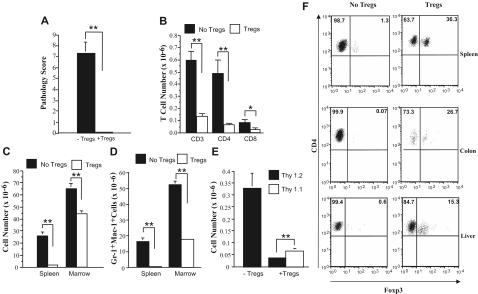
Given the more complete protective effect conferred by CD4+ T cells, we then examined whether CD4+CD25+ regulatory T cells (Tregs) were the primary population responsible for the prevention of autoimmunity. The co-administration of Tregs along with GVHD spleen cells resulted in complete protection from pathological damage when compared with animals that received GVHD spleen cells alone (P = .006) (Figure 3A). Cotransfer of Tregs also resulted in statistically significant reductions in the absolute numbers of CD3+, CD4+, and CD8+ T cells in the spleen (Figure 3B). Overall cellularity in the spleen and bone marrow (Figure 3C), and more specifically, myeloid expansion in both the spleen and bone marrow (Figure 3D), were also significantly lower in mice that were administered Tregs at the time of transfer. There was also a significant reduction in Thy 1.2+ cells and preferential survival of Tregs (Figure 3E). This was confirmed by foxp3 staining of splenic CD4+ T cells, where a sizable population of Tregs was detected, while these cells were virtually absent in animals that did not receive Tregs (Figure 3F). Similarly, approximately 15% to 25% of colonic intraepithelial (IEL) and hepatic CD4+ T cells from protected mice expressed foxp3, indicating that Tregs were able to traffic to target tissues (Figure 3F). Conversely, no detectable foxp3 expression was evident in animals that were not administered Tregs.
Figure 3.
CD4+CD25+ regulatory T cells protect mice from developing autoimmunity. Lethally irradiated (900 cGy) Balb/c mice received transplants of TCD B6 BM (10 × 106) plus 3-4 × 105 B6 spleen cells. At 20 days after BMT, mice were killed, and spleen cells (adjusted to yield 0.5 × 106 T cells) were transferred either alone (n = 7) or together with an equivalent number of CD4+ CD25+ T cells (n = 7) into nonirradiated B6 Rag animals. Mice were killed 42 to 87 days after BMT. (A) Overall pathology score of mice that received transplants of GVHD spleen cells alone (■) or together with Tregs (□). (B) Absolute number of CD3+, CD4+, and CD8+ T cells in the spleens of mice that received transplants of GVHD spleen cells alone (■) or with CD4+CD25+ Tregs (□). (C,D) Overall cellularity and absolute number of Gr-1+ Mac-1+ cells in the spleen and bone marrow of mice that received transplants of GVHD spleen cells alone (■) or together with Tregs (□). (E) Absolute number of Thy 1.2+ (■) or Thy1.1+ (□) T cells in the spleens of mice that received transplants of GVHD spleen cells alone or together with CD4+CD25+ T cells. Data in Figure 3A-E are presented as the mean plus or minus SEM and are the cumulative results of 2 experiments. (F) Spleen cells, hepatic lymphocytes, and IELs from the colon were isolated from mice with autoimmunity and similarly from animals that had received transplants that were coadministered Tregs and had no evidence of pathological damage. The percentage of foxp3-expressing cells in the gated CD4+ T-cell population from each tissue site is depicted by the numbers in the quadrants. Statistics: *P < .05; **P < .01.
Acute GVHD deleteriously affects the reconstitution of CD4+Foxp3+ T cells
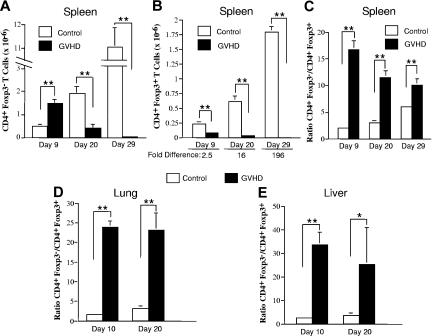
The absence of Tregs in mice with autoimmunity was evidence that there was loss of these cells during acute GVHD prior to the transfer of spleen cells into secondary recipients. To further address this issue, lethally irradiated Balb/c mice received transplants of TCD B6 BM alone (control) or together with 3 × 105 B6 spleen cells (GVHD). Cohorts of animals were then killed at 10-day intervals after transplantation, and the relative and absolute numbers of CD4+Foxp3− and CD4+Foxp3+ T cells were examined in the spleen, lung, and liver. A progressive increase in the absolute numbers of both splenic CD4+Foxp3− and CD4+Foxp3+ T cells was observed in control mice (Figure 4A,B). In contrast, the absolute number of both cell populations declined over time in acute GVHD animals due to impaired splenic reconstitution and was significantly lower than in control mice at all time points. The decrease in splenic CD4+Foxp3+ T cells in GVHD animals, however, was proportionately greater than for CD4+Foxp3− T cells, with the net result being a significantly higher ratio of CD4+Foxp3− to CD4+Foxp3+ T cells (Figure 4C). When the lung and liver were examined, we also observed a significantly higher ratio of CD4+ Foxp3− to CD4+Foxp3+ T cells in GVHD animals compared with controls (Figure 4D,E), indicating that acute GVHD deleteriously affected the reconstitution of Tregs in target organs as well.
Figure 4.
Acute GVHD results in a significant reduction in the ratio of CD4+ Foxp3+ to CD4+ Foxp3− T cells in the spleen and in GVHD target tissues. Lethally irradiated (900 cGy) Balb/c mice received transplants of TCD B6 BM alone (control) or together with 3 × 105 B6 spleen cells (GVHD). Cohorts of mice (n = 6/group per time point) were then killed on days 9, 20, or 29 after transplantation. (A,B) The absolute number of CD4+Foxp3− and CD4+Foxp3+ T cells in the spleens of control or GVHD animals is shown in panels A and B, respectively, for each time point. (C) The ratio of CD4+Foxp3− to CD4+Foxp3+ T cells in the spleens of control and GVHD mice. Data in panels A-C are the cumulative results from 2 experiments and are presented as the mean plus or minus SEM. (D,E) Lethally irradiated (900 cGy) Balb/c mice were transplanted with TCD B6 BM alone (control) or together with 3 × 105 B6 spleen cells (GVHD). Lymphocytes were isolated from the lungs or livers of control or GVHD mice (n = 2-3/group per time point) and pooled suspensions were analyzed for the proportion of CD4+Foxp3− versus CD4+Foxp3+ T cells. The ratio of CD4+Foxp3− to CD4+Foxp3+ T cells in the lung and liver of control and GVHD mice at the indicated time points is shown in panels D and E, respectively. Data are derived from 3 to 4 experiments per time point and are presented as the mean plus or minus SEM. Statistics: *P ≤ .05, **P < .01.
Absence of CD4+ CD25+ Foxp3+ T cells results in TH1- and TH17-mediated proinflammatory cytokine production in autoimmune mice
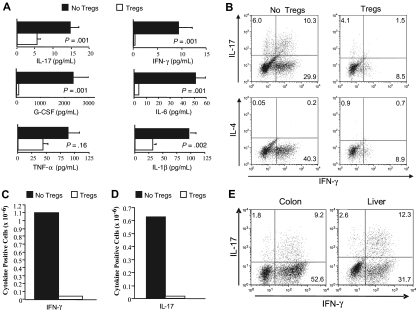
Studies were then performed to determine the mechanism by which absence of Tregs led to the development of GVHD-associated autoimmunity. Serum cytokine analysis revealed that autoimmune mice had significantly higher levels of proinflammatory mediators such as IFN-γ, IL-6, G-CSF, IL-1β, and IL-17 than animals that received Tregs (Figure 5A). Given the higher levels of IFN-γ and IL-17 in autoimmune animals and the requirement for CD4+ T cells in the pathogenesis of disease (Figure 1A), we performed studies to confirm that CD4+ T cells from these mice were capable of producing these cytokines. Intracellular staining of splenic CD4+ T cells demonstrated both IFN-γ– and IL-17–producing cells, while IL-4–secreting T cells were not detected (Figure 5B). The average percentage of TH1 and TH17 cells in the spleens of these animals was 34.7 (± 3.3) and 9.3 (± 1.9), respectively (mean 6 independent experiments). Co-administration of Tregs to B6 Rag recipients resulted in a substantial reduction in the absolute number of IFN-γ (Figure 5C) and IL-17–producing CD4+ T cells (Figure 5D). Notably, there was a preferential reduction in the number of CD4+ T cells that secreted both IFN-γ and IL-17, which constituted the majority of IL-17–producing CD4+ T cells in autoimmune animals (Figure 5B). To determine whether TH1 and TH17 cells also were present in target organs, colon and liver tissue were examined for the presence of these cell populations. In both organs, TH1 and TH17 cells were present in similar percentages as observed in the spleen, supporting a role for these cells in mediating pathological damage (Figure 5E).
Figure 5.
Autoimmunity is characterized by TH1- and TH17-mediated proinflammatory cytokine production that is suppressed by CD4+CD25+Foxp3+ T cells. Lethally irradiated Balb/c mice received transplants of TCD BM (10 × 106) plus 3 × 105 B6 spleen cells. At 20 days after transplantation, spleen cells (adjusted to yield a T-cell dose of 0.5 × 106 cells) were transplanted into B6 Rag mice alone (n = 7) or together with 0.5 × 106 purified CD4+CD25+ T cells from B6.PL animals (n = 7). (A) B6 Rag mice that received GVHD spleen cells alone or GVHD spleen cells plus Tregs were bled 42 to 83 days after BMT, and serum was analyzed for presence of IL-1β, TNF-α, G-CSF, IL-6, IFN-γ, and IL-17. Data are presented as the mean plus or minus SEM and are cumulative results from 2 experiments. IFN-γ levels were undetectable in 4 of 6 mice that received Tregs. (B) CD4+ T cells were purified from the spleens of B6 Rag mice that received GVHD spleen cells only (■) or together with Tregs (□) 42 to 83 days after transplantation. Cells were then restimulated with PMA and ionomycin in the presence of GolgiStop and then intracellularly stained with IFN-γ, IL-17, or IL-4–specific antibodies. Dot plots show the percentage of CD4+ T cells that stained positive for each of the cytokines. (C,D) The average absolute number of IFN-γ and IL-17–positive CD4+ T cells from pooled spleen cell suspensions obtained from mice (n = 3/group) that received transplants of primary GVHD spleen cells alone (■) or together with an equivalent number of Tregs (□). Data are from 1 of 2 experiments that yielded similar results. (E) Lymphocytes were isolated from the colon lamina propria or livers of B6 Rag mice (n = 5) 72 days after transplantation with primary GVHD spleen cells. Dot plots show the percentage of CD4+ T cells that were positive for IL-17 and/or IFN-γ. Data are from 1 of 2 experiments that yielded similar results.
Donor-derived TH1 and TH17 cells emerge during acute GVHD
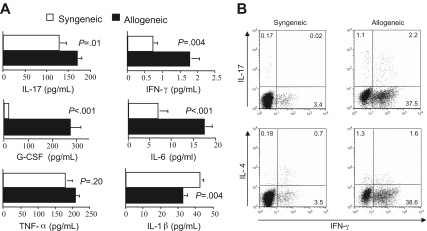
The presence of a TH1- and TH17-mediated proinflammatory milieu in autoimmune mice raised the question as to whether these cells were present early in the course of GVHD or only emerged later after transfer into secondary recipients. To address this question, we first examined whether the cytokine milieu in mice with acute GVHD was similar to that observed in animals with autoimmunity. Lethally irradiated Balb/c mice received transplants of either TCD B6 BM plus 5 × 105 B6 spleen cells (allogeneic) or TCD Balb/c BM plus an equivalent number of Balb spleen cells (syngeneic) as control animals. Recipients of allogeneic marrow grafts had significantly higher levels of G-CSF, IL-17, IFN-γ, and IL-6 than syngeneic controls 21 days after BMT (Figure 6A). Thus, with the exception of IL-1β, proinflammatory cytokines that were present in autoimmune mice also were elevated in animals with acute GVHD. Studies were then conducted to examine the CD4+ T-cell cytokine phenotype in mice with acute GVHD. Although serum IFN-γ levels were quite low in animals with acute GVHD at the time of transfer into secondary recipients (Figure 6A), due to the fact that levels peaked on day 7 after transplantation before rapidly declining (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), intracellular cytokine analysis demonstrated a significantly increased percentage of IFN-γ–secreting CD4+ T cells (∼40%) (Figure 6B). There was also an increased population of CD4+ IL-17–producing cells, relative to syngeneic controls, with the majority of these cells secreting both IL-17 and IFN-γ, similar to what was observed in mice with autoimmunity (Figure 5B). These data indicated that the proinflammatory environment was comparable in mice with acute GVHD and those with autoimmunity, and that potentially autoreactive donor-derived TH1 and TH17 cells emerged during the course of acute GVHD.
Figure 6.
Donor-derived TH1 and TH17 cells that mediate autoimmunity emerge during acute GVHD. (A) Lethally irradiated (900 cGy) Balb/c mice received transplants of TCD Balb BM plus 3 × 105 Balb spleen cells (syngeneic) (□) or TCD B6 BM (10 × 106) plus 3 × 105 B6 spleen cells (allogeneic) (■). Mice in both cohorts were bled 21 days after transplantation, and serum was analyzed for presence of IL-17, G-CSF, TNF-α, IL-6, IL-1β, and IFN-γ. Data are presented as the mean plus or minus SEM and are cumulative results from 2 experiments. (B) Lethally irradiated Balb mice were transplanted with TCD BM (10 × 106) plus 3 × 105 B6 spleen cells. At 20 days after transplantation, CD4+ T cells were purified from pooled spleen cell suspensions by magnetic bead separation. Cells were then restimulated with PMA and ionomycin in the presence of GolgiStop and then intracellularly stained with IFN-γ, IL-17, or IL-4–specific antibodies. Dot plots show the percentage of CD4+ T cells that were positive for each of these cytokines.
CD4+CD25+Foxp3+ regulatory T cells prevent the propagation of GVHD mediated through the indirect alloreactive pathway
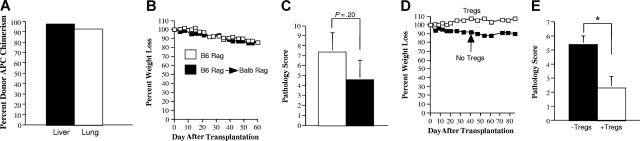
During chronic GVHD in man, donor T-cell recognition of host antigens occurs in the context of donor APCs through the indirect alloreactive pathway. We therefore examined whether Tregs that were able to prevent the development of autoimmunity also were capable of protecting mice from GVHD induced by way of indirect allorecognition. To address this question, B6 Rag BM→ Balb Rag chimeric mice were created so that allorecognition of Balb host antigens would occur in the context of B6 donor APCs. To verify that APCs from chimeric mice were of donor origin, animals were killed 60 days after transplantation, and CD11c+ cells from both the liver and lung were determined to be of donor (B6) origin (Figure 7A). In subsequent experiments, spleen cells from mice with acute GVHD were transplanted into chimeric (B6 Rag BM→ Balb Rag) or B6 Rag animals to determine whether T cells that induced autoimmunity also were capable of causing pathological damage by way of indirect allorecognition. Transplantation of the same spleen cell inoculum into both groups of mice resulted in comparable weight loss (Figure 7B) and pathological damage (Figure 7C). We then examined whether Tregs, which protected mice from autoimmunity, could prevent pathological damage in chimeric animals. The co-administration of Tregs along with spleen cells obtained from mice with acute GVHD protected chimeric animals from both weight loss (Figure 7D) and pathological damage (Figure 7E), indicating that Tregs were also capable of preventing GVHD that was propagated through the indirect alloreactive pathway.
Figure 7.
CD4+CD25+Foxp3+ regulatory T cells prevent the propagation of GVHD mediated through the indirect alloreactive pathway. (A) Lethally irradiated Balb Rag mice (900 cGy) (n = 3) received transplants of 5-10 × 106 B6 Rag BM cells. Sixty-eight days after transplantation, animals were killed, and CD11c+ cells were isolated from the liver and lung of recipients as described in “Cell isolation.” The percentage of donor-derived H-2Kb+ CD11c+ dendritic cells in each of these organs is depicted. Data are presented as the mean plus or minus 1 SD and are representative of 3 individual experiments. Actual values are 97.5 (± 0.35) for liver and 92.6 (± 1.1) for lung. (B,C) Lethally irradiated Balb/c mice received transplants of TCD B6 BM (10 × 106) plus 3 × 105 B6 spleen cells. At 20-22 days after transplantation, spleen cells (adjusted to yield a T-cell dose of 0.5 × 106 cells) were transplanted into either B6 Rag (■) (n = 5) or B6 Rag BM→ Balb Rag chimeric mice that were 60 to 70 days after transplantation of B6 Rag BM cells (□) (n = 7). B6 Rag and B6 Rag BM→ Balb Rag mice that received spleen cells from primary GVHD animals were then killed 60 to 62 days after transplantation. Serial weight curves (B) and overall pathological score for the colon and liver (C) are depicted. Data are presented as the mean plus or minus SEM and are the cumulative results from 2 experiments. (D,E) Lethally irradiated Balb/c mice received transplants of TCD B6 BM (10 × 106) plus 3 × 105 B6 spleen cells. At 20 days after transplantation, spleen cells (adjusted to yield a T-cell dose of 0.5 × 106 cells) were transplanted into B6 Rag BM→ Balb Rag chimeric mice alone (■) (n = 5) or together with an equivalent number of purified CD4+CD25+ B6.PL T cells (□) (n = 4). Mice were killed 85 days after transplantation. Serial weight curves (D) and overall pathological score (± SD; E) are depicted. Statistics: *P < .05.
Discussion
Chronic GVHD is a major cause of mortality after allogeneic BMT and deleteriously affects the quality of life in surviving patients who otherwise have been cured of their underlying disease.13 The major criteria for the diagnosis of chronic GVHD in man are based on pathologic changes occurring in the skin, lung, mucous membranes (ie, mouth, genitalia), gastrointestinal tract, and musculoskeletal system. Many of these organ-specific manifestations that are diagnostic of chronic GVHD such as lichen planus,14 bronchiolitis obliterans,15,16 lichen sclerosus,17 and morphea18 are presumed to be of autoimmune origin when they occur in non-BMT recipients. Moreover, discrete autoimmune disorders that involve the hematopoietic, endocrine, and neurologic systems have all been reported in these patients.5–7 The occurrence of these abnormalities in GVHD recipients, therefore, strongly points to autoimmunity as an integral and unique component of the pathophysiology of chronic GVHD.
In these studies, we have demonstrated that autoimmunity, which develops as a consequence of GVHD, is attributable to donor-derived CD4+ T cells with TH1 and TH17 cytokine phenotypes. The emergence of these cells is due to the disproportionate loss of CD4+ CD25+ Foxp3+ cells, which unleashes TH1 and TH17 cells to expand, release proinflammatory cytokines, and cause pathological damage. Both TH1 and TH17 cells are present early after transplantation, providing evidence that the pathophysiological events that lead to the development of chronic GVHD are set in motion during the acute phase of GVHD. Thus, these cells serve as a critical link between the alloreactivity of acute GVHD and the autoreactivity of chronic GVHD, although the relative contribu-tions of TH1 and TH17 cells in the induction of pathological damage are not resolved by these studies.
Classically, CD4+ T cells have been assigned to TH1 or TH2 lineages based on the cytokines produced by these cells.19 A role for TH1 cells in the transition from acute to chronic GVHD is supported by clinical data showing that CD4+ T-cell clones, which produce IFN-γ, have been identified in patients with acute GVHD,20 while increased levels have also been demonstrated in chronic GVHD patients.21–23 Increased transcription of IFN-γ has also been detected in the involved skin of patients with chronic GVHD,21 supporting a role for this cytokine in tissue injury. While a large percentage of CD4+ T cells in autoimmune animals were TH1 cells, there was also a distinct population of CD4+ T cells that produced IL-17, which is a developmental lineage distinct from TH1 and TH2 cells.24–26 IL-17 has been shown to play an etiological role in autoimmune disorders such as experimental allergic encephalomyelitis27 and collagen-induced arthritis,28 but has not been previously studied in allogeneic BMT recipients. T-cell production of IL-17 induces epithelial, endothelial, and stromal cells to secrete proinflammatory and hematopoietic cytokines such as IFN-γ, IL-6, TNF-α, IL-1β, and G-CSF,29,30 all of which were elevated in mice with autoimmunity. Interestingly, these cytokines have also been shown to be increased in patients with chronic GVHD,20,22,31,32 raising the possibility that an IL-17–mediated cytokine cascade may be important in the pathogenesis of chronic GVHD. Co-administration of Tregs resulted in significant reductions in nearly all proinflammatory cytokines as well as a substantial decrease in TH1 and TH17 cells (Figure 5C,D). Notably, the TH17 cell population that was most profoundly reduced by Tregs secreted both IL-17 and IFN-γ, whereas CD4+ T cells that produced only IL-17 were less affected (Figure 5D). CD4+ IL-17+ IFN-γ+ T cells recently have been postulated to have an etiological role in the pathogenesis of experimental autoimmune encephalomyelitis33 and may therefore be an IL-17–producing subpopulation that is important in autoimmunity that occurs as a consequence of GVHD.
The major event that led to the development of autoimmunity and, by extension, chronic GVHD, was the lack of proportionate Treg expansion in the setting of a strong alloresponse. Rather, there was a significant increase in the ratio of CD4+Foxp3− to CD4+Foxp3+ cells in the spleen and in GVHD target organs, which led to the emergence of TH1 and TH17 cells in secondary recipients. A decline in Tregs as a critical event in the pathophysiology of chronic GVHD is supported by several studies that have documented a direct correlation between a reduction in the number of Foxp3+ regulatory T cells in both the peripheral blood and intestinal tract and the presence of chronic GVHD,34–36 although this observation has not been confirmed in all studies.37,38 Of particular relevance to our report are studies from Rieger and colleagues,36 who demonstrated that, in patients without GVHD or in nontransplant patients with either diverticulitis or cytomegalovirus (CMV) colitis, there is a marked increase in the number of Tregs relative to effector CD8+ T cells. This is in contrast to patients with either acute or chronic GVHD, where the ratio of these 2 cell populations was no different from that observed in healthy controls. Moreover, consistent with our data (Figure 4), these investigators observed that the ratio of CD4+Foxp3+ to CD4+non-Foxp3+ T cells also was significantly reduced in GVHD recipients, indicating that the decline in Treg numbers was not attributable to a more general reduction in the number of CD4+ T cells. Thus, unlike other inflammatory disorders, GVHD appears to be characterized by the failure to mount a counter-regulatory response to the underlying inflammatory process.
The reason for the lack of Treg expansion in GVHD is not clear but may be attributable to the cytokine milieu that exists in acute GVHD animals. IL-6, for example, which was significantly elevated in the sera of these mice, has been shown to deleteriously affect the generation of adaptive Tregs induced by transforming growth factor (TGF)-β39,40 by blocking foxp3 expression.41 Furthermore, IL-6 produced by dendritic cells after activation through Toll-like receptors is able to inhibit the suppressive function of natural Tregs.42,43 Interestingly, clinical studies have shown that patients with elevated plasma levels of IL-6,31,32 as well as those with a recipient or donor IL-6 genotype that results in increased IL-6 production,44,45 have an increased incidence and severity of GVHD. The failure to mount a counter-regulatory response to GVHD-induced inflammation may therefore be, in part, attributable to the proinflammatory release of IL-6. Alternatively, IL-2 is required for the expansion and conversion of CD4+Foxp3− T cells into CD4+Foxp3+ cells46 and for both the function and maintenance of natural Tregs.47,48 IL-2 levels, unlike many other cytokines, have not been reported to be increased during GVHD,49,50 and therefore the relative absence of IL-2 could also serve as a limiting factor in Treg expansion and inhibit Treg function.
The observation that TH1 and TH17 cells were present within the first 2 to 3 weeks after transplantation is evidence that the pathophysiological events that lead to chronic GVHD are set in motion during the acute phase of GVHD. In fact, the proinflammatory environment induced during GVHD may actually favor the generation of these autoreactive T cells. For instance, IL-6 can, in the presence of TGF-β, lead to the differentiation of TH17 cells from TH0 precursors.41 IL-6 also has been shown to enhance the expansion and survival of antigen-activated CD4+ T cells.51 Thus, the dual effects of IL-6 to enhance differentiation and survival of TH17 cells and restrain Treg development may serve to promote autoreactivity, while at the same time inhibiting a counter-regulatory response. Additionally, IL-2 signaling has been shown to play a critical role in inhibiting the development of TH17 cells.52 The relative absence of IL-2 in the post-transplantation period also could serve in a dual capacity to constrain Treg development while promoting TH17 differentiation. The emergence of CD4+ T cells during acute GVHD with the potential to induce subsequent autoimmunity is supported by studies from Parkman and colleagues,53,54 who demonstrated that autoreactive noncytotoxic CD4+ T cells could be cloned from the peripheral blood of mice within 10 to 14 days after BMT. Our finding that CD4+IL-17+IFN-γ+ T cells, which have been implicated in the pathogenesis of autoimmunity,13 constituted the dominant IL-17–producing population in both acute GVHD and autoimmune mice is also consistent with the premise that potentially autoreactive T cells are present early after transplantation.
While acute GVHD is initiated by donor T-cell allorecognition of host antigens and host APCs or through the direct alloreactive pathway, the progression to chronic GVHD occurs by way of indirect allorecognition due to the repopulation of the APC compartment by donor-derived APCs. During acute GVHD, donor T cells recognize polymorphic alloantigens due to major or minor histocompatibility differences between donor and host. The fact that T cells from GVHD mice acquire the capability to recognize self antigens suggests that, during the evolution to chronic GVHD, antigenic recognition may no longer be restricted to polymorphic antigens but may also include nonpolymorphic antigens shared by donor and host. The ability of CD4+ T cells to recognize donor antigens to which they would otherwise be tolerant would, under these circumstances, be attributable to the loss of peripheral tolerance mechanisms that are the result of the relative absence of CD4+ CD25+ Foxp3+ T cells. If this is true, one would predict that the ability of donor T cells to recognize nonpolymorphic antigens would expand the breadth of the alloimmune response. In fact, clinically, this is exactly what is observed, as patients with chronic GVHD have much broader organ involvement than observed in acute GVHD patients, where tissue involvement is more restricted. What remains to be determined is the extent to which the T-cell populations that mediate autoreactivity and indirect alloreactivity are the same or distinct T cells. The identification of these T cells, however, may not be critical from a therapeutic perspective, given that both populations can be effectively prevented from causing pathological damage by co-transfer of Tregs (Figure 7). Thus, the direct administration of Tregs or, alternatively, strategies designed to alter the cytokine milieu to favor Treg expansion, may be viable approaches for the amelioration of chronic GVHD due to the ability of these cells to prevent autoreactivity as well as indirect alloreactivity.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health (HL64603 and 081650) and by awards from the Midwest Athletes Against Childhood Cancer Fund and the Ridin' for Research Foundation.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.C. designed experiments, performed animal studies, generated figures, and wrote the manuscript. S.V.J. and M.K. performed transplantations, flow cytometric analysis, and cell isolations. B.J. designed experiments and analyzed data. R.K. performed pathological analysis of all tissue samples. W.R.D. designed experiments, supervised the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William R. Drobyski, Bone Marrow Transplant Program, 9200 West Wisconsin Ave, Milwaukee, WI 53226; e-mail: wdrobysk@mcw.edu.
References
- 1.Glucksberg H, Storb R, Fefer A. Clinical manifestations of graft versus host disease in human recipients of marrow from HLA-identical sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Higman MA, Vogelsang GB. Chronic graft versus host disease. Br J Haematol. 2004;25:435–454. doi: 10.1111/j.1365-2141.2004.04945.x. [DOI] [PubMed] [Google Scholar]
- 3.Rouquette-Gally AM, Boyeldieu D, Prost AC, Gluckman E. Autoimmunity after allogeneic bone marrow transplantation: A study of 53 long-term-surviving patients. Transplantation. 1988;46:238–240. doi: 10.1097/00007890-198808000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Rouquette-Gally AM, Boyeldieu D, Gluckman E, Abuaf N, Combrisson A. Autoimmunity in 28 patients after allogeneic bone marrow transplantation: comparison with Sjogren's syndrome and scleroderma. Br J Haematol. 1987;66:45–47. doi: 10.1111/j.1365-2141.1987.tb06888.x. [DOI] [PubMed] [Google Scholar]
- 5.Sherer Y, Shoenfeld Y. Autoimmune diseases and autoimmunity post-bone marrow transplantation. Bone Marrow Transplant. 1998;22:873–881. doi: 10.1038/sj.bmt.1701437. [DOI] [PubMed] [Google Scholar]
- 6.Mackey JR, Desai S, Larratt L, Cwik V, Nabholtz JM. Myasthenia gravis in association with allogeneic bone marrow transplantation: clinical observations, therapeutic implications and review of literature. Bone Marrow Transplant. 1997;19:939–942. doi: 10.1038/sj.bmt.1700759. [DOI] [PubMed] [Google Scholar]
- 7.Drobyski WR, Potluri J, Sauer D, Gottschall JL. Autoimmune hemolytic anemia following T cell depleted bone marrow transplantation. Bone Marrow Transplant. 1996;17:1093–1099. [PubMed] [Google Scholar]
- 8.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health Consensus Development Project on criteria for clinical trails in chronic graft versus host disease, I: diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–955. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Tivol EA, Komorowski R, Drobyski WR. Emergent autoimmunity in graft versus host disease. Blood. 2005;105:4887–4893. doi: 10.1182/blood-2004-12-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke KR, Hill GR, Crawford JM, et al. Tumor necrosis factor alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft versus host disease. J Clin Invest. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 12.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice: disease development is prevented by co-transfer of purified CD4+ T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft versus host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108:2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugerman PB, Savage NW, Walsh LJ, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Med. 13:350–365. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 15.Snyder LD, Palmer SM. Immune mechanisms of lung allograft rejection. Sem Resp Crit Care Med. 2002;27:534–543. doi: 10.1055/s-2006-954610. [DOI] [PubMed] [Google Scholar]
- 16.Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+ CD25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799–1808. doi: 10.1111/j.1600-6143.2006.01383.x. [DOI] [PubMed] [Google Scholar]
- 17.Regauer S. Immune dysregulation in lichen sclerosus. Eur J Cell Biol. 2005;84:273–277. doi: 10.1016/j.ejcb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Takehara K, Sato S. Localized scleroderma is an autoimmune disorder. Rheumatology. 2005;44:274–279. doi: 10.1093/rheumatology/keh487. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clones, I: definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1985;136:2348–2357. [PubMed] [Google Scholar]
- 20.Faber LM, van Luxemburg-Heijs SA, Veenhof WF, Willemze R, Falkenburg JH. Generation of CD4+ cytotoxic T lymphocyte clones from a patient with severe graft versus host disease after allogeneic bone marrow transplantation: implications for graft versus leukemia reactivity. Blood. 1995;86:2821–2828. [PubMed] [Google Scholar]
- 21.Ochs LA, Blazar BR, Roy J, Rest EB, Weisdorf DJ. Cytokine expression in human cutaneous chronic graft versus host disease. Bone Marrow Transplant. 1996;17:1085–1092. [PubMed] [Google Scholar]
- 22.Ritchie D, Seconi J, Wood C, Walton J, Watt V. Prospective monitoring of tumor necrosis factor α and interferon γ to predict the onset of acute and chronic graft versus host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:706–712. doi: 10.1016/j.bbmt.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Körholz D, Kunst D, Hempel L, et al. Decreased interleukin 10 and increased interferon-γ production in patients with chronic graft versus host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1997;19:691–695. doi: 10.1038/sj.bmt.1700718. [DOI] [PubMed] [Google Scholar]
- 24.Kolls JK, Linden A. Interleukin 17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 26.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 28.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17 deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 29.Jovanovic DV, Di Battista JA, Martel-Pelletier J, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1β and TNF-α by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 30.Fossiez F, Djossou O, Chomarat P, et al. T cell interleukin 17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imamura M, Hashino S, Kobayashi H, et al. Serum cytokine levels in bone marrow transplantation: synergistic interaction of interleukin 6, interferon gamma, and tumor necrosis factor alpha in graft versus host disease. Bone Marrow Transplant. 1994;13:745–751. [PubMed] [Google Scholar]
- 32.Barak V, Levi-Schaffer F, Nisman B, Nagler A. Cytokine dysregulation in chronic graft versus host disease. Leuk Lymphoma. 1995;17:169–173. doi: 10.3109/10428199509051718. [DOI] [PubMed] [Google Scholar]
- 33.Axtell RC, Xu L, Barnum SR, Raman C. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17 expressing T cells in the central nervous system. J Immunol. 2006;177:8542–8549. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miura Y, Thoburn CJ, Bright EC, et al. Association of Foxp3 regulatory gene expression with graft versus host disease. Blood. 2004;104:2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 35.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of Foxp3+ CD4+ CD25+ regulatory T cells in patients with chronic graft versus host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieger K, Loddenkemper C, Maul J, et al. Mucosal Foxp3+ regulatory T cells are numerically deficient in acute and chronic GVHD. Blood. 2006;107:1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 37.Meignin V, de Latour R, Zuber J, et al. Numbers of foxp3-expressing CD4+ CD25high T cells do not correlate with the establishment of long term tolerance after allogeneic stem cell transplantation. Exp Hematol. 2005;33:894–900. doi: 10.1016/j.exphem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Arimoto K, Kadowaki N, Ishikawa T, Ichinohe T, Uchiyama T. Foxp3 expression in peripheral blood rapidly recovers and lacks correlation with the occurrence of graft versus host disease after allogeneic stem cell transplantation. Int J Hematol. 2007;85:154–162. doi: 10.1532/IJH97.06160. [DOI] [PubMed] [Google Scholar]
- 39.Fu S, Zhang N, Yopp AC, et al. TGF-β induces Foxp3+ T regulatory cells from CD4+ CD25− precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 40.Fantini M, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-β induces a regulatory phenotype in CD4+ CD25− T cells through foxp3 induction and down regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 41.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 42.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell–mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 43.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+ CD25+ T cell regulatory functions. J Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 44.Cavet J, Dickinson AM, Norden J, Taylor PRA, Jackson GH, Middleton PG. Interferon γ and interleukin 6 gene polymorphisms associate with graft versus host disease in HLA-matched sibling bone marrow transplantation. Blood. 2001;98:1594–1600. doi: 10.1182/blood.v98.5.1594. [DOI] [PubMed] [Google Scholar]
- 45.Socie G, Loiseau P, Tamouza R, et al. Both genetic and clinical factors predict the development of graft versus host disease after allogeneic hematopoietic stem cell transplantation. Transplantation. 2001;72:699–706. doi: 10.1097/00007890-200108270-00024. [DOI] [PubMed] [Google Scholar]
- 46.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+ CD25− cells to CD25+ Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 47.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4+ regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin 2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abu-Ghosh A, Goldman S, Slone V, et al. Immunological reconstitution and correlation of circulating serum inflammatory mediators/cytokines with the incidence of acute graft versus host disease during the first 100 days following unrelated umbilical cord blood transplantation. Bone Marrow Transplant. 1999;24:535–544. doi: 10.1038/sj.bmt.1701921. [DOI] [PubMed] [Google Scholar]
- 50.Imamura MS, Hashino S, Kobayashi H, et al. Hyperacute graft versus host disease accompanied by increased serum interleukin 6 levels. Int J Hematol. 1994;60:85–89. [PubMed] [Google Scholar]
- 51.Rochman I, Paul WE, Ben-Sasson SZ. IL-6 increases primed cell expansion and survival. J Immunol. 2005;174:4761–4767. doi: 10.4049/jimmunol.174.8.4761. [DOI] [PubMed] [Google Scholar]
- 52.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Parkman R. Clonal analysis of murine graft-vs-host disease, I: phenotypic and functional analysis of T lymphocyte clones. J Immunol. 1986;136:3543–3548. [PubMed] [Google Scholar]
- 54.DeClerck Y, Draper V, Parkman R. Clonal analysis of murine graft-vs-host disease, II: leukokines that stimulate fibroblast proliferation and collagen synthesis in graft-vs-host disease. J Immunol. 1986;136:3549–3552. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.