Abstract
Background
Little is known about the specific contribution of serotonin (5-HT) to the neurobiology of emotion and mood in healthy people. In an exploratory study, we sought to investigate the effect of rapid and sustained changes of emotional state on the trapping of 11C-labelled α-methyl-L-tryptophan (11C-αMtrp) used as a proxy of 5-HT synthesis, using positron emission tomography (PET).
Method
In a within-subject repeated-measure design, participants recalled autobiographical memories to self-induce sadness, happiness and a neutral emotional state during scanning to measure brain trapping of 11C-αMtrp. Three separate scan acquisitions, counterbalanced for order across subjects, took place at the McConnell Brain Imaging Center, Montréal.
Results
Whole brain analysis revealed positive and negative correlations between experienced levels of emotions and 11C-αMtrp trapping in the right anterior cingulate cortex.
Conclusion
These findings point to a mechanism whereby state-related changes in a proxy of 5-HT synthesis underscore aspects of the self-regulation of normal mood.
Medical subject headings: brain; depression; emotions; gyrus, cingulate; positron emission tomography; serotonin; tryphophan
Abstract
Contexte
La contribution spécifique de la sérotonine (5-HT) à la neurobiologie des émotions et de l'humeur chez des sujets sains est peu connue. Dans le cadre d'une étude exploratoire en tomographie par émission de positons, nous avons entrepris d'étudier l'effet d'un changement aigu et soutenu de l'état émotionnel sur le taux de capture de l'α-méthyl-L-tryptophane marqué au 11C (11C-αMtrp), liguant employé comme indicateur de la synthèse de 5-HT.
Méthode
Selon un devis expérimental intra-sujet à mesures répétées, les participants se remémoraient des souvenirs autobiographiques afin d'autoinduire la tristesse, la joie et un état émotionnel neutre lors de l'acquisition de scanographies mesurant la capture de l'11C-αMtrp. Trois acquisitions en TEP, contrebalancés à travers les sujets, ont été réalisées au Centre d'Imagerie Cérébrale McConnell de Montréal.
Résultats
Une analyse du cerveau complet a révélé des corrélations positives et négatives dans le cortex antérieur cingulaire droit entre la capture d'11C-αMtrp et l'intensité émotionnelle subjective.
Conclusion
Ses résultats suggèrent l'existence d'un mécanisme par lequel les variations du taux de capture de l'11C-αMtrp reliés aux changements d'états émotionnels mettent en évidence des aspects de l'autorégulation de l'humeur normale.
Introduction
Brain serotonin (5-HT) is believed to modulate the generation and regulation of emotion and mood.1 In healthy volunteers, the transient reduction of serotonergic neurotransmission by means of dietary depletion of tryptophan has often been associated with mood-lowering.2,3 Conversely, selective serotonin reuptake inhibitors, whose mechanism of action is believed to rely on the enhancement of serotonergic function in the brain, are effective antidepressants.4 In vivo measurements of various components of 5-HT neurotransmission in patients with major depressive disorder and their relatives all support the theory of an absolute or relative deficit in cortico-limbic pathways believed to regulate the expression of mood.5 Consistent with this theory is the recent report of a decreased trapping of 11C-labelled α-methy-L-tryptophan (11C-αΜtrp), used as a proxy for the measurement of 5-HT synthesis, in the anterior cingulate cortex (ACC), in medication-free patients with major depression.6
Little is known about the functional brain neurochemical correlates of normal mood. In this study, we focused on measuring in vivo the putative changes in brain tryptophan– 5-HT metabolism in healthy volunteers during the self-induction of nonpathologic sadness and happiness. Specifically, we investigated the effects of rapid and sustained changes of emotional state in professional actors, using positron emission tomography (PET) and measuring in vivo the brain trapping constant for 11C-αMtrp. 11C-αΜtrp is a radioactive-labelled synthetic analog of L-tryptophan that crosses the blood–brain barrier and is in part metabolized into 11C-α-methyl-serotonin in 5-HT neurons, the latter not being a substrate for the monoamine oxydase enzyme.7–9 In the rat brain, the unidirectional trapping of 11C-αΜtrp correlates with the rate of metabolic conversion of L-tryptophan into 5-HT, thereby providing a justification for the use of tracer trapping as a proxy estimate for brain 5-HT synthesis.10
Methods
All participating subjects (n = 7, mean [standard deviation] age 35 [5.4] years) were male professional actors of French-Canadian descent, physically healthy, medication-free and devoid of current Axis I DSM-IV diagnosis,11 determined according to the Structured Clinical Interview for DSM-IV Axis I disorder (SCID-I).12 The emphasis was on men only because of previously reported in vivo sex differences in brain 5-HT synthesis.13 The study was approved by the Montréal Neurological Institute Research Ethics Board. After the process was completely descirbed, all subjects provided written informed consent.
All subjects were trained to reliably induce and maintain happiness, sadness and a neutral emotional state for 60 minutes (scan duration) while recalling autobiographical memories. To encourage subjects to maintain the target states, we let the subjects use their discretion as to what images and memories to use. Professional actors were recruited because of their ability to experience intense emotional states for long periods. They were instructed to relive and re-enact genuine emotions while understanding the outcome of their compliance to the emotion induction for the validity of the study. During prescan training sessions, subjects had to self-induce and maintain the 3 target states for a minimum of 60 minutes. All subjects participated in a PET sham scanning a few days before entering the protocol to familiarize themselves with the scanning environment.
All subjects underwent 3 measurements of 11C-αΜtrp brain uptake and trapping on separate days, each acquired at the same time of the day under a specific self-induced emotional condition (happy, sad, neutral), with the order of condition being counterbalanced across subjects. Self-induction of mood state was initiated 5 minutes before injection of 11C-αΜtrp. Subjects were asked to maintain the target emotional state at high intensity for the entire duration of the scan (60 min). 11C-αΜtrp was prepared as described by Mzengeza and colleagues.14 Before data acquisition, transmission scans for attenuation correction were obtained with use of a 68Ge/68Ga source. Sixty-minute dynamic scans were acquired with an ECAT HR+ scanner (CTI/Siemens, Knoxville, Tenn.), after intravenous administration of 7–9.8 mCi of 11C-αΜtrp over 2 minutes. Eleven venous blood samples were drawn during each scanning period to compute the 11C-αΜtrp input function and measure free and total plasma L-tryphophan levels.7 Three-dimensional dynamic images were blurred by using a 6-mm full width at half maximum Hanning filter transaxially. All subjects underwent high-resolution 1.5T MRI for PET–MR correlations (Gyroscan, Philips Medical Systems, Eindhoven, The Netherlands).
Heart rate (HR) and electrodermal responses (EDRs) were recorded during each of the 3 conditions in 6 blocks of 10 minutes each. After each scanning period, subjects reported the intensity of primary emotions they experienced on a visual analog scale ranging from “not at all” (0) to “extremely” (10).
Functional brain images of 11C-αΜtrp trapping (K*) were computed as detailed, and the computation was based on a 2-tissue 3-rate constant biological model and the use of venous sinus–venous plasma input function.15 The functional images were analyzed with use of Statistical Parametric Mapping (SPM99) software (Wellcome Functional Imaging Laboratory, London, UK). To remove the effect of global differences on regional values among subjects, functional images were normalized by the mean global K* values of the grey matter, with proportional scaling. The t value of each voxel was then converted to a normal standard distribution (z values) that, according to the Gaussian random field theory, is independent of the error's degree of freedom.16 These values constitute a statistical parametric map, as described by Friston and associates.17 SPM parameters were thresholded as follows: pic height (u) was set at p = 0.005 uncorrected, and extend threshold (k) was set to 75 voxels to remove small noisy clusters that could reach statistical significance by chance.18 Finally, only clusters with a probability level of z > 3.0 (p ≤ 0.001) were used to interpret statistical results.
Image analysis focused on calculating regression maps, with single subject covariates used to explore possible relations between changes of regional cerebral 11C-αΜtrp trapping and the felt intensity of happiness and sadness.
Results
Plasma concentrations of free and total tryptophan did not differ significantly across scans (repeated-measures analysis of variance, free tryptophan: p = 0.14; total tryptophan: p = 0.98; plasma concentration of free tryptophan per condition in nmol/mL: sad 8.8, happy 9.9, neutral 9.7; plasma concentration of total tryptophan per condition in nmol/mL: sad 43.6, happy 42.9, neutral 43.0).
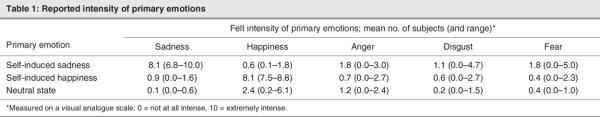
All subjects reported significantly experiencing the target emotional state during the 3 experimental conditions; the perceived intensity did not differ across the 3 conditions (Table 1). Modulation of emotional states was associated with various nonspecific changes in HR and EDR: average EDR responses were significantly greater during blocks 5 and 6 in the happy condition, relative to neutral, and HR average responses were significantly higher during block 1 in the sad condition, relative to neutral.
Table 1
Normalized K* values (μL/g/min) ranged from 99 to 127 in Brodmann's area (BA) 32 and from 84 to 132 in BA 25 during the sad condition, for a reported intensity in sadness varying between 6.80 and 10, whereas normalized K* values (μL/g/min) ranged from 96 to 114 in BA 32 and from 92 to 127 in BA 25 during the happy condition, for a reported intensity of happiness varying between 7.50 and 8.80.
Correlation analyses revealed that the reported level of happiness was positively correlated with K* in the right ACC (BA 32: x = 12, y = 11, z = 34, t = 11.45; r = 0.98; p < 0.005; z = 3.92). In addition, the reported level of sadness was negatively correlated with K* in the subcallosal area of the right ACC (BA 25: x = 12, y = 28, z = –17, t = 9.59; r = –0.97; p < 0.005; z = 3.71) (Fig. 1).
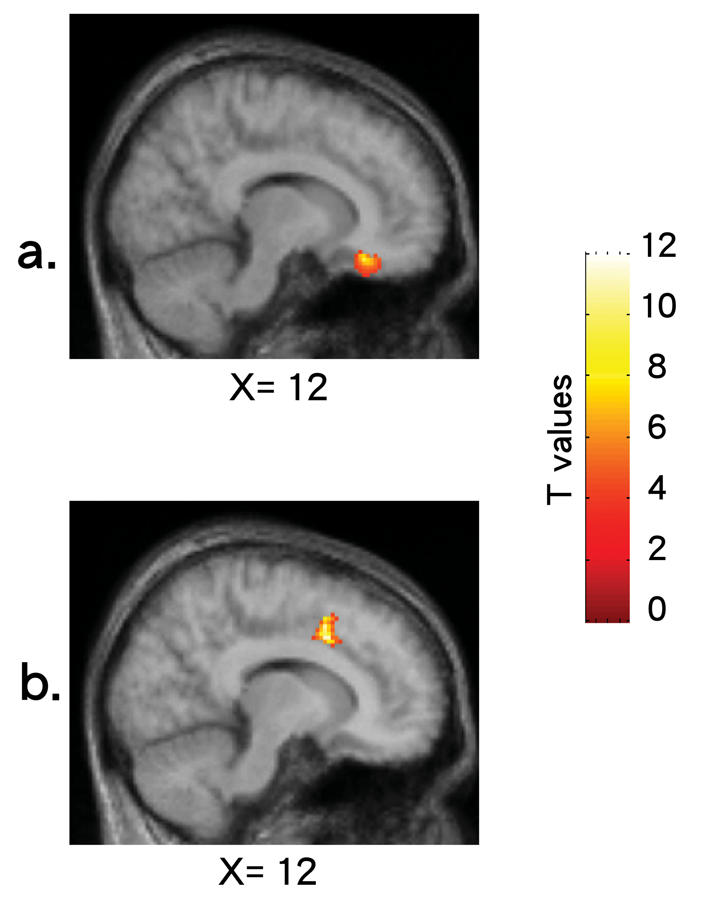
Fig. 1: Correlation maps visualizing significant negative correlations between the reported level of sadness and K* in (a) the subcallosal area of the anterior cingulate cortex (ACC) (Brodmann's Area [BA] 25) and a significant positive correlation between the reported level of happiness and K* in (b) in the right ACC (BA 32). All images represent sagittal sections for the data averaged across subjects.
Discussion
In this study, transient self-induced changes in mood correlated with corresponding changes in 11C-αΜtrp brain trapping used as a proxy of 5-HT synthesis in the ACC, a cerebral structure known to be part of the functional circuitry of mood regulation.19 The variance of 11C-αΜtrp brain trapping noted here, resulting from the experimental manipulation of mood states, could not be accounted for solely by within-subject variability because it exceeded the magnitude of change determined in healthy volunteers in a separate test–retest study, which was found to be less than 5%,20 nor by changes in the plasma concentrations of free tryptophan, which remained relatively stable across conditions.
Those findings point to a mechanism whereby state-related changes in 5-HT synthesis capacity, estimated by trapping of 11C-αΜtrp, may in part underscore aspects of the self-regulation of normal mood. Serotonergic neurotransmission is generally deemed a stable trait,21 although it can be modulated by environment, experience or drugs. In experimental animals, acute manipulations of arousal and emotional states are commonly associated with activation of the serotonergic system in cortical or paralimbic (or both) brain structures but not in the striatum.22,23 Further, modelling of “depressive” states in rodents is reportedly associated with a decreased releasable pool of 5-HT.24,25 Although generally limited, the most recent data examining the interrelations, in humans, between subjective mood states at rest and peripheral indices of serotonergic neurotransmission26,27 suggest that positive, but not negative, mood correlates positively with 5-HT neurotransmission. The present finding of a positive correlation in the right ACC between self-induced happiness and K* is consistent with animal work emphasizing increased 5-HT function in the face of increased arousal. Conversely, the finding of a negative correlation in the right subgenual ACC between self-induced sadness and K* is deemed consistent with the abnormal metabolic activity reported in this anterior cingulate area in patients with major depression28 and is also consistent with the recent report by our group of reduced K* in the ACC in medication-free depression patients.6
Although preliminary, the present findings suggest that self-induced high and low mood, respectively, may involve serotonergic mechanisms of seemingly opposite directions in overlapping limbic and paralimbic mood circuits, of which the ACC, and most particularly BA 25, appears to be a central relay.28 This interpretation, however, rests on the following methodologic considerations:
• The sample size is modest, although the repeated-measures within-subjects design adds power.
• The results rest on agreement that PET measurement of the regional trapping of 11C-αΜtrp represents an acceptable proxy for 5-HT synthesis. Among the concerns expressed by some is the suggestion that 11C-αΜtrp K* might better reflect blood–brain barrier transport of tryptophan than synthesis of 5-HT itself.29 A recent independent report describes linear Patlak plots of 11C-αΜtrp trapping in primates, with a slope significantly different from zero; this suggests that 11C-αΜtrp behaves as an irreversible tracer9 and is a significant contribution, albeit cautious, in support of a considered use of the method. This being said, the report of a correlation between mood states and blood–brain barrier tryptophan transport would by itself have merit.
• Variations of regional K* as a function of self-induced mood states could be interpreted as a consequence of mood-related changes of cerebral blood flow or plasma tryptophan. However, this is unlikely because tracers with low plasma–brain rate constant (less than 10%30), such as 11C-αΜtrp, are insensitive to variations in blood flow.31 Plasma-free tryptophan did not differ among all 3 conditions and, as such, could not account for the observed differences.
• All subjects were asked to maintain the target emotion for the duration of the scan, a task that might involve other cognitive components apart from the self-generation of emotion. Theoretically, differences in strategy among subjects or conditions could confound the results and account for some of the variance, even though such modest effects might cancel out across conditions.
• To increase the ecologic validity of the present findings, the protocol should be replicated in normal control subjects who are not experts in emotional self-regulation, as are professional actors.
Footnotes
Contributors: Ms. Perreau-Linck, Mr. Gravel, Mr. Paquette, and Drs. Beauregard and Benkelfat designed the study. Ms. Perreau-Linck, Mr. Gravel, and Drs. Beauregard, Soucy and Diksic acquired the data, which Ms. Perreau-Linck, Mr. Gravel, and Drs. Beauregard, Soucy, Diksic and Benkelfat analyzed. Ms. Perreau-Linck, Mr. Gravel and Drs. Benkelfat and Beauregard wrote the article, and all authors revised it. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Chawki Benkelfat, Department of Psychiatry, Research and Training Building, 1033 Pine Ave. W., Montréal, QC H3A 1A1; fax 514 398-4866; chawki.benkelfat@mcgill.ca
References
- 1.Davidson RJ. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. Am Psychol 2000;55:1196-214. [DOI] [PubMed]
- 2.Benkelfat C, Ellenbogen MA, Dean P, et al. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective-disorders. Arch Gen Psychiatry 1994;51:687-97. [DOI] [PubMed]
- 3.Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels [review]. Pharmacol Biochem Behav 2002;71:857-65. [DOI] [PubMed]
- 4.Blier P, DeMontigny C. Serotonin and drug-induced therapeutic responses in major depression, obsessive-compulsive and panic disorders. Neuropsychopharmacology 1999;21(2 Suppl):91S-8S. [DOI] [PubMed]
- 5.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry 1998;44:151-62. [DOI] [PubMed]
- 6.Rosa-Neto P, Diksic M, Okazawa H, et al. Measurement of brain regional alpha-[11C]methyl-L-tryptophan trapping as a measure of serotonin synthesis in medication-free patients with major depression. Arch Gen Psychiatry 2004;61:556-63. [DOI] [PubMed]
- 7.Nishizawa S, Leyton M, Okazawa H, et al. Validation of a less-invasive method for measurement of serotonin synthesis rate with alpha-[11C]methyl-tryptophan. J Cereb Blood Flow Metab 1998;18:1121-9. [DOI] [PubMed]
- 8.Chugani DC, Muzik O. Alpha[C-11]methyl-L-tryptophan PET maps brain serotonin synthesis and kynurenine pathway metabolism. J Cereb Blood Flow Metab 2000;20:2-9. [DOI] [PubMed]
- 9.Lundquist P, Hartvig P, Blomquist G, et al. 5-Hydroxy-L-[beta-11C]tryptophan versus alpha-[11C]methyl-L-tryptophan for positron emission tomography imaging of serotonin synthesis capacity in the rhesus monkey brain. J Cereb Blood Flow Metab 2007;27:821-30. [DOI] [PubMed]
- 10.Diksic M, Tohyama Y, Takada A. Brain net unidirectional uptake of alpha-[(14)C]methyl-L-tryptophan (alpha-MTrp) and its correlation with regional serotonin synthesis, tryptophan incorporation into proteins, and permeability surface area products of tryptophan and alpha-MTrp. Neurochem Res 2000;25:1537-46. [DOI] [PubMed]
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental disorders-IV-TR. 4th ed. Text revision. Washington: The Association; 2000.
- 12.First MB, Spitzer RL, Gibbon M. Axis I disorders. New York: New York State Psychiatric Institute; 2002.
- 13.Sakai Y, Nishikawa M, Leyton M, et al. Cortical trapping of alpha-[(11)C]methyl-L-tryptophan, an index of serotonin synthesis, is lower in females than males. Neuroimage 2006;33:815-24. [DOI] [PubMed]
- 14.Mzengeza S, Venkatachalam TK, Diksic M. Asymmetric radiosynthesis of alpha-[11C]methyl-L-tryptophan for PET studies. Nucl Med Biol 1995;22:303-7. [DOI] [PubMed]
- 15.Diksic M, Young SN. Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. J Neurochem 2001;78:1185-200. [DOI] [PubMed]
- 16.Worsley KJ, Cao J, Paus T, et al. Applications of random field theory to functional connectivity. Hum Brain Mapp 1998;6:364-7. [DOI] [PMC free article] [PubMed]
- 17.Friston KJ, Frith CD, Liddle PF, et al. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 1991;11:690-9. [DOI] [PubMed]
- 18.Friston KJ, Tononi G, Reeke GN Jr, et al. Value-dependent selection in the brain: simulation in a synthetic neural model. Neuroscience 1994;59:229-43. [DOI] [PubMed]
- 19.Beauregard M, Lévesque J, Paquette V. Neural basis of conscious and voluntary self-regulation of emotion. In: Beauregard M, editor. Consciousness, emotional self-regulation and the brain. Amsterdam (NL): John Benjamins Publishing; 2004. p. 163-94.
- 20.Rosa-Neto P, Diksic M, Leyton M, et al. Stability of alpha-[(11)C]methyl-L-tryptophan brain trapping in healthy male volunteers. Eur J Nucl Med Mol Imaging 2005;32:1199-204. [DOI] [PubMed]
- 21.Hildebrand J, Bourgeois F, Buyse M, et al. Reproducibility of monoamine metabolite measurements in human cerebrospinal fluid. Acta Neurol Scand 1990;81:427-30. [DOI] [PubMed]
- 22.Haller J, Toth M, Halasz J. The activation of raphe serotonergic neurons in normal and hypoarousal-driven aggression: a double labeling study in rats. Behav Brain Res 2005;161:88-94. [DOI] [PubMed]
- 23.Yokoyama M, Suzuki E, Sato T, et al. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett 2005;379: 37-41. [DOI] [PubMed]
- 24.Jaffe EH, De Frias V, Ibarra C. Changes in basal and stimulated release of endogenous serotonin from different nuclei of rats subjected to two models of depression. Neurosci Lett 1993;162:157-60. [DOI] [PubMed]
- 25.Hasegawa S, Nishi K, Watanabe A, et al. Brain 5-HT synthesis in the Flinders Sensitive Line rat model of depression: an autoradiographic study. Neurochem Int 2006;48:358-66. [DOI] [PubMed]
- 26.Flory JD, Manuck SB, Matthews KA, et al. Serotonergic function in the central nervous system is associated with daily ratings of positive mood. Psychiatry Res 2004;129:11-9. [DOI] [PubMed]
- 27.Williams, E, Stewart-Knox B, Helander A, et al. Associations between whole-blood serotonin and subjective mood in healthy male volunteers. Biol Psychol 2006;71:171-4. [DOI] [PubMed]
- 28.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999;156:675-82. [DOI] [PubMed]
- 29.Shoaf SE, Carson R, Hommer D, et al. Brain serotonin synthesis rates in rhesus monkeys determined by [11C]alpha-methyl-L-tryptophan and positron emission tomography compared to CSF 5-hydroxyindole-3-acetic acid concentrations. Neuropsychopharmacology 1998;19:345-53. [DOI] [PubMed]
- 30.Muzik O, Chugani DC, Chakraborty P, et al. Analysis of [C-11] alpha-methyl-tryptophan kinetics for the estimation of serotonin synthesis rate in vivo. J Cereb Blood Flow Metab 1997;17:659-69. [DOI] [PubMed]
- 31.Fenstermacher JD, Blasberg RG, Patlak CS. Methods for quantifying the transport of drugs across brain barrier systems. Pharmacol Ther 1981;14:217-48. [DOI] [PubMed]