Abstract
Steroid modulation of cognitive function has focused on estrogen (E2), but progestins naturally co-vary with E2 and may also influence cognitive performance. Spatial performance in the object placement task over endogenous hormonal states in which E2 and progestins vary, and when E2 and/or progestins were administered, was examined. Experiment 1: Rats in proestrus or estrus had significantly better performance in the object placement task than did diestrous rats. Experiment 2: Rats in the third trimester, post-partum, or lactation exhibited significantly better performance in the object placement task than did rats in the first trimester. Experiment 3: Ovariectomized (ovx) rats administered 17β-estradiol (0.9 mg/kg, subcutaneously (sc), progesterone (P; 4 mg/kg, sc), or E2 and P, immediately after training in the object placement task, performed significantly better when tested 4 hours later, than did control rats administered vehicle (sesame oil 0.2 cc). Experiment 4: Ovx rats administered E2 or P with a one and a half hour delay after training in the object placement task, did not perform differently than vehicle-administered controls. Experiment 5: Ovx rats administered post-training E2, which has a high affinity for both E2 receptor (ER)α and β isoforms, or propyl pyrazole triol (PPT; 0.9 mg/kg, sc), which is more selective for ERα than ERβ, had significantly better performance in the object placement task than did rats administered vehicle or diarylpropionitrile (DPN; 0.9 mg/kg, sc), an ERβ selective ligand. Experiment 6: Ovx rats administered P, or its metabolite, 5α-pregnan-3α-ol-20-one (3α,5α-THP; 4 mg/kg, sc), immediately post-training performed significantly better in the object placement task than did vehicle control rats. Thus, performance in the object placement task is better when E2 and/or P are naturally elevated or when E2, the ERα selective ER modulator PPT, P, or its metabolite, 3α,5α-THP, are administered post-training.
1. Introduction
Ovarian steroids, such as estradiol (E2), can influence cognitive processes of female rodents. Performance in the eye-blink conditioning, passive avoidance, and object recognition tasks, which involve the hippocampus and other regions of the brain (i.e. amygdala, cortex), is better during behavioral estrus when E2 levels are acutely elevated than during low E2 phases of the estrous cycle (Frye & Bayon, 1999; Rhodes & Frye, 2004; Shors et al., 1998; Walf et al., 2006; Wood et al., 2001). When E2 is administered to ovariectomized (ovx) rats, such that physiological E2 concentrations are achieved during training and consolidation, performance in hippocampally-mediated tasks, such as the water maze, radial arm maze, 4-arm plus maze, and passive avoidance are improved over that produced by vehicle administration (Bimonte & Denenberg, 1999; Daniel et al., 1997; Davis et al., 2005; Diaz-Veliz et al., 1991; Fader et al., 1998; Frye & Rhodes, 2002; Gibbs, 1999; 2000; as reviewed in Korol, 2004; Korol & Kolo, 2002; Luine et al., 1998; Mariott & Korol, 2003; O’Neal et al., 1996; Packard, 1998; Sandstrom & Williams, 2001; 2004). These data suggest a role of the hippocampus as a target for steroid hormones’ effects for cognitive processes and that the timing of hormone exposure and testing is critical. Indeed, when rats are trained and tested in different phases of the estrous cycle, little evidence for cognitive-enhancing effects of endogenous ovarian steroids in the water maze are observed (Frye, 1995). We have utilized the object recognition task, which has a four-hour intertrial interval, to assess steroids’ effects on object memory in a task that relies on functioning of the prefrontal cortex and hippocampus. Given the four-hour intertrial interval, we were able to assess these effects over the estrous cycle when rats are trained and tested in the same estrous cycle state (Walf et al., 2006). As such, using a hippocampally-mediated task with a short interval between training and testing, such as the object placement task, to investigate effects of endogenous changes in ovarian hormones is essential to assess the role of the hippocampus for these effects.
Estradiol binds with a high affinity to both E2 receptor (ER) isoforms, ERα and ERβ. Although there is differential distribution of ERα and ERβ throughout the central nervous system, both ERα and ERβ are expressed in the hippocampus and cortex (Shughrue et al., 1997; 1998; Shughrue and Mercenthaler, 2001) and may influence cognitive processes that rely on hippocampal and cortical function. Indeed, ERα and ERβ selective ER modulators (SERMS) that have high affinity for ERα, enhance exploration and performance in tasks that involve exploration, hippocampal, and cortical function, such as the object recognition task (Luine et al., 2003; Morgan et al., 2004; Walf et al., 2006). SERMS or dietary phytoestrogens, which have selective actions at ERβ, enhance performance in hippocampal tasks, such as the water and radial arm mazes and passive avoidance (Lephart et al., 2002; Rhodes and Frye, 2006). Thus, actions at ERα and/or ERβ may underlie E2-enhanced performance in hippocampally-mediated tasks.
Progesterone and its metabolites, dihydroprogesterone (DHP), and 5α-pregnan-3α-ol-20-one (3α,5α-THP), co-vary with E2 over reproductive cycles and may influence cognitive performance, yet few studies have addressed the role and/or mechanisms of progestins for spatial, hippocampus-dependent learning. Although pregnancy is characterized by more marked elevations in progestin than E2 levels, one of the few animal studies investigating spatial learning over pregnancy attributed differences to E2 concentrations (Galea et al., 2000). Notably, E2 enhances P’s metabolism to DHP and 3α,5α-THP (Cheng & Karavolas, 1973; Vongher & Frye, 1999) and P or its metabolites can enhance performance in various cognitive tasks. Administration of P, DHP, or 3α,5α-THP to ovx rats enhances performance in the object recognition and Y-maze tasks, both of which involve prefrontal and hippocampal processes, as well as conditioned and passive avoidance, tasks (Diaz-Veliz et al., 1994; Ebner et al., 1981; Frye & Lacey, 2000, van Wimersma Greidanus, et. al. 1977; Walf et al., 2006). Indeed, regression analyses revealed significant positive correlations between E2 and 3α,5α-THP levels in the hippocampus and 3α,5α-THP levels in the prefrontal cortex for performance in the object recognition task (Walf et al., 2006). Thus, it is necessary to ascertain the nature and extent to which E2 and progestins can have integrated and/or independent effects on hippocampus-dependent spatial performance.
To further address the respective roles and/or mechanisms by which E2 and/or progestins may influence cognitive performance in a hippocampally-mediated task, the effects of endogenous fluctuations in ovarian steroids, removal of the ovaries, and selective replacement of estrogens and progestins on performance in the object placement task were examined. We hypothesized that endogenous fluctuations and exogenous administration of E2 and/or progestins would alter object placement performance. To test this hypothesis, we examined performance of rats across the estrous cycle (Experiment 1), various stages of pregnancy (Experiment 2), and following ovx and administration of E2 and/or P (Experiments 3 & 4), SERMs (Experiment 5), or progestins (Experiment 6).
2. Methods
The Institutional Animal Care and Use Committee pre-approved these methods.
Animals & Housing
Subjects were 2-4 month old, female Long-Evans rats (N=161), bred at S.U.N.Y.-Albany from stock originally purchased from Taconic Farms, Germantown, NY. Rats were housed 4-5 per cage (45 × 24 × 21 cm) with ad libitum access to Purina Rat Chow and tap water, in a temperature-controlled room (21 ± 1° C), that had a 12/12 hour reversed-light cycle (lights off at 0800) in The Laboratory Animal Care Facility in The Life Sciences Research Building. After testing in this experiment, rats became breeder females or were tested in other ongoing experiments in the laboratory.
Determination of estrous cycle/pregnancy
For some rats, phase of estrous cycle was determined by daily examination of vaginal cytology between 0800-0900 (Frye & Bayon, 1999) and were trained immediately after status was determined (and tested 4 hours after training in the same cycle phase). Vaginal lavages of rats in proestrus, estrus, and diestrus are differentiated by the preponderance of nucleated, cornified epithelial, and heterogeneous cell types, respectively. Some rats in behavioral estrous were not tested that day, but were mated and were utilized in Experiment 1 as the pregnant, post-partum, and/or lactating rats. Only rats that showed typical cycling for at least 3 cycles were utilized in this study.
Extirpation and Steroid Replacement
Some rats had the primary endogenous sources of E2 and progestins, the ovaries, removed under Rompum (12 mg/kg; Bayer Corp., Shawnee Mission, KS) and Ketaset (80 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) anesthesia, one week prior to testing. Steroids were then administered subcutaneously (sc) in sesame oil vehicle to control the concentration and timing of the hormones.
Behavioral Testing- Object Placement Task
The object placement task is a spatial memory task that is considered hippocampus-dependent, but also relies on functioning of the cortex (Ennaceur et al., 1997). Indeed, although some studies suggest that extensive hippocampal lesions do not adversely affect object recognition/placement memory, a recent report suggests that dorsal hippocampal lesions impair object placement memory (Aggelton et al., 1986; Lee, Hunsaker, & Kesner, 2005; reviewed in Mumby, 2001). This task was used as modified from previously published methods (Ennaceur & Delacour, 1988; Luine et al., 2003). The advantages of this task are that cognitive performance does not depend upon retention of a rule, does not involve a physical or psychological stressor, is not influenced by changes in response to reward, and involves rats’ natural inclination to seek novel stimuli.
In all experiments, rats were trained in this task between 0800 and 1200 and were tested 4 hours later to ensure that intact rats would be in the same hormonal milieu during training and testing. During the training session, which lasts 3 minutes, rats are placed in a white open field (76 cm × 57 cm with 35 cm high walls) with no shavings in the brightly-lit testing room. Before initiation in this study, experimental rats were naïve to this environment. During training, the open field has two identical objects in adjacent corners (NW and NE) and the time spent exploring these objects were recorded; rats that spent less than 1 s exploring each object were excluded from statistical analyses for not reaching training criterion. The operation definition of exploring utilized was that the animal spent a body’s length or less from the object and sniffed, climbed on, or touched the object. The identical objects were colored spheres (“orange,” “lemon,” etc plastic toys) that were wiped cleaned with Quatricide between animals and trials as was the open field. During the testing session, one sphere is moved to the opposite corner of the open field (i.e. SW or SE). The rats are placed back in the open field with the same two objects, with one object having been displaced (i.e. objects are in NW and SE or NE and SW). In both the training and testing trials, the time spent exploring the two objects during 3 minutes are recorded simultaneously by an observer blind to the experimental condition of the subject who is located in the experimental room and a video-tracking/computer system (Any-Maze; Stoelting). Cursory examination group means and absolute values of biases did not reveal that rats’ had a bias towards exploring objects on the right vs. left side of the open field. However, to eliminate any possible effects of a spatial bias, displacement of the object was counterbalanced across all treatment groups and testing sessions. An index of enhanced cognitive performance in this task is demonstrated by an increased percentage of time spent exploring the object in a new location compared to the total amount of time spent exploring both objects during testing (duration spent with displaced object/(duration spent with displaced object + duration spent with non-displaced object) × 100).
Procedure
Rats were repeatedly tested in each experiment under conditions that were counterbalanced. We and others have found little evidence of repeated testing effects using this task or the object recognition task (Luine et al., 2003; Walf et al., 2006) or in the present study. Experiment 1: Intact rats in proestrus, estrus, or diestrus were trained in the object placement task and tested 4 hrs later. Experiment 2: Rats that were pregnant and in the first trimester (gestational days 5-7), pregnant and in the third trimester (gestational days 16-21), post-partum (1-2 days post-parturition), and lactating (5-13 days post-parturition with pups) were trained in the object placement task and tested 4 hrs later. Experiment 3: Ovx rats were subcutaneously (SC) administered 17β-estradiol (17β-E2; Sigma Chemical Co.; 0.9 mg/kg), P (Sigma Chemical Co.; 4 mg/kg), 17β-E2 (0.9 mg/kg) & P (4 mg/kg), or sesame oil vehicle (0.2 cc), immediately after training in the object placement task and were tested 4 hrs later. Dosing utilized was based upon previous results that these regimen produce proestrus-like E2 and progestin levels in plasma, hippocampus and cortex (Walf et al., 2006). Experiment 4: We wanted to be able to elucidate the mnemonic vs. anxiolytic effects of E2 and/or progestins in the object placement task. As such we utilized an approach described by Packard (reviewed in Packard, 1998) that has been used with success with the object recognition task (Walf et al., 2006), in which steroids were administered outside the timeframe when memory consolidation occurs (i.e. 1-2 hours post-training). As such, in this experiment, ovx rats were SC administered E2 (0.9 mg/kg), P (4mg/kg), E2 (0.9 mg/kg) & P (4 mg/kg; n=16), or sesame oil vehicle (0.2 cc), one and a half hours after training in the object placement task and were tested 4 hrs after training. Experiment 5: Ovx rats were administered 17β-estradiol (E2; 0.9 mg/kg, which binds to both ERα and ERβ; Steraloids), an ERα–selective SERM, propyl pyrazole triol (PPT; 0.9 mg/kg; Stauffer et al., 2000; Sigma Chemical Co.) or an ERβ-selective SERM, diarylpropionitrile (DPN; 0.9 mg/kg; Meyers et al., 2001; Sigma Chemical Co.) or sesame oil vehicle (0.2 cc), immediately after training in the object placement task and were tested 4 hrs later. Dosing utilized was based upon previous results that these and similar regimen enhance learning, anxiety, and/or reproductive behavior (Walf & Frye, 2005; Walf et al., 2006). Experiment 6: Ovx rats were administered sc P (4 mg/kg), 3α,5α-THP (4 mg/kg), or sesame oil vehicle (0.2 cc), immediately after training in the object placement task and were tested 4 hrs later. Dosing utilized was based upon previous results that these regimen produce proestrus-like progestin levels in plasma, hippocampus and cortex (Walf et al., 2006).
Statistical analyses
To determine effects of hormone condition on performance in the object placement task, data were analyzed using analyses of variance (ANOVAs). When an α level of p<0.05 was achieved, differences between groups were investigated with Fisher PLSD comparisons.
3. Results
3.1 Experiment 1- Rats in proestrus or estrus performed better in the object placement task than did diestrous rats
The significant main effect of the estrous cycle F(2,72)=6.25, p=0.003 was due to proestrous or estrous rats spending a greater percentage of time with the object in a novel location than did control, diestrous rats (Figure 1). Means of groups for total time spent with objects during training and testing are included in Table 1 (n.s.).
Figure 1.
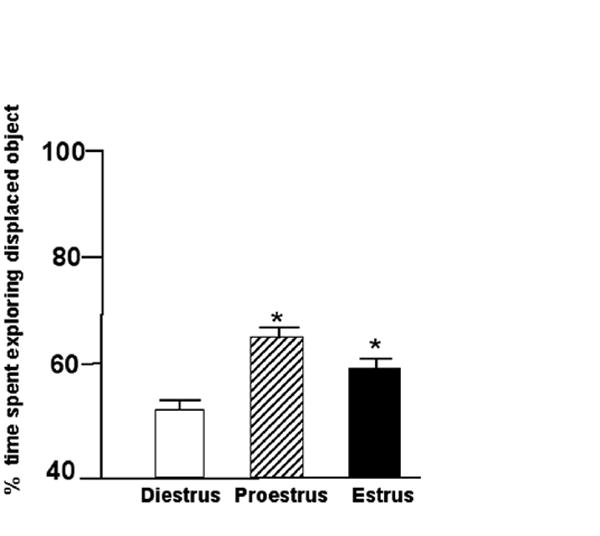
Mean percent of time (± standard error) proestrous (n=25; stripped bar) and estrous (n=25; filled bar) rats spent exploring the displaced object as compared to diestrous controls (n=25; open bar). * Indicates significant difference from diestrus (p ≤ 0.05).
Table 1.
Means (± sem) of total time spent with objects during training, or testing when objects were in familiar location or were displaced.
| Experimental Condition | n | Total duration spent with objects during training | Total duration spent with object in familiar location | Total duration spent with displaced object | |
|---|---|---|---|---|---|
| Experiment 1 | Diestrous | 25 | 6.8 (± 1.2) | 3.1 (± 0.6) | 2.9 (± 0.5) |
| Estrous | 25 | 7.6 (± 1.3) | 2.7 (± 0.4) | 3.7 (± 0.5) | |
| Proestrous | 25 | 4.3 (± 0.6) | 2.7 (± 0.5) | 3.6 (± 0.5) | |
| Experiment 2 | Ist Trimester | 25 | 8.6 (± 1.4) | 4.5 (± 0.6)^ | 4.2 (± 0.7) |
| 3rd Trimester | 25 | 5.7 (± 0.7) | 3.6 (± 0.7) | 4.0 (± 0.5) | |
| Post-partum | 23 | 7.9 (± 1.4) | 2.7 (± 0.6) | 4.7 (± 0.8) | |
| Lactating | 22 | 6.0 (± 0.8) | 1.8 (± 0.3) | 3.9 (± 0.7) | |
| Experiment 3-Steroids administered immediately post-training | Vehicle | 21 | 6.3 (± 0.9) | 2.7 (± 0.5) | 2.7 (± 0.5) |
| E2 | 18 | 7.7 (± 1.6) | 3.3 (± 0.5) | 5.2 (± 0.6)* | |
| P | 21 | 6.6 (± 0.8) | 3.3 (± 0.8) | 5.2 (± 1.1)* | |
| E2 + P | 18 | 7.5 (± 1.3) | 2.8 (± 0.6) | 4.2 (± 0.5) | |
| Experiment 4-Steroids administered 1.5 hours post-training | Vehicle | 16 | 5.1 (± 0.8) | 1.9 (± 0.3) | 2.3 (± 0.5) |
| E2 | 16 | 4.9 (± 0.7) | 1.8 (± 0.4) | 2.3 (± 0.5) | |
| P | 16 | 6.9 (± 1.0) | 2.6 (± 0.6) | 2.7 (± 0.7) | |
| E2 + P | 16 | 7.7 (± 1.0)* | 2.8 (± 0.5) | 3.0 (± 0.5) | |
| Experiment 5-Steroids administered immediately post-training | Vehicle | 17 | 7.7 (± 1.0) | 4.0 (± 0.6) | 4.0 (± 0.8) |
| E2 | 17 | 6.8 (± 1.1) | 3.7 (± 0.7) | 5.5 (± 0.7) | |
| PPT | 17 | 7.1 (± 1.1) | 2.5 (± 0.6) | 4.0 (± 0.7) | |
| DPN | 17 | 7.0 (± 1.0) | 3.2 (± 0.7) | 4.3 (± 0.9) | |
| Experiment 6-Steroids administered immediately post-training | Vehicle | 21 | 7.5 (± 1.0) | 4.9 (± 1.1) | 3.8 (± 0.7) |
| P | 19 | 7.0 (± 1.0) | 2.9 (± 0.6) | 5.1 (± 0.7) | |
| 3α,5α -THP | 18 | 7.6 (± 1.1) | 3.9 (± 0.7) | 5.5 (± 1.0) |
significant difference vs. postpartum or lactating (p ≤ 0.05).
significant difference vs. vehicle (p ≤ 0.05).
3.2 Experiment 2- Rats in the 3rd trimester, post-partum, or lactating performed better in the object placement task than did rats in the 1st trimester of pregnancy
The significant main effect of testing at different points in pregnancy, parturition, and/or lactation F(3,91)=3.06, p=0.03 was due to rats in the third trimester, post-partum, or lactating spending more time with the object in the novel location than did control rats in the first trimester of pregnancy (Figure 2). Means of groups for total time spent with objects during training and testing are included in Table 1. Rats in first trimester spent more time exploring object in the familiar location than did post-partum or lactating rats.
Figure 2.
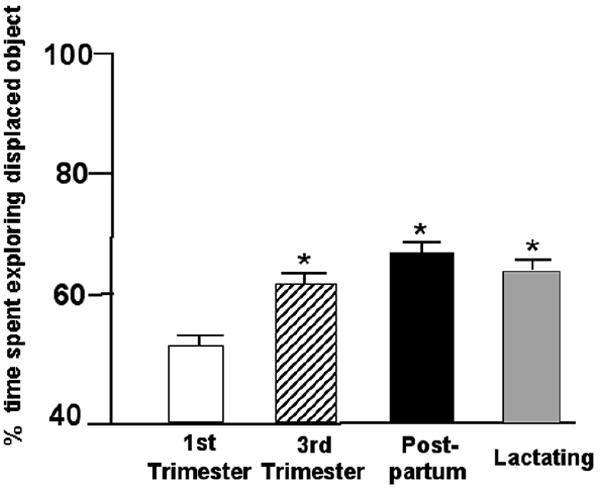
Mean percent of time (± standard error) 3rd trimester (n=25; stripped bar), post-partum (n=23; filled bar), and lactating (n=22; shaded bar) rats spent exploring the displaced object as compared to 1st trimester controls (n=25). * Indicates significant difference from 1st trimester (p ≤ 0.05).
3.3 Experiment 3- Administration of 17β-E2 and/or P4 to OVX rats immediately post-training improve performance in the object placement task
The significant main effects of post-training E2 and/or P F(3,74)=3.09, p≤0.03 was due to rats administered 17β-E2, P4, or 17β-E2 & P4 spending significantly more time exploring the object in a novel location than did rats administered vehicle (Figure 3). Means of groups for total time spent with objects during training and testing are included in Table 1 (n.s.).
Figure 3.
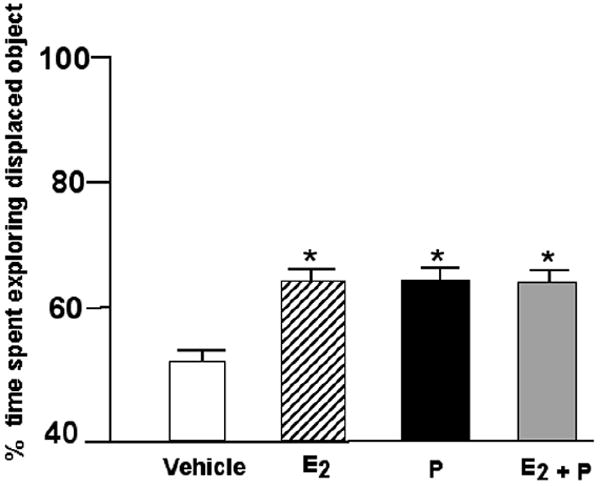
Mean percent (± standard error) of time spent exploring the displaced object of ovx rats administered E2 (n=18; stripped bar), P (n=21; filled bar) or E2 + P (n=18; shaded bar) immediately after training compared to vehicle (n=21) * Indicates significant difference from ovx vehicle (p ≤ 0.05).
3.4 Experiment 4- When administration of E2 and/or P4 was delayed 1.5 hour post-training, performance in the object placement task was not improved
There was no significant main effect when administration of E2 and/or P was delayed an hour and a half post-training F(3,60)=0.027, p=0.99. Rats administered 17β-E2, P4, 17β-E2 & P4, or vehicle one and half hours post-training, did not differ in the duration of time spent exploring the object in a novel location (Figure 4). Means of groups for total time spent with objects during training and testing are included in Table 1 (n.s.). Rats in the 17β-E2 & P4 condition spent more time investigating the objects during training than did vehicle control rats.
Figure 4.
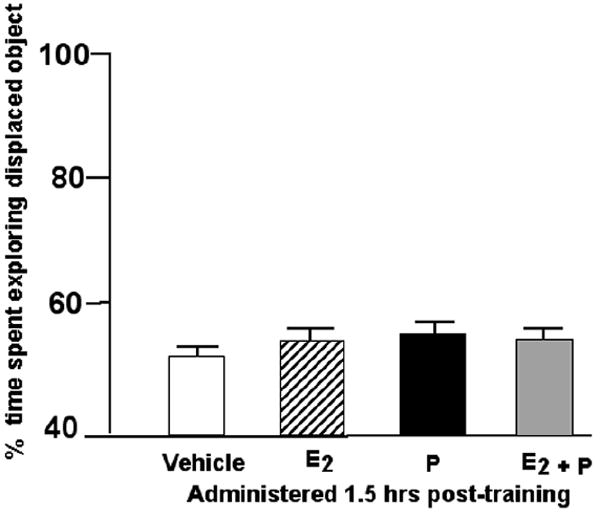
Mean percent (± standard error) of time spent exploring the displaced object of ovx rats administered E2 (n=16; stripped bar), P (n=16; filled bar) or E2 + P (n=16; shaded bar) 1.5 hours after training compared to vehicle (n=16).
3.5 Experiment 5- Administration of 17β-E2 or PPT to OVX rats immediately post-training improved performance in the object placement task
The significant main effect of estrogen administration F(3,64)=2.87, p=0.04 was due to rats receiving 17β-E2 or PPT, but not DPN, spending a greater percentage of time with the object in the novel location than did rats administered vehicle (Figure 5). Means of groups for total time spent with objects during training and testing are included in Table 1 (n.s.).
Figure 5.
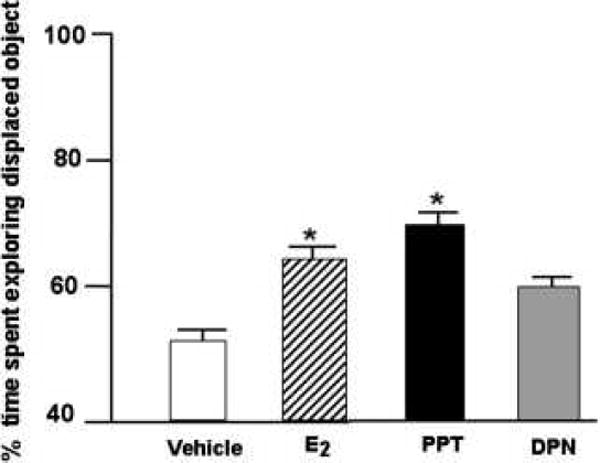
Mean percent (± standard error) of time ovx rats administered post-training E2 (n=17; stripped bar), PPT (n=17; filled bar), or DPN (n=17; shaded bar) rats spent exploring the displaced object as compared to ovx vehicle controls (n=17). * Indicates significant difference from ovx (p ≤ 0.05).
3.6 Experiment 6- Administration of P or 3α,5α-THP to OVX rats immediately post-training improved performance in the object placement task
The significant main effect of progestin condition F(2,54)=3.28, p=0.04 on object placement task performance was due to administration of P4 or 3α,5α-THP significantly increased the percentage of time spent with the object in a novel location compared to vehicle-administration (Figure 6). Means of groups for total time spent with objects during training and testing are included in Table 1 (n.s.).
Figure 6.
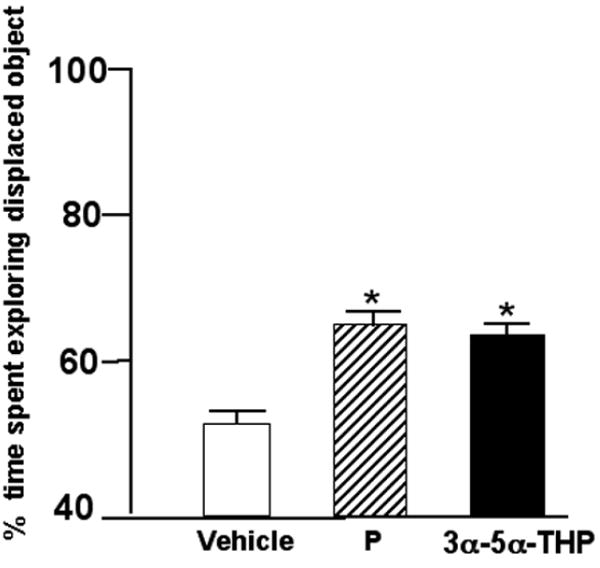
Mean percent (± standard error) of time ovx rats administered post-training P (n=19; stripped bar) or 3α-5α-THP (n=18; filled bar) spent exploring the displace object as compared to ovx vehicle controls (n=21). * Indicates significant difference from ovx vehicle (p ≤ 0.05).
4. Discussion
The data from the present study supported our hypotheses that endogenous fluctuations in, or exogenous administration of, E2 or progestins would influence performance in the object placement task. In support, rats in proestrus or estrus spent more time with the object in a novel location than did diestrous rats. Rats in the 3rd trimester, post-partum, or lactating spent more time exploring the object in novel location than did rats in the 1st trimester of pregnancy. Further, administration of physiological regimen of E2 and/or progestins immediately (but not with a 1.5 hour delay) post-training, increased time spent with the object in the novel location compared to that of ovx rats administered vehicle. Administration of E2 or PPT, which is selective for ERα (but not ERβ selective DPN), enhanced time spent with the object in the novel location compared to vehicle controls. Administration of P or 3α,5α-THP enhanced time spent with the object in the novel location compared to vehicle controls. Together these data suggest that performance in the object placement task is better when E2 and/or P are naturally elevated or when E2, PPT (an ERα selective ER modulator), P or its metabolite 3α,5α-THP are administered immediately post-training. Thus, both short- and long-term increases in ovarian steroids can enhance spatial performance in the object placement task.
The present results confirm and extend previous findings on variations in cognitive performance of rats over the estrous cycle. Rats in proestrus and estrus spent more time with the object in a novel location than did diestrous rats, suggesting that acute increases in endogenous E2 and/or progestin levels may be beneficial for cognitive performance in a spatial task. In studies utilizing tasks where animals can be trained and tested in the same hormonal milieu, such as the classical eye-blink conditioning task, passive avoidance task, and one-day waer maze, performance is better during proestrus compared to other phases of the cycle (Frick and Berger-Sweeny, 2001; Rhodes & Frye, 2004; Shors et al., 1998; Wood et al., 2001). In contrast, rodents in proestrus perform no differently or worse in the water maze, radial arm maze, and active avoidance (Farr et al., 1995; Frye, 1995; Rubinow et al., 2004; Stackman et al., 1997; Warren and Juraska, 1997). Together, these findings suggest that effects on spatial ability of hormones over the cycle may differ with task demand, stress, and/or shifts in cognitive strategy (Galea et al., 2000; Korol et al., 2004; Korol & Kolo, 2002; Rubinow et al., 2004). As such, using a task, e.g. the object recognition or object placement, which does not incorporate aversive/noxious stimuli (electric shock, food deprivation) or physical demand (swimming), but incorporates rats’ natural exploratory tendencies may reveal effects of steroids for spatial memory that can be parsed out from effects on stress/anxiety. Although this task is considered to be less demanding than tasks that do incorporate such stimuli, indices of stress/arousal are increased following exposure to novel objects/arenas in some animals (Cavigelli and McClintock, 2003), and improved performance in object memory tasks is dependent upon some ‘emotional arousal’ of the animal during training, an effect that is abolished with post-training injections of high dosages of corticosterone (Okuda et al., 2003). However, we found little evidence of consistent differences in exploration of the objects during training in intact or ovx rats that would imply differences were due to exploration/anxiety rather than mnemonic processes. Given that immediate, but not delayed, post-training administration of E2, P, or E2 & P to ovx rats enhances performance further suggest that effects observed were on memory consolidation rather than motoric and affective confounds. Future studies could investigate this point further by using steroids that rapidly metabolize and would thus not be at appreciable levels during training and testing.
Furthermore, the present study suggests that chronic exposure to increased P levels may also be beneficial. 3rd trimester, post-partum, and lactating animals all similarly outperformed 1st trimester animals; despite post-partrum and lactating rats having lower steroid levels during training and testing. There is evidence to suggest that beneficial effects of pregnancy on cognitive performance may persist beyond the gestational period. Water maze performance is better among multiparous than nulliparous rats (Gatewood et al., 2005). Multiparous rats have enhanced working memory performance and primiparous rats have enhanced reference memory performance compared to nulliparous rats which suggested that hormone-induced hippocampal modifications in pregnant rats may improve learning and memory ((Kinsley et al. 1999) albeit it is not clear whether some of these effects are due to motor or mnemonic processes). These pregnancy-related effects observed in animal models may be analogous to that of some women. Six months after pregnancy, women’s word recall was enhanced compared to that of non-pregnant controls (Sharp et al., 1993). Although we may infer that E2 and/or progestins during pregnancy influence cognitive performance, 1st trimester rats did not have enhanced performance in this task, which may be due to effects of the many pregnancy hormones that are changing at this time. Prolactin, oxytocin, and glucocorticoids are all elevated during pregnancy in rats, are known to influence brain structures, and may be involved in the modulation of spatial cognition (Grattan, 2002; Oitzl et al., 1998; Roozendaal et al., 1996; Shingo et al., 2003; Tomizawa et al., 2003). For that reason, it is essential to examine effects on cognitive performance of both endogenous variations in hormones and effects of systemic hormone-replacement.
In the present study, administration of E2 and/or P4 immediately post-training increased the percentage of time spent exploring the object in the novel location. We have previously shown that administration of E2 and P increase E2 and P levels in the hippocampus (similar to that observed in proestrus) 4 hours post subcutaneous administration (Walf et al., 2006). These data confirm other reports that suggest administration of E2 and P can enhance cognitive performance. Co-administration of E2 and P4 enhances performance of young OVX rats in spatial memory tasks (Sandstrom & Williams, 2001; Sato et al., 2004). Notably, few studies have looked at effects of E2 and P and P alone for cognitive performance in a “healthy” animal as most have used pharmacological agents or neurodegeneration models. For instance, administration of E2 alone, or with P, to ovx rats attenuated scopolamine-induced deficits in T- maze alternations (Dohanich et al., 1994). Co-administration of E2 and P4 enhances performance of young oxv rats with neurodegeneration in the hippocampally-mediated spatial task (Vongher & Frye, 1999). Indeed, beneficial effects of E2 for water maze performance are abrogated by P administration to aged mice in a dose-dependent manner (Harburger et al., 2007). Notably, a 1.5 hour post training delay in E2 and/or P administration abrogated their mnemonic effects, which suggest that the beneficial effects of these steroids in a young/healthy system occur when they are present during early consolidation. Thus, the present data suggest that E2 and P have beneficial effects in a normally-functioning system to enhance cognitive performance.
The present results that E2 and the ERα-specific ligand, PPT, enhanced performance in the object placement task suggest that E2’s effects at ERs may be important for its beneficial effects on cognitive performance. That E2 and PPT enhanced performance, unlike DPN and vehicle, suggest that ERα may be important for performance in this spatial task. This confirms previous reports that ERα-selective SERMs enhance performance in the object recognition and object placement tasks (Luine et al., 2003; Walf et al., 2006). However, other reports have suggested that in object recognition or spatial or emotional memory tasks, such as the water maze or passive avoidance, are enhanced by ERα and/or β-specific SERMs (Fugger et al., 1998; Rhodes & Frye, 2006; Walf et al., 2006). Notably, in these previous studies and in the present one, dose-dependent effects of these compounds were not assessed, which may be particularly important to investigate given their differential affinity for ERα and ERβ. It may be that both ERα and ERβ are important for cognitive performance and that these effects may be task-specific and dose-dependent. Furthermore, given that we observed that the ERa agonist0treated, but not ERb agonist-treated, rats had enhanced performance and ERα is less abundant than ERβ in the cortex (Shughrue & Mercenthaler, 2001; Shughrue et al., 1998), perhaps for object placement the cortex may be a less important brain target for estrogens’ effects than other sites. This is speculative and was not investigated in the present study. Future studies investigating the task- and dose-dependency and site-specificity of the observed effects are warranted.
The present results suggest that progestins can have mnemonic effects. In support, post-training P or 3α,5α-THP administration, compared to vehicle, increased the percentage of time spent exploring the object in a novel location. In other tasks that utilize the hippocampus, young, ovx rats show decrements in water maze, passive avoidance, and object recognition that can be ameliorated with administration of P or 3α,5α-THP (Frye & Lacey, 2000; Frye & Sturgis, 1995; Walf et al., 2006). 3α,5α-THP in particular appeared to have beneficial effects in the object placement task. Ovx rats administered 3α,5α-THP out-performed ovx animals administered P or vehicle. In addition, 3α,5α-THP cannot back convert to P, which implies that P’s effects are due to conversion to 3α,5α-THP and 3α,5α-THP’s subsequent effect. Other studies have not demonstrated a beneficial effect of progestins for cognitive performance (Freeman et al., 1993; Zou et al., 2000); however, these effects may be due to 3α,5α-THP’s dose-dependent effects for behavior in this task. Notably, higher dosages of 3α,5α-THP can have anxiolytic or sedative effects (Bitran et al., 1991; 1999; Frye et al., 2000; Korneyev & Costa, 1996; Mok & Krieger, 1990; Seyle, 1941), which would interfere with consolidation and performance. However, we have shown that the dosages used in the present study produce physiological progestin levels in the hippocampus (Walf et al., 2006).
In summary, we have found that acute and/or chronic endogenous increases in E2 and progestins, such as occur over the estrous cycle and pregnancy, can enhance cognitive performance in the object placement task. Furthermore, acute administration of E2 and/or progestins immediately after training improved performance in the object placement task The mnemonic effects of E2 and/or progestins were abrogated when they were administered 1.5 hours after training, which suggests that steroids may have an enhancing effects that are relegated to the early phase of consolidation. As well, there were similar effects of E2 and/or the ERα selective SERM to enhance performance in this task, which suggest that actions at ERα may be important. Finally, the similar effects of P and 3α,5α-THP suggest that actions of 3α,5α-THP may underlie some of the beneficial effects of progestins on cognitive performance. Together, these data suggest that both short-term and chronic changes in E2 and progestins can alter spatial performance in the object placement task.
Acknowledgments
Technical (Alicia Babson, Rob Streger) and intellectual input (Dr. Madeline Rhodes) provided by others was instrumental in the completion of this project. Caryn Duffy received SUNY-Albany’s Presidential Award for Undergraduate Research for this work, which was funded with support from NIMH (MH 06-76980) and NSF (IBN03-16083).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behavioural Brain Research. 1986;19:133–46. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–73. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Research. 1999;850:217–24. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α-pregnan-20-one-endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Research. 1991;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proceedings of the National Academy of Science. 2003;100:16131–6. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YJ, Karavolas HJ. Conversion of progesterone to 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregan-20-one by rat medial basal hypothalamus and the effects of stage on the estrus cycle on the conversion. Endocrinology. 1973;93:1157–1162. doi: 10.1210/endo-93-5-1157. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Hormones and Behavior. 1997;32:217–25. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiology of Learning and Memory. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Urresta F, Dussaubat N, Mora S. Effects of estradiol replacement in ovariectomized rats on conditioned avoidance responses and other behaviors. Physiology and Behavior. 1991;50:61–5. doi: 10.1016/0031-9384(91)90498-d. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Urresta F, Dussubat N, Mora S. Progesterone effects on the acquisition of conditioned avoidance responses and other motoric behaviors intact and overiectomized rats. Psychoneuroendocrinology. 1994;37:889–904. doi: 10.1016/0306-4530(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behavioral Neuroscience. 1994;108:988–92. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- Ebner DL, Richardson R, Riccio DC. Ovarian hormones and retention of learned fear in rats. Behavioral and Neurobiology. 1981;33:44–58. doi: 10.1016/s0163-1047(81)92215-9. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural Brain Research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Experimental Brain Research. 1997;113:509–19. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiology of Learning and Memory. 1998;69:225–40. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE. Effect of ovarian steroids on footshock avoidance learning and retention in female mice. Physiology and Behavior. 1995;58:715–23. doi: 10.1016/0031-9384(95)00124-2. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Purdy RH, Coutifaris C, Rickels K, Paul SM. Anxiolytic metabolites of progesterone: correlation with mood and performance measures following oral progesterone administration to healthy female volunteers. Neuroendocrinology. 1993;58:478–84. doi: 10.1159/000126579. [DOI] [PubMed] [Google Scholar]
- Frick KM, Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice. Behavioral Neuroscience. 2001;115:229–37. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiology and Behavior. 1995;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. Journal of Neuroendocrinology. 1999;11:839–47. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. Progestins influence performance on cognitive tasks independent of change in affective behavior. Psychobiology. 2000;28:550–563. [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacology, Biochemistry & Behavior. 2000;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Enhancing effects of estrogen on passive avoidance performance may be in part independent of intracellular estrogen receptors in the hippocampus. Brain Research. 2002;956:285–93. doi: 10.1016/s0006-8993(02)03559-x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Sturgis JD. Neurosteriods affect spatial/reference, working and long-term memory of female rats. Behavioral & Neural Biology. 1995;64:83–96. doi: 10.1006/nlme.1995.1046. [DOI] [PubMed] [Google Scholar]
- Fugger HN, Cunningham SG, Rissman EF, Foster TC. Sex differences in the activational effect of ERα on spatial learning. Hormones and Behavior. 1998;34:163–70. doi: 10.1006/hbeh.1998.1475. [DOI] [PubMed] [Google Scholar]
- Galea LA, Ormerod BK, Sampath S, Kostaras X, Wilkie DM, Phelps MT. Spatial working memory and hippocampal size across pregnancy in rats. Hormones and Behavior. 2000;37:86–95. doi: 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Research Bulletin. 2005;66:91–8. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Hormones and Behavior. 1999;36:222–33. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of Aging. 2000;21:107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Grattan DR. Behavioural significance of prolactin signaling in the central nervous system during pregnancy and lactation. Reproduction. 2002;123:497–506. doi: 10.1530/rep.0.1230497. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiology of Aging. 2007;28:602–10. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- Korneyev A, Costa E. Allopregnanolone (THP) mediates anesthetic effects of progesterone in rat brain. Hormones and Behavior. 1996;30:37–43. doi: 10.1006/hbeh.1996.0006. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiology of Learning and Memory. 2004;82:309–23. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behavioral Neuroscience. 2002;116:411–20. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Hormones and Behavior. 2004;45:330–8. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicology and Teratology. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Hormones and Behavior. 1998;34:149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiology of Learning and Memory. 2003;80:315–22. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. Journal of medicinal chemistry. 2001;44:4230–5. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Mok WM, Krieger NR. Evidence that 5α-pregnane-3α-ol-20-one is the metabolite responsible for progesterone anesthesia. Brain Research. 1990;533:42–45. doi: 10.1016/0006-8993(90)91792-f. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neuroscience Biobehavioral Reviews. 2004;28:55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behavioural Brain Research. 2001;127:159–81. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- O’Neal MF, Means LW, Poole MC, Hamm RJ. Estrogen affects performance of ovariectomized rats in a two-choice water-escape working memory task. Psychoneuroendocrinology. 1996;21:51–65. doi: 10.1016/0306-4530(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Fluttert M, de Kloet ER. Acute blockade of hippocampal glucocorticoid receptors facilitates spatial learning in rats. Brain Research. 1998;797:159–162. doi: 10.1016/s0006-8993(98)00387-4. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proceedings of the National Academy of Sciences. 2004;101:853–8. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG. Posttraining estrogen and memory modulation. Hormones and Behavior. 1998;34:126–39. doi: 10.1006/hbeh.1998.1464. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Estrogen has mnemonic enhancing effects in the passive avoidance task. Pharmacology, Biochemistry, and Behavior. 2004a;78:551–558. doi: 10.1016/j.pbb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERβ-selective SERMs produce mnemonic effects in the passive avoidance and water maze tasks. Neurobiology of Learning and Memory. 2006;85:183–191. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Arseneau LM, Beverly JL, Juraska JM. Effect of the estrous cycle on water maze acquisition depends on the temperature of the water. Behav Neurosci. 2004;118:863–8. doi: 10.1037/0735-7044.118.4.863. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behavioral Neuroscience. 2001;115:384–93. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Hormones and Behavior. 2004;45:128–35. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Sato T, Tanaka K, Ohnishi Y, Teramoto T, Irifune M, Nishikawa T. Effects of estradiol and progesterone on radial maze performance in middle-aged female rats fed a low-calcium diet. Behavioral Brain Research. 2004;150:33–42. doi: 10.1016/S0166-4328(03)00249-3. [DOI] [PubMed] [Google Scholar]
- Selye H. Anaesthetic effects of steroid hormones. Proc Soc Exp Biol. 1941;46:116–121. [Google Scholar]
- Sharp K, Brindle PM, Brown MW, Turner GM. Memory loss during pregnancy. British Journal of Obstetrics and Gynaecology. 1993;100:209–15. doi: 10.1111/j.1471-0528.1993.tb15232.x. [DOI] [PubMed] [Google Scholar]
- Shingo T, Cregg C, Enware E, Fujikawa H, Hassam R, Geary C, Cross J, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–23. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. Journal of Comparative Neurology. 2001;436:64–81. [PubMed] [Google Scholar]
- Shughrue PJ, Scrimo PJ, Merchenthaler I. Evidence for the colocalization of estrogen receptor-β mRNA and estrogen receptor α immunoreactivity in neurons of the rat forebrain. Endocrinology. 1998;139:5267–70. doi: 10.1210/endo.139.12.6525. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS. Stability of spatial working memory across the estrous cycle of Long-Evans rats. Neurobiology of Learning and Memory. 1997;67:167–71. doi: 10.1006/nlme.1996.3753. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. Journal of medicinal chemistry. 2000;43:4934–47. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu Y-F, Moriwaki A, Matsushita M, Li S-T, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nature Neuroscience. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- van Wimersma Greidanus TB. Pregnene-type steroids and impairment of passive avoidance in rats. Hormones and Behavior. 1977;9:49–56. doi: 10.1016/0018-506x(77)90049-6. [DOI] [PubMed] [Google Scholar]
- Vongher JM, Frye CA. Progesterone in conjunction with estradiol has neuroprotective effects in an animal model of neurodegeneration. Pharmacology, Biochemistry, and Behavior. 1999;64:777–85. doi: 10.1016/s0091-3057(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally-cycling and overiectomized, hormone-primed rats. Neurobiology of Learning and Memory. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behavioral Neuroscience. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behavioral Neuroscience. 2001;115:175–87. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Zou LB, Yamada K, Sasa M, Nakata Y, Nabeshima T. Effects of sigma(1) receptor agonist SA4503 and neuroactive steroids on performance in a radial arm maze task in rats. Neuropharmacology. 2000;39:1617–1627. doi: 10.1016/s0028-3908(99)00228-2. [DOI] [PubMed] [Google Scholar]


