Abstract
Quinine has been increasingly utilized as a placebo in cystic fibrosis (CF) clinical trials, including those leading to FDA approval of inhaled tobramycin, recent studies of anti-inflammatory aerosols such as glutathione, and clinical testing of hypertonic saline aerosols to augment mucous clearance. The drug effectively masks taste of experimental therapeutics, but could also confer changes in processes contributing to CF pathogenesis, including chloride secretion and paracellular ion permeability. In the Ussing chamber, concentrations of quinine (1 mg/ml) anticipated in the airways of CF subjects after aerosolization led to changes in chloride transport in Calu-3 (airway serous glandular) cell monolayers. Tissue resistance was significantly disrupted by the compound in both Calu-3 and primary airway epithelial cells in vitro. Lower doses of quinine (between 10 and 100 μg/ml) strongly inhibited the chloride secretory mechanism that utilizes CFTR, and forskolin activated ISC was reduced by approximately 24% and 44% in the presence of 10 and 100 μg/ml quinine, respectively. Our findings indicate that quinine disrupts airway epithelial functional integrity and blocks transepithelial chloride transport. The use of quinine as a taste-masking agent may have bioelectric effects relevant to CF trials using aerosolized drug delivery.
Introduction
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), an integral membrane protein that governs ion transport across epithelial tissues (1). Abnormalities in CFTR structure and function lead to hyperviscous secretions in the lungs, pulmonary infection and progressive respiratory decline. Life expectancy in CF has steadily increased due to cumulative improvements in supportive care. Anti-inflammatory agents and new therapies delivered by nebulization such as recombinant human DNase, inhaled tobramycin, and hypertonic saline have been shown to decrease CF morbidity in randomized controlled trials (2-7). Experimental aerosols for CF therapy (hypertonic sodium chloride, chloride secretagogues, anti-inflammatory agents, airway surface volume expanders, and sodium channel blockers) are in progress or envisioned within multicenter, placebo controlled trials in the immediate future.
Quinine has served as a placebo in CF clinical studies, including trials utilizing nebulized tobramycin, glutathione, xylitol, hypertonic saline, and a number of other therapeutic strategies (4, 8-10). The compound masks the taste of therapeutic agents, presumably due to its ability to block ion channels crucial to gustatory sensation (11). Quinine is known to alter the bioelectric properties of human cell types such as bronchial epithelial cells (12). Quinine has also been shown to have ion transport effects in tissues including cardiac muscle (13) and colon (14). In the present experiments, we examined whether quinine might influence bioelectric properties of airway epithelia in a fashion that could compromise its role as an inert placebo for CF therapeutic drug development. We tested effects of quinine on chloride dependent short circuit current (ISC) and epithelial resistance, two parameters fundamental to normal lung physiology and cystic fibrosis lung disease pathogenesis.
Methods
Cell culture
Calu-3 (human airway serous glandular) cells were obtained from the American Type Culture Collection (Manassas, VA) and propagated in minimal essential media (MEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin supplemented with non-essential amino acids. For Ussing chamber, transepithelial electrical resistance (TER), and immunofluorescence experiments, polarized Calu-3 monolayers at an air-liquid interface were grown on Costar® Transwell®-polyester membrane filters (6.5 mm diameter, 0.4 μM pore size; Fisher Scientific, Pittsburgh, PA) coated with human placental collagen (HPC, Becton Dickinson, Franklin Lakes, NJ) at 5 μg/mL and seeded at ∼1×106 cells/cm2. Apical medium was removed at confluency (5-7 days after seeding) and filters incubated for another 48 hrs to confer a polarized phenotype. Monolayers were studied at resistances of 800-1500 Ω across a 0.33 cm2 filter area (∼250-500 Ω•cm2). All experiments were internally controlled by utilizing direct comparisons between cells of the same passage frequency and identical culture conditions.
Primary human airway cells were isolated from CF sinus tissues obtained through the University of Alabama at Birmingham (UAB) Tissue Procurement Office and following approval by the UAB Institutional Review Board. After treatment with 1.5 mg/mL pronase for 24- 48 hrs (Roche Diagnostics, Indianapolis, IN), cells were grown as explant cultures on Vitrogen treated Costar® Transwell® filters (6.5 mm, 0.4 μm) using DMEM/F12 medium with supplements. Cells were studied approximately 2 weeks after plating.
Transepithelial current measurements
Calu-3 polarized monolayers were mounted in Ussing chambers (Jim's Instruments, Iowa City, IA) in Ringers solution on both the apical and basolateral surfaces (120 mM NaCl, 25 mM NaHCO3, 3.3 mM KH2P04, 0.83 mM K2HP04, 1.2 mM CaC12, 1.2 mM MgC12, and 10 mM D-Glucose at pH 7.4; osmolarity ∼320 mM). Chambers and bath solutions were maintained at 37°C with continuous 5% CO2. An epithelial voltage clamp (University of Iowa Bioengineering, Iowa City, IA) was used to measure short-circuit current (ISC) with resistance monitored by 1 sec, 3 mV pulses initiated every 100 sec. To measure stimulated ISC, quinine (1 mg/mL, 100 μg/mL, or 10 μg/mL) was added to either the mucosal or serosal surface in Ringers solution. In some experiments, the mucosal or serosal bath was changed to a low Cl− solution (1.2 mM NaCl, 115 mM sodium gluconate, and other components as above) to determine the effect of a chloride secretory (low mucosal chloride) or resorptive (low serosal chloride) directed gradient. Forskolin (20 μM, dissolved in 20 mM DMSO stock solution) was used to activate CFTR through cyclic AMP, and glybenclamide (200 uM in 200 mM DMSO stock solution) or CFTRinh−172 (20 uM in 20mM H20 stock solution) were used to inhibit CFTR mediated anion transport.
Immunofluorescence studies
Calu-3 cells were grown as polarized monolayers on 12 mm tissue culture inserts coated with 5 μg/cm2 HPC. Cells were treated with quinine, rinsed 3 times with cold PBS, and fixed with either −20°C methanol for 10 min or 4% formaldehyde for 30 min. Following three rinses with cold PBS, a 1:20 dilution of goat serum (20 min at room temperature) was used to block nonspecific protein-binding sites.
Filters were then cut from inserts and incubated for 2 hrs at room temperature with primary antibody obtained from ATCC (HB-11947) [1:100 anti-CFTR 24-1 (methanol fixed); 1:50 anti-CFTR M3A7 (Upstate) (formaldehyde fixed); or non-immune mouse IgG (negative control)] (15). After washing, an additional antibody [1:100 anti-ZO1 or 5 μg/ml non-immune rabbit IgG (negative control)] was added for 1 hr at room temperature. Antibody detection was with 1:500 dilution of anti-mouse IgG Alexa Fluor 488 or anti-rabbit ISG Alexa Fluor 594 for 1 hr at room temperature. The cells were then washed and mounted on slides in Vectashield/DAPI by folding the filters so that the apical surfaces were exposed (16).
Statistical analysis
For ISC and resistance measurements, descriptive statistics (mean and S.E.M.) were reported. Statistical comparisons utilized Student's t-tests, and were performed using SigmaStat software (Jandel, CA) and/or Microsoft Excel (Seattle, WA). All statistical tests were two-sided and were performed at α=0.05. For statistical analysis of short-circuit current, summary data was used to describe the specific change in ISC compared to the stable baseline prior to drug treatment unless otherwise expressly noted.
Results
Quinine alters paracellular chloride permeability of airway epithelial monolayers
Quinine is typically aerosolized at doses of 1.25 mg/ 5 mL as a taste masking agent in CF therapeutic trials (4-6, 9). Because the final concentration of quinine in the airway surface liquid following aerosolized delivery is not known, we tested a range of concentrations from 10 – 1000 μg/mL. Higher concentrations were studied in part because hyperabsorption of fluid has been reported in CF surface epithelium and could have concentrating effects on small molecules aerosolized to the periciliary layer. In either case, a range of concentrations (including levels below those anticipated in vivo, see below) were evaluated.
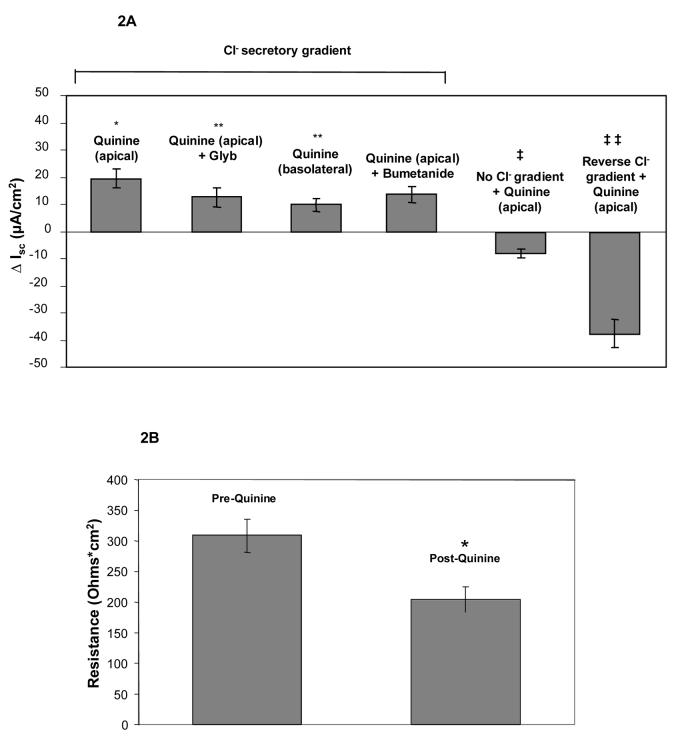
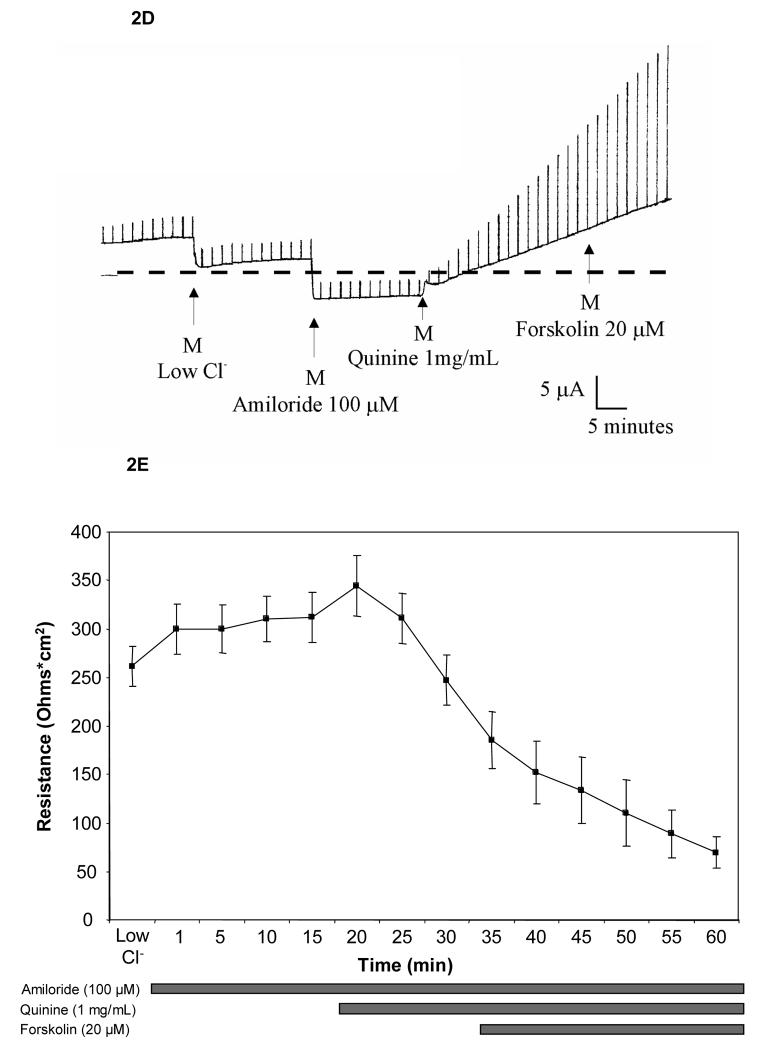
Ussing chamber measurements in Calu-3 cells grown as monolayers at an air-liquid interface demonstrated augmentation of chloride permeability by quinine at 1 mg/mL (Figure 1, Panel A). The effect was observed with either apical or basolateral addition of compound. Inhibition of CFTR using glybenclamide (200 μM) caused small, inconsistent blockade (Figure 1, Panel A), while experiments utilizing the highly specific CFTR inhibitor CFTRinh-172 (20 μM) in lieu of glybenclamide resulted in no inhibition of ion transport (mean change +1.5 μA/cm2, n=8). Control experiments (omitting chloride on both sides of the monolayer to remove the chloride secretory gradient) revealed chloride dependence of the change in permeability (measured as μA, Figure 1, Panel B; summarized in Figure 2, Panel A). The observed change of chloride movement in a reverse (resorptive) chloride gradient suggested effects on paracellular (junctional) pathways present in airway monolayers. Addition of bumetanide (a blocker of basolateral chloride entry) had no effect, further indicating a paracellular mechanism (Figure 2, Panel A). One mg/ml quinine resulted in a significant reduction of resistance over a 10 minute period in Calu-3 cells (p = 0.004, Figure 2, Panel B), although tight junction complexes were not grossly disrupted under the same conditions (Figure 2, Panel C). Similar effects were also observed in primary cultures of human airway surface epithelial cells (Figure 2, Panel D and E).
Figure 1. Apical addition of high dose quinine to Calu-3 monolayers.
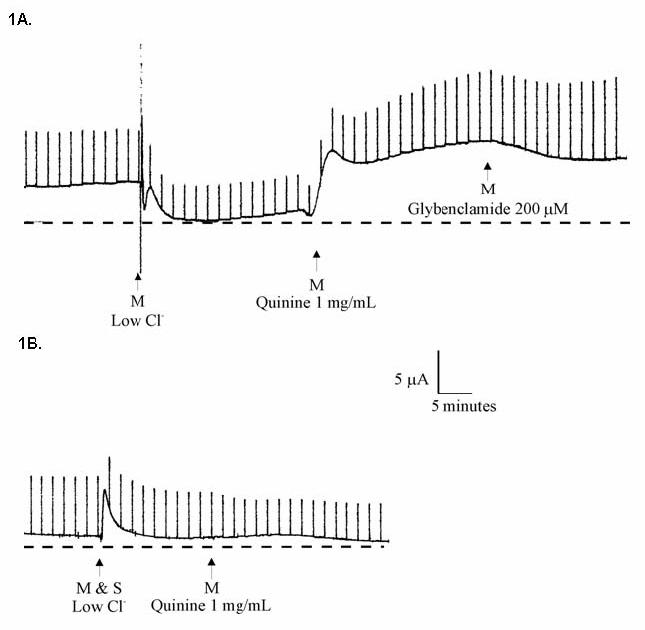
Cells were grown at an air-liquid interface and studied in Ussing chambers. A: Calu-3 cells in Ringers solution followed by 1) mucosal (M) low- Cl− solution, 2) addition of 1 mg/mL quinine, and 3) addition of 200 μM glybenclamide. B: Same experiment as in A, with mucosal and serosal low- Cl− solution (i.e. no chloride gradient) prior to addition of quinine.
Figure 2. Quinine increases Cl− permeability in airway epithelia through a paracellular pathway associated with decreased epithelial resistance.
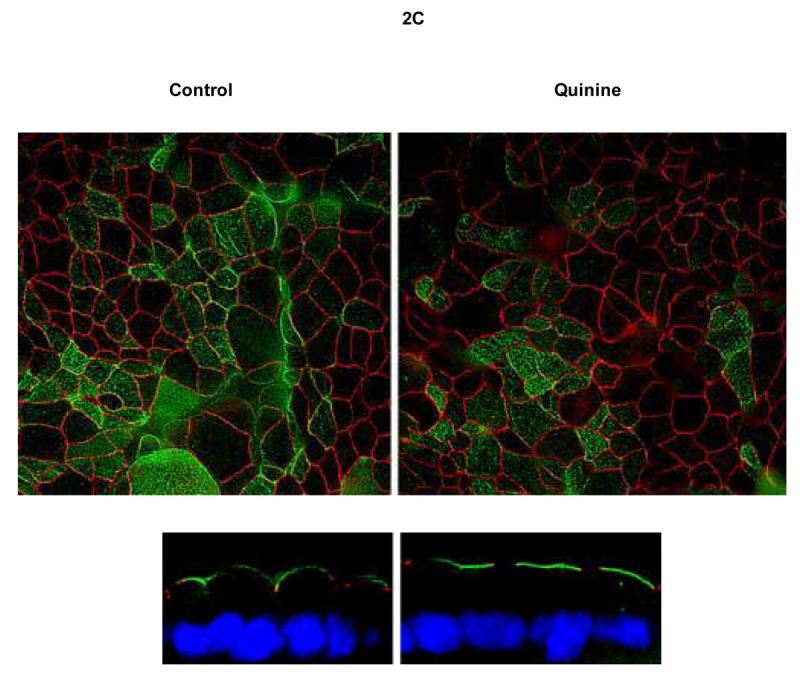
A: In Calu-3 monolayers, apical quinine (1 mg/mL) increased the monolayer permeability (19.4 ± 3.4 μA/cm2, as also shown in Figure 1A,* p < 0.001 compared with untreated controls, n = 16, ± SEM) in the presence of a Cl− secretory gradient (low Cl− on mucosal surface) and was only partially blocked by the addition of glybenclamide (change of 6.9 ± 1.8 μA/cm2, **p< 0.05 vs. apical quinine, n=16, ± SEM). The effect of quinine applied to the basolateral surface was less pronounced than apical administration (**p<0.05 vs. apical quinine, n=16, ± SEM). Bumetanide applied to the basolateral surface several minutes after quinine had no effect compared to apical quinine alone. The change attributable to quinine decreased significantly upon omission of the chloride gradient (‡ p <0.001 vs. apical quinine in Cl− secretory gradient, n=16, ± SEM). In a reversed chloride gradient, the direction of apparent Cl− movement was reversed (‡‡ p <0.001 vs. apical quinine in Cl− secretory gradient, n = 12, ±SEM). B: In Calu-3 monolayers, apical quinine (1 mg/mL) added in a Cl− secretory gradient caused a decrease in airway epithelial resistance (*p= 0.004, n=16, ±SEM) in Calu-3 cells. C: Quinine (1 mg/mL) had no effect on gross epithelial morphology in Calu-3 monolayers studied for localization of tight junctions (Z0-1, green) and CFTR (red). D: Effects of quinine (1 mg/mL) on Ussing chamber recording and transepithelial electrical resistance (E) in primary airway surface epithelial cell monolayers (n = 8 filters tested, ±SEM).
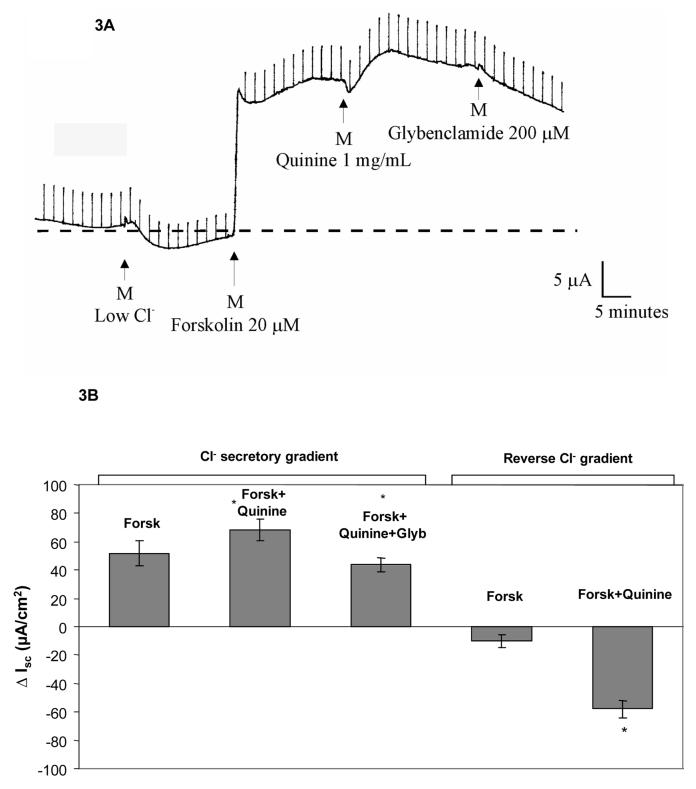
To evaluate the effects of high concentration quinine on cyclic AMP mediated CFTR activity, Calu-3 cells grown as monolayers were studied in Ringers solution followed sequentially by 1) mucosal low Cl−, 2) 20 μM forskolin, 3) 1 mg/mL quinine, and 4) 200 μM glybenclamide. After stimulation of CFTR with forskolin, a mean ISC of 52 μA/cm2 was observed, which increased modestly following addition of quinine (Figure 3, Panels A and B). Reversal of the gradient abrogated forskolin dependent ISC, with a further negative deflection again observed following quinine indicating dependence on the imposed chloride gradient. The absolute magnitude of ion transport seen with quinine in a reverse (chloride absorptive) gradient was similar to that seen in the chloride secretory configuration, reflecting the dominant role of the paracellular transport pathway (compared to the transcellular mechanism) at this concentration of quinine.
Figure 3. Influence of 1 mg/ml quinine on transepithelial chloride secretion activated by forskolin.
A: Representative ISC tracing. Calu-3 cells initially studied in Ringers solution followed by 1) mucosal low- Cl− solution, 2) addition of 20 μM forskolin, 3) addition of 1mg/mL quinine, and 4)addition of 200 μM glybenclamide. B: After maximal activation of CFTR, addition of high-dose quinine (1 mg/ml) modestly increased apparent ISC in a chloride secretory gradient (17.0 ± 3.9 μA/cm2, *p<0.005 compared to forskolin alone, n=18, ±SEM) and was partially inhibited by glybenclamide (*p<0.005, n=18, ±SEM). The apparent reversal of ISC due to quinine in a reversed chloride gradient is attributable to increased paracellular permeability (*p<0.001, n=14, ±SEM).
Quinine also inhibits CFTR-dependent transepithelial short-circuit current
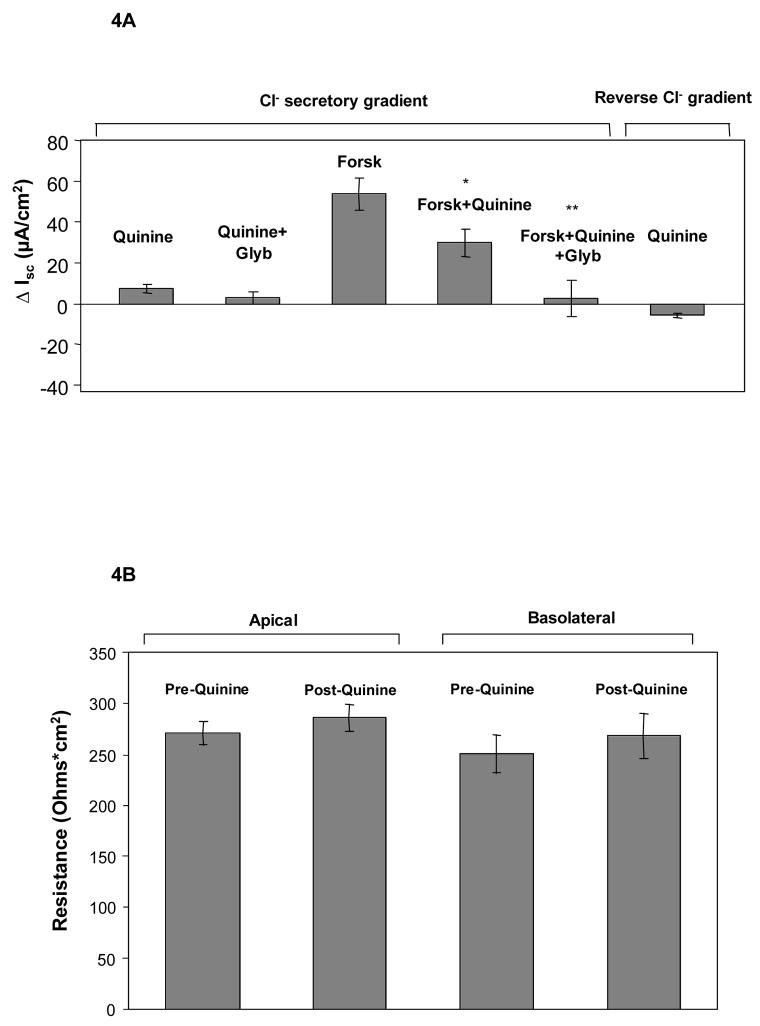
To better discriminate the paracellular versus transcellular effects of quinine, we investigated whether concentrations of apically administered drug that do not disrupt tissue resistance could alter airway epithelial ion movement by an independent (transcellular) mechanism. Polarizing Calu-3 monolayers treated with quinine at concentrations of 100 μg/mL showed little activation of chloride dependent junctional permeability in a gradient directed towards chloride secretion (Figure 4, Panel A). Quinine itself at this concentration also had minimal effect on transepithelial electrical resistance (Figure 4, Panel B). Although this concentration of drug had minimal effects on the paracellular pathway, significantly reduced transepithelial chloride transport (directly or indirectly) through a pathway regulated by CFTR was detected by effects on forskolin (20 μM) activated ISC. This occurred whether quinine was added before glybenclamide (44% reduction in ISC, p<0.05, Figure 4, Panel A) or after (63% reduction in ISC, p<.001, data not shown), implying effects on both CFTR dependent and CFTR independent pathways. A reduced effect was also observed at 10-fold lower concentrations of quinine (10 μg/ml, 24% reduction in forskolin mediated Isc when administered prior to glybenclamide).
Figure 4. Quinine blocks CFTR dependent Cl− secretion at concentrations that do not alter airway epithelial resistance.
A: In the presence of a Cl− gradient, apical quinine added at 100 μg/mL led to a modest change (7.4 μA/cm2) in ISC. After activation of CFTR with forskolin (20 μM), substantial reduction in short-circuit current was seen with apical quinine (100 μg/mL, *p<0.05, n=12, ±SEM). Addition of glybenclamide (Glyb, 200 μM) further reduced ISC(**p<0.005, n=12, ±SEM). In a reversed Cl− gradient, low dose quinine by itself had minimal effect (−5.8 μA/cm2, n=13, ±SEM), indicating functionally intact junctional complexes. B: No significant change in monolayer resistance was observed with 100 μg/mL quinine (n=12, ±SEM).
Discussion
The present findings suggest important effects of quinine on pulmonary epithelial cells at concentrations that might occur at both the airway epithelial surface and within serous cells located at the base of respiratory submucosal glands. Concentrations of quinine similar to those tested here have been administered to several hundred human CF subjects in recent therapeutic trials as a taste-masking agent (4, 8-10). Administration of quinine at 1 mg/ml to either Calu-3 monolayers or primary human airway epithelium disrupts transepithelial electrical resistance and establishes a paracellular chloride transport pathway dependent on the chloride gradient. Lower doses of quinine (10 and 100 μg/ml) had minimal effects on TER but blocked transepithelial chloride dependent ISC through a CFTR-dependent mechanism. Effects such as these were noted for at least 40 minutes (Figures 1-3); in unpublished studies, duration of activity lasting over 3 hours has been observed in the Ussing chamber system.
Quinine is typically aerosolized at concentrations of 0.25 mg/ml, and administered in doses of 1-1.25 mg two to four times daily (5, 6, 9). The concentration of quinine exposed to the surface of airway cells in vivo has not been measured, and is likely to vary depending on the particular pulmonary milieu. Despite this, it seems reasonable to imagine that aerosol droplets might deposit quinine at or near this concentration within non-obstructed airway surfaces. It could also be argued that hyperabsorption of fluid and electrolytes suggested previously in CF airways might have concentrating effects on small molecules delivered to the periciliary layer. Estimates of total airway surface liquid (2-3 ml in the conducting, proximal airways) suggest that a regimen of 1.25 mg quinine nebulized twice daily would deliver substantial amounts of the compound to surface epithelial cells, in the same way that aerosols of other small molecules (hypertonic sodium chloride, amiloride and analogues, chloride secretagogues, tobramycin) have been achieved at relatively high concentrations to the mucosal surfaces of CF lungs in the past.
Quinine has known inhibitory effects on calcium activated potassium channels in human cardiac tissue, animal models of colonic epithelium, and a number of other cell types (13, 17-19). The results presented in this study establish that quinine also causes pronounced alterations of ion permeability across airway epithelium, and these effects exhibit dose-dependence. Aerosolization would be expected to deposit variable concentrations of quinine in specific regions of the lung (e.g. large airways, small airways, terminal bronchioles, alveoli, and submucosal glands) so that disruption of either transepithelial chloride secretion and/or TER might be a concern in human subjects. In either case, the failure of forskolin to activate Isc following quinine administration (Figure 3) suggests increased junctional permeability established by the compound. Under these conditions, paracellular permeability may undermine the ability to generate vectoral chloride transport through CFTR or other pathways by dissipating the necessary gradients.
Agents that alter TER may have important biologic effects. For example, azithromycin, a compound shown to improve lung function in CF clinical trials, increases TER and alters the expression of various tight junction proteins in vitro (20). The relationship between tight junction assembly and function (including ion transport regulation) is complex. Disruption of tight junctions without gross abnormalities in structural components (e.g. Figure 2C) has been described previously (21, 22), and typically reflects permeability to ions but not proteins or other macromolecules. Ion selectivity of the paracellular pathway is sensitive to small changes in tight junction protein expression (e.g. claudins) without producing gross changes in epithelial structure/function (23-26). The effects of protein expression vary among particular epithelial tissues. For example, expression of claudin-4 increases TER in Madin-Darby canine kidney II and LLC-PK1 cells, but increases Na permeability only in the former cell type (25). Whether the acute effects of quinine on TER are explained by changes in tight junction protein assembly in human airway cells deserves further study.
No direct effect of quinine on single CFTR channels has been reported previously. If quinine blocked potassium channels present at the basolateral surfaces of airway epithelium, chloride entry (which occurs in exchange for K+ exit through Na/K/2Cl− co-transporters) would be impaired. Devor et al. have previously shown the existence of a quinine sensitive basolateral K+ conductance in human bronchial epithelial cells (12). In contrast, the outwardly rectified chloride channel (ORCC) present in a variety of epithelial tissues (including those derived from lungs) can be blocked by quinine (14). Because CFTR dependent chloride secretion recruits ORCCs, effects of quinine in the present experiments might be attributable to blocked transepithelial chloride secretion through this ion channel (27). Further studies, including direct measurements of quinine on single CFTR chloride channels, will be necessary to distinguish these possibilities.
The present study is germane to CF clinical trials that support the use of aerosolized tobramycin (4, 9, 28), and more recently hypertonic saline (5, 6). While the results presented here raise concern regarding the use of quinine as a taste-masking agent in CF trials, the Calu-3 model obviously cannot replicate many aspects of the in vivo situation. Despite the potential effects of quinine on human airways, we do not believe our findings alter the interpretation of recent phase III trials utilizing quinine. For example, because the majority of patients enrolled in phase III testing of inhaled tobramycin were homozygous for ΔF508 CFTR (a mutation that confers defective protein processing), minimal CFTR would be expected at the cell surface in these subjects. Although it remains possible that partial function of the ΔF508 mutation (or other compensatory chloride secretory channels) might be inhibited by quinine, direct effects of the drug on CFTR are likely negligible. In trials evaluating hypertonic saline aerosols, both the control and experimental arms were administered quinine. Therefore, even if CFTR mediated ion transport were blunted, a significant therapeutic benefit could still be discerned.
Because quinine influences the fundamental pathophysiologic defect in cystic fibrosis (transepithelial chloride secretion) in vitro, alternatives should be considered in future, placebo-controlled CF interventional trials. This may be particularly important in studies evaluating new agents (so called ‘potentiators’) that restore activity to mutant CFTR located at the cell surface (e.g. Class III, IV, and VI CFTR mutations). Compounds such as these are expected to begin human clinical testing in the very near future (1). Moreover, since elements of gustatory sensation are mediated by mechanisms that rely on ion transport, compounds to mask taste in CF should be selected with particular attention to effects on epithelial chloride secretion and/or junctional permeability. The finding that quinine decreases resistance at moderate to high doses also raises the possibility of injury to airway epithelial cells, including disruption of the tight junctions and other structures crucial to pulmonary mucosal integrity. This could predispose to infection or impair normal mucosal defense against the pathogens that chronically persist in CF lungs.
In summary, we found that quinine has important effects on airway epithelia at doses expected to be encountered after aerosolizion of this agent as a taste-masking placebo. The present findings should be confirmed in vivo, and considered in the design of placebo controlled trials for cystic fibrosis and other pulmonary diseases in the future.
Acknowledgments
The authors wish to thank Mrs. Jane Schell, Mrs. Melody Kingsley, and Ms. Carolyn Cox for their assistance preparing this manuscript. We also are grateful to Drs. A. Smith and J.P. Clancy for several useful discussions.
Financial support: National Institutes of Health (P50 DK053090, 1K23 DK075788-01) and the Cystic Fibrosis Foundation.
Abbreviations
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane regulator
- FBS
fetal bovine serum
- HPC
human placental collagen
- ISC
short-circuit current
- MEM
minimal essential media
- ORCC
outwardly rectified chloride channel
- PBS
sodium phosphate-buffered isotonic saline
- TER
transepithelial electrical resistance
- Sec
seconds
- UAB
University of Alabama at Birmingham
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. Jama. 2003;290(13):1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs HJ, Borowitz DS, Christiansen DH, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331(10):637–42. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 4.Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 5.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354(3):241–50. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 7.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med. 1995;332(13):848–54. doi: 10.1056/NEJM199503303321303. [DOI] [PubMed] [Google Scholar]
- 8.Sorscher EJ, Zabner J. Novel Therapeutic Approaches for CF Lung Disease; Workshop 23. North American Cystic Fibrosis Conference; St. Louis, MO. 2004.2004. [Google Scholar]
- 9.Ramsey BW, Dorkin HL, Eisenberg JD, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med. 1993;328(24):1740–6. doi: 10.1056/NEJM199306173282403. [DOI] [PubMed] [Google Scholar]
- 10.Bye P, Elkins M, Robinson M, Moriarty C, Rose B, Harbour C. Long-term inhalation of hypertonic saline in patients with cystic fibrosis - a randomized controlled trial [Abstract] Pediatr Pulmonol. 2004;38(S27):329. [Google Scholar]
- 11.Scott TR, Plata-Salaman CR. Taste in the monkey cortex. Physiol Behav. 1999;67(4):489–511. doi: 10.1016/s0031-9384(99)00115-8. [DOI] [PubMed] [Google Scholar]
- 12.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol. 2000;279(2):C461–79. doi: 10.1152/ajpcell.2000.279.2.C461. [DOI] [PubMed] [Google Scholar]
- 13.Imaizumi Y, Giles WR. Quinidine-induced inhibition of transient outward current in cardiac muscle. Am J Physiol. 1987;253(3 Pt 2):H704–8. doi: 10.1152/ajpheart.1987.253.3.H704. [DOI] [PubMed] [Google Scholar]
- 14.Gogelein H, Capek K. Quinine inhibits chloride and nonselective cation channels in isolated rat distal colon cells. Biochim Biophys Acta. 1990;1027(2):191–8. doi: 10.1016/0005-2736(90)90084-2. [DOI] [PubMed] [Google Scholar]
- 15.Varga K, Jurkuvenaite A, Wakefield J, et al. Efficient Intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem. 2004 doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- 16.Bebok Z, Varga K, Hicks JK, et al. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl- secretion in airway epithelia. J Biol Chem. 2002;277(45):43041–9. doi: 10.1074/jbc.M203154200. [DOI] [PubMed] [Google Scholar]
- 17.Iwatsuki N, Petersen OH. Inhibition of Ca2+-activated K+ channels in pig pancreatic acinar cells by Ba2+, Ca2+, quinine and quinidine. Biochim Biophys Acta. 1985;819(2):249–57. doi: 10.1016/0005-2736(85)90180-4. [DOI] [PubMed] [Google Scholar]
- 18.Mancilla E, Rojas E. Quinine blocks the high conductance, calcium-activated potassium channel in rat pancreatic beta-cells. FEBS Lett. 1990;260(1):105–8. doi: 10.1016/0014-5793(90)80078-w. [DOI] [PubMed] [Google Scholar]
- 19.Richards NW, Dawson DC. Single potassium channels blocked by lidocaine and quinidine in isolated turtle colon epithelial cells. Am J Physiol. 1986;251(1 Pt 1):C85–9. doi: 10.1152/ajpcell.1986.251.1.C85. [DOI] [PubMed] [Google Scholar]
- 20.Asgrimsson V, Gudjonsson T, Gudmundsson GH, Baldursson O. Novel effects of azithromycin on tight junction proteins in human airway epithelia. Antimicrob Agents Chemother. 2006;50(5):1805–12. doi: 10.1128/AAC.50.5.1805-1812.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jornot L, Rochat T, Lacroix JS. Nasal polyps and middle turbinates epithelial cells sensitivity to amphotericin B. Rhinology. 2003;41(4):201–5. [PubMed] [Google Scholar]
- 22.Schmitz H, Fromm M, Bentzel CJ, et al. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112(Pt 1):137–46. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- 23.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci. 2005;118(Pt 12):2683–93. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 24.Gye MC. Changes in the expression of claudins and transepithelial electrical resistance of mouse Sertoli cells by Leydig cell coculture. Int J Androl. 2003;26(5):271–8. doi: 10.1046/j.1365-2605.2003.00423.x. [DOI] [PubMed] [Google Scholar]
- 25.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285(6):F1078–84. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 26.Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284(6):C1346–54. doi: 10.1152/ajpcell.00547.2002. [DOI] [PubMed] [Google Scholar]
- 27.Schwiebert EM, Morales MM, Devidas S, Egan ME, Guggino WB. Chloride channel and chloride conductance regulator domains of CFTR, the cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1998;95(5):2674–9. doi: 10.1073/pnas.95.5.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss RB. Administration of aerosolized antibiotics in cystic fibrosis patients. Chest. 2001;120(3 Suppl):107S–113S. doi: 10.1378/chest.120.3_suppl.107s. [DOI] [PubMed] [Google Scholar]