Abstract
Neurochemical, electrophysiological and behavioural evidence indicates that certain forms of goal-directed behaviours are mediated by complex and reciprocal interactions between limbic and dopamine (DA) inputs in the nucleus accumbens (NAc). Mesoaccumbens DA transmission appears to be compartmentalized; synaptic DA transmission is mediated by phasic burst firing of DA neurons, whereas extrasynaptic tonic DA levels are regulated by DA neuron population activity and limbic glutamatergic inputs to the NAc. DA release facilitated by limbic inputs and acting on D1 receptors can either potentiate or suppress neural activity driven by separate limbic inputs converging on the same postsynaptic NAc neurons. In turn, D1 receptors in the NAc mediate accuracy of search behaviour regulated by hippocampal–ventral striatal circuitries; D2 receptors appear to mediate motivational aspects of task performance. These findings suggest that dopaminergic modulation of limbic afferents to the NAc may be a cellular mechanism for input selection that governs the smooth coordination of behaviour by permitting information processed by one limbic region to temporarily exert control over the type and intensity of adaptive behavioural responses.
Medical subject headings: nucleus accumbens, dopamine, hippocampus, amygdala, electrophysiology
Abstract
Des données probantes neurochimiques, électrophysiologiques et comportementales indiquent que des interactions complexes et réciproques entre les intrants limbiques et la dopamine (DA) dans le noyau accumbens (NAc) servent de médiateur à certaines formes de comportements dictés par des objectifs. La transmission de la DA dans le mesoaccumbens semble être compartimentée; le déclenchement par rafales en phases de neurones de DA sert de médiateur à la transmission de la DA dans les synapses, tandis que les concentrations extrasynaptiques de DA tonique sont régularisées par l'activité des neurones DA et les intrants glutamatergiques limbiques dans le NAc. La libération de DA facilitée par les intrants limbiques et agissant sur les récepteurs D1 peut activer ou réduire l'activité neuronale mue par des intrants limbiques distincts convergeant sur les mêmes neurones NAc postsynaptiques. En retour, les récepteurs D1 dans le NAc servent de médiateur à l'exactitude du comportement de recherche régularisé par les circuits striés hippocampiques–ventraux; les récepteurs D2 semblent servir de médiateur dans les aspects motivation de l'exécution des tâches. Ces constatations indiquent que la modulation dopaminergique des afférents limbiques du NAc peut constituer un mécanisme cellulaire de sélection des intrants qui régit la coordination fluide du comportement en permettant à l'information traitée par une région limbique de contrôler temporairement le type et l'intensité des réactions comportementales d'adaptation.
Introduction
Since the pioneering anatomic and electrophysiological studies of Gordon Mogenson and Lennart Heimer, the nucleus accumbens (NAc) has been viewed as a site where the integration of limbic inputs with motor effector regions occurs. This region of the ventral striatum receives excitatory glutamatergic inputs from several cortical and limbic regions, including the hippocampus and the basolateral amygdala (BLA),1 and in turn, sends projections to both pallidal and mesencephalic motor effector sites.2,3 This anatomic arrangement places the NAc in an ideal position to regulate the control that limbic and cortical regions exert over behaviour, which has led to the hypothesis that this nucleus serves as a “limbic-motor interface” that plays a critical role in processes that determine the response priorities of an organism.4
The anatomic organization of the NAc is heterogeneous, and subregions of this nucleus have been segregated on the basis of histochemical markers and intrinsic afferent and efferent connectivity.1 Initial studies segregated the ventral striatum on the basis of the regional distribution of neuropeptides such as cholecystokinin, substance P and enkephalin.1,5–8 As such, the NAc is viewed as an area consisting of 2 primary segments: a medial “shell” subregion and a more lateral “core” component. Afferent connections from limbic and cortical areas display distinct topographical organization throughout the core and shell subregions of the NAc. For example, excitatory glutamatergic inputs from the ventral subiculum (vSub), the main output of the hippocampal formation, terminate primarily in the medial shell regions, whereas projections from the dorsal subiculum terminate more in the lateral core segments of the NAc.8,9 A similar pattern of connectivity is displayed by glutamatergic afferents from the BLA.9,10 The topographically organized arrangement of these inputs has led to the speculation that the ventral striatum is a collection of “neuronal ensembles” consisting of separate clusters of neurons subserving different functional roles determined by their afferent connections.11–13
Another anatomic feature of the NAc is the degree to which inputs from different limbic regions converge. The NAc shell receives overlapping input from both the vSub and the BLA,1 and in some instances, inputs from both regions converge on the same individual medium spiny neuron.14–18 This pattern of connectivity deserves particular consideration when viewed in light of the potential mechanisms by which information processed by each of these regions may access the motor systems via the NAc. Specifically, separate memory systems incorporating the hippocampus or the BLA may be characterized by fundamentally different rules of operation, with each individual system addressing a specialized set of functional problems that cannot be solved readily by the cognitive operations regulated by another system.19 Whereas separate systems may process similar environmental stimuli, patterns of behaviour elicited by these stimuli can differ substantially from system to system. For example, following presentation of a novel stimulus, patterns of activation in these brain regions may promote either fear-related responses mediated by the BLA (such as behavioural arrest or active avoidance) or an approach response toward the novel stimulus, mediated by the hippocampus.20 Under some circumstances, these separate systems may work cooperatively to facilitate a particular behavioural response, whereas in other situations, these memory systems may work in an antagonistic manner.2,11,21 Given that individual neurons in the NAc receive information from both the hippocampus and the BLA, the intrinsic connectivity of these circuits would necessitate some sort of neural biasing or gating mechanism that would allow a particular cognitive processing system to have preferential access to motor effector sites while inhibiting other potentially competing inputs.
The NAc receives a dense dopaminergic projection from the ventral tegmental area (VTA),1,22 and owing to their anatomic arrangement and neurophysiological actions, dopamine (DA) inputs are ideally positioned to differentially gate excitatory limbic inputs to the NAc. Ultrastructural anatomic studies have shown that mesoaccumbens DA terminals are located in close apposition to excitatory afferents from either the hippocampus or the BLA.23–25 The close anatomic arrangement between dopaminergic and glutamatergic inputs suggests that DA may be ideally situated to modulate both the intrinsic membrane properties of postsynaptic NAc neurons and presynaptic glutamate inputs. Further, this connectivity suggests that glutamatergic inputs may, in turn, regulate the release of DA via local presynaptic mechanisms. Electrophysiological studies have shown that DA can either inhibit or augment synaptic activity of medium spiny neurons evoked by excitatory glutamatergic afferents, depending on several factors.26–32 These opposing actions suggest that mesoaccumbens DA may mediate the integration and gating of different limbic signals to the NAc by amplifying one subset of inputs while concurrently inhibiting activation of NAc neurons evoked by other afferent projections.2,11,33–35 It follows that mesoaccumbens DA transmission may play a particularly important role in mediating behaviours in situations where ambiguity about the environmental stimuli may have motivational relevance.
This review summarizes the findings of studies conducted by the author and fellow collaborators. The experiments in these studies were designed to further elucidate the interactions between limbic and DA inputs to the ventral striatum. In so doing, we used a multidisciplinary approach combining neurochemical, electrophysiological and behavioural methodologies to obtain a more complete understanding of the role that mesoaccumbens DA plays in facilitating behaviours mediated by the hippocampus and amygdala and of the potential cellular mechanisms that underlie these processes.
Regulation of DA transmission in the NAc
Much of the research investigating the mechanisms that regulate DA release in the ventral striatum has focused on activity of the DA cell bodies located in the VTA. It is becoming increasingly apparent, however, that DA transmission within the ventral striatum is not a unitary phenomenon but, rather, may be segregated into dissociable compartments, each of which is regulated by different neural mechanisms. One component is mediated by burst firing of DA neurons that induces a fast-acting and spatially restricted “phasic” signal. Increases in burst firing of DA neurons occur in response to primary or conditioned rewarding stimuli and have been proposed to mediate prediction error for anticipated rewards.36,37 Phasic release of DA would be expected to affect a relatively restricted number of postsynaptic neurons within the NAc because diffusion of DA from the synapse is curtailed by reuptake mechanisms involved in eliminating DA from the synaptic cleft by the high-affinity DA transporter. Burst firing of DA neurons is mediated by excitatory inputs to the VTA from regions such as the pedunculopontine nucleus, and we have shown that chemical stimulation of these excitatory inputs induces a selective increase in burst firing of VTA DA neurons without altering the overall number of DA cells that are active38 (Fig. 1A). However, under normal conditions, activation of the pedunculopontine nucleus does not evoke a discernable change in extrasynaptic DA levels within the NAc when measured with microdialysis. However, if DA reuptake is blocked, the same manipulation causes a dramatic increase in DA efflux (Fig. 1B). This finding indicates that selective increases in burst firing of DA neurons induce a massive increase in DA release at the terminal level. However, under normal conditions, the diffusion of DA is limited by reuptake mechanisms to regions in and around the synapse.
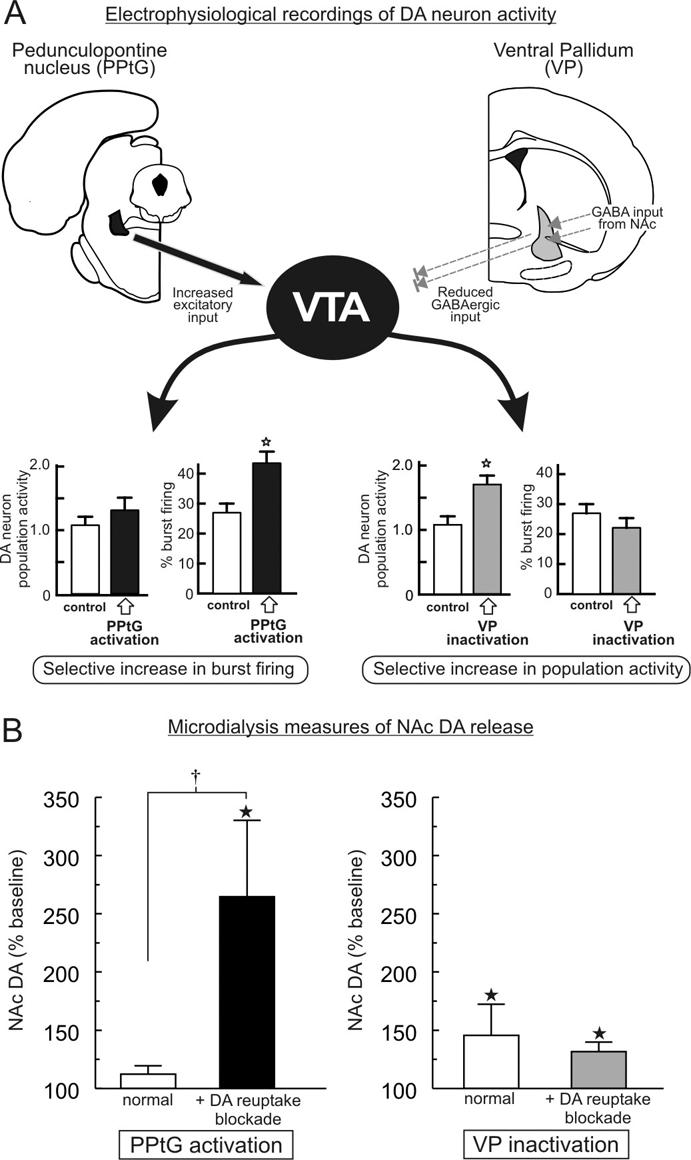
Fig. 1: Excitatory and inhibitory subcortical afferents to the ventral tegmental area (VTA) dissociably regulate different aspects of dopamine (DA) neuron activity and extrasynaptic DA levels in the nucleus accumbens (NAc). (A) Pharmacologic activation of excitatory glutamatergic and cholinergic outputs from the pedunculopontine tegmental nucleus (PPtG) causes a selective increase in burst firing of DA neurons but does not affect the overall number of spontaneously active DA neurons in the VTA. In contrast, inactivation of the inhibitory γ-aminobutyric acid-ergic (GABAergic) projection from the ventral pallidum (VP) exerts the opposite effect by selectively increasing DA neuron population activity but not affecting burst firing. Stars denote p < 0.05 versus control. (B) In vivo microdialysis data showing that increased DA neuron burst firing induced by activation of the PPtG under normal conditions does not cause a discernable increase in extrasynaptic DA levels in the NAc. However, when DA reuptake is blocked by local perfusion of nomifensine, increased burst firing causes a massive increase in DA release. In contrast, increased DA neuron population activity induced by inactivation of the VP significantly increases DA efflux in the NAc, a phenomenon that is not influenced by DA reuptake. Stars denote p < 0.05 versus baseline (not shown), and dagger denotes p < 0.01 comparing normal versus blockade of DA reuptake. Adapted from Floresco and colleagues.38
In addition to the phasic DA signal, extrasynaptic or “tonic” DA transmission represents a DA pool present in the extracellular space that changes on a much slower time course than transmission mediated by burst firing of DA neurons (second to minute v. millisecond). It is this compartment of DA transmission that is measured with conventional neurochemical techniques such as microdialysis. The neural circuitries that regulate changes in DA concentrations within this compartment appear to be distinct from those regulating phasic DA transmission. One factor that contributes to the extrasynaptic levels of DA is the overall number of spontaneously active DA neurons in the VTA (i.e., population activity). Like phasic DA neuronal firing, this profile of DA neuron activity is also under the control of subcortical afferents to the VTA. In particular, γ-aminobutyric acid-ergic (GABAergic) projection neurons from the ventral pallidum appear to exert a tonic inhibition over subsets of VTA DA neurons that, under basal conditions, limit the number of DA neurons that display spontaneous activity. Inhibition of pallidal neural activity, either by direct infusion of GABA agonists or activation of GABAergic output neurons of the NAc via stimulation of the hippocampus releases these “silent” neurons from tonic GABAergic inhibition. This, in turn, results in an overall increase in the population activity of DA neurons in the VTA, an effect that occurs in the absence of any changes in average firing rate or burst firing of DA neurons38,39 (Fig. 1A). With respect to the impact on DA terminal release, we observed that manipulations that increase the DA neuron population activity induce consistent increases in extracellular DA levels in the NAc as measured with in vivo microdialysis (Fig. 1B). Notably, blockade of DA reuptake does not alter the magnitude of change in tonic DA levels induced by disinhibition of nonfiring DA neurons, indicating that, once DA seeps out of the synapse, tonic DA levels are not heavily influenced by reuptake and that other mechanisms (e.g., extraneuronal metabolism) eliminate extrasynaptic DA. Thus increases in the overall population activity of VTA DA neurons lead to an increase in extrasynaptic DA levels in the NAc.
It is not clear why reuptake should curtail escape of phasically released DA into the extrasynaptic space but not affect extrasynaptic DA levels mediated by DA neuron population activity. However, these effects may be related to changes in the reuptake kinetics of the dopamine transporter induced by activation of D2 autoreceptors. Burst firing of a subpopulation of DA neurons in the absence of changes in DA neuron population activity would be expected to activate D2 receptors in the synaptic cleft, which could in turn cause a compensatory increase in DA reuptake kinetics and further limit diffusion of DA out of the synapse.40 Conversely, activating a greater proportion of DA neurons would increase the number of active mesoaccumbens DA terminals that “leak” small amounts of DA into the extrasynaptic space, which over time contributes to a rise in tonic DA levels (Fig. 2A). Thus it is plausible that changes in the kinetics of the DA reuptake may set the absolute amount of DA that leaks into the extrasynaptic space to a relatively fixed concentration, regardless of whether a DA neuron is firing in a slow, irregular manner or in a burst mode.
Fig. 2: Regulation of extrasynaptic (tonic) dopamine (DA) levels. (A) Diagram of potential mechanisms that control extrasynaptic DA levels. Under “basal conditions,” some DA neurons fire in a slow, irregular mode, whereas others are inactive. The dopamine transporter (DAT) curtails the amount of DA that can escape into the extrasynaptic space. Increased burst firing in a subpopulation of DA neurons induces a massive release of DA in the synapse, but also stimulates D2 autoreceptors, which may increase the reuptake kinetics of the DAT. Thus, even though more DA is being released in the synapse, the amount that escapes into the extrasynaptic space may remain relatively constant. However, an increase in the number of DA neurons that are spontaneously active (population activity) leads to more DA terminals that can “leak” DA into the extrasynaptic space, which over time leads to a gradual increase in tonic DA levels. (B) Brief activation of hippocampal inputs to the nucleus accumbens (NAc) (ventral subiculum [vSub] stim, arrows) enhances tonic DA levels in the NAc, measured with in vivo microdialysis. However, this effect is completely abolished by local perfusion of the N-methyl-D-aspartate (NMDA) antagonist 2-amino-5-phosphonovalerate (APV) in the NAc (white bar). Adapted from Taepavarapruk and colleagues.41
Tonic DA concentrations are also under the direct control of limbic glutamatergic afferents to the NAc. In a series of studies, we demonstrated that relatively brief, higher-frequency activation (20 Hz for 10 s) of either vSub or BLA inputs to the NAc induces a prolonged (30-min) increase in extrasynaptic DA levels.41–46 These stimulation patterns resemble the firing patterns of neurons in either the vSub or BLA observed when animals are presented with motivationally relevant stimuli.47–49 These effects have been observed in both anesthetized and awake rats measured with either in vivo voltammetry42,43,46 or microdialysis.41,44,45 Moreover, facilitation of DA release by these inputs appears to be mediated by local glutamatergic mechanisms within the ventral striatum and is independent of activity of DA neurons in the VTA. DA release evoked by brief activation of the vSub or BLA is abolished by blockade of either N-methyl-D-aspartate (NMDA) or α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) glutamate receptors within the NAc41–43,45 (Fig. 2B). This suggests that glutamate released by brief, higher-frequency activation of vSub or BLA inputs may act presynaptically on DA terminals in the NAc to promote the release of DA. This contention is supported by the findings that, first, inputs from both the hippocampus and the BLA form synapses that are in close apposition to DA inputs to the ventral striatum24,25 and, second, that NMDA receptors have been localized on the intravaricose segments of DA axons as well as on postsynaptic medium spiny neurons in the NAc.50 In addition, pharmacologic blockade of glutamate receptors or sodium ion (Na+) channels in the VTA does not affect DA release evoked in this manner. Thus brief bursts of higher-frequency activity in glutamatergic limbic inputs to the NAc, as may occur when an organism is engaged in cognitive or reward-related activity,47–49 may enhance glutamatergic transmission in the NAc. This, in turn, may facilitate mesoaccumbens DA efflux via local, glutamatergic-dependent mechanisms but is not dependent on the activity of DA cell bodies in the VTA. It should be noted, however, that chemical stimulation of the vSub can also increase DA neuron population activity that may contribute to the facilitation of mesoaccumbens DA efflux.39,51,52 This latter finding suggests that prolonged activation of the vSub-NAc pathway may promote increases in extrasynaptic levels of DA via mechanisms that are distinct from those induced by brief electrical activation of hippocampal or BLA inputs to the NAc.
Modulation of limbic-driven activity of NAc neurons by DA
The above-mentioned findings indicate that activation of hippocampal or amygdalar neurons that project to the NAc serves to augment tonic DA levels in addition to stimulating postsynaptic medium spiny neurons. One question that arose from these findings was, What are the neurophysiological actions of DA released in this manner on the activity of NAc neurons driven by these same glutamatergic inputs? The physiological actions of mesoaccumbens DA on NAc neurons are complex: under different conditions, it can either enhance or suppress neural activity. These effects of DA may occur via postsynaptic actions on medium spiny neurons or presynaptically, on glutamate terminals. For example, application of DA or its agonists hyperpolarizes medium spiny neurons in the NAc in vitro.53,54 Similarly, endogenous or exogenous DA suppresses spontaneous or glutamate-evoked firing activity of NAc neurons.26,55 These inhibitory actions of DA appear to be comediated by both D1-type and D2-type receptors in the NAc and are manifested primarily through actions on Na+ and potassium ion (K+) channels.55–57 However, other studies have shown that iontophoretic administration of low doses of DA can enhance glutamate-evoked firing activity of striatal neurons.55,58 These effects may be attributable to D1 receptor-mediated augmentation of L-type calcium ion (Ca2+) and glutamate-mediated NMDA currents.30–32,59 Thus DA can exert different postsynaptic effects on NAc neurons that depend on several variables, including the membrane potential of the neuron, the concentration of DA and the specific DA receptor subtypes that are stimulated.
DA also exerts differential effects on synaptically evoked activity in the NAc. Initial studies revealed that DA attenuates evoked excitatory responses in NAc neurons driven by inputs from the hippocampus or the BLA via an apparent presynaptic action on DA heteroreceptors localized on glutamatergic nerve terminals.27,28,60 However, this effect of DA depends on both the frequency at which glutamatergic inputs are activated and the timing of DA release relative to the activation of glutamatergic inputs. When these inputs are activated at a higher frequency (e.g., > 5 Hz) or when DA is applied coincidentally with activation of glutamate inputs, DA no longer suppresses evoked activity and, in some instances, can potentiate evoked excitatory responses of striatal neurons.28,29,61,62 These findings indicate that DA may play a particularly important role in time-domain filtering of glutamate inputs to the ventral striatum, augmenting a particular subset of inputs that are active coincidental with DA release while having no such effect on inputs that are inactive when DA is released.
In light of the above-mentioned findings, we conducted a series of experiments to assess whether mesoaccumbens DA release induced by activation of glutamatergic inputs could differentially modulate firing of NAc neurons driven by inputs from the hippocampus or the BLA. In these studies, we concurrently monitored changes in both NAc DA efflux firing of NAc neurons induced by stimulation of excitatory afferent input.17,63 In our initial studies, we observed that brief tetanic stimulation of either hippocampal or BLA inputs to the NAc caused a time-locked increase in tonic DA levels, measured with in vivo voltametry (Fig. 3A). Further, in these same preparations, tetanic stimulation of either input also produced a robust short-term potentiation in firing evoked by excitatory afferent stimulation. This effect was not specific to one type of limbic input, as they were observed in separate populations of NAc neurons that only responded to stimulation of the hippocampus17 (Fig. 3B) or the BLA.63

Fig. 3: Combined neurochemical and electrophysiological studies investigating interactions between limbic and dopamine (DA) inputs to the nucleus accumbens (NAc). (A) Tetanic activation of hippocampal inputs causes a brief, and then more prolonged, increase in mesoaccumbens DA efflux, measured with in vivo voltametry. (B) In these same preparations, firing of NAc neurons evoked by hippocampal stimulation was potentiated (black squares). Augmentation of hippocampal-evoked activity was dependent on both DA D1 and N-methyl-D-aspartate (NMDA) receptors; administration of antagonists for either D1 (black circles) or NMDA (grey squares) receptors abolished this effect. (C) In a separate population of NAc neurons, brief tetanic activation of hippocampal inputs caused a potentiation of hippocampal-evoked firing (black bars) while, at the same time, inducing a suppression of firing evoked by basolateral amygdala (BLA) inputs converging on the same neuron (grey bars). Both of these opposing effects were blocked by antagonists for D1 (R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepine-7-ol [SCH 23390]), but not D2 (sulpiride), receptors. Simultaneous activation of both the hippocampus and BLA potentiated firing evoked by both inputs, suggesting that DA exerts an activity-dependent modulation over different limbic inputs to the NAc. Adapted from Floresco and colleagues.17
This facilitation of hippocampal-or BLA-evoked firing is comediated by both DA D1 and NMDA receptors; pharmacologic blockade of either of these receptors before tetanic activation of the hippocampus or BLA abolished this effect (Fig. 3B). In contrast, D2 receptor antagonism does not affect this phenomenon. In addition, we observed that D1 and NMDA receptors each played distinct roles in mediating the potentiation of hippocampal-or BLA-evoked firing in NAc neurons. Blockade of D1 receptors after the initial increase in DA had no effect on the potentiation of evoked firing, whereas administration of an NMDA receptor antagonist at this same time point was still effective in blocking this potentiation. These results indicate that brief activation of D1 receptors is sufficient to induce long-lasting increases in the responsivity of NAc neurons to different excitatory afferents from the hippocampus or BLA. Moreover, the potentiation of these limbic-striatal pathways is likely mediated by a D1 receptor facilitation of NMDA currents in medium spiny neurons. When viewed collectively, these findings imply that the intrinsic connectivity of limbic and dopaminergic inputs to the ventral striatum seems designed to promote increases in DA release concomitant with increased activation of the hippocampus or BLA, as may occur in response to salient environmental stimuli, such as novelty or reward, that activate these regions. These mechanisms working in concert play a biasing role whereby increases in mesoaccumbens DA release facilitated by these limbic inputs would ensure activation of D1 receptors coincidental with activation of postsynaptic NAc neurons. This simultaneous activation in turn augments the responsivity of NAc neurons to subsequent inputs from a particular limbic region.
As noted previously, a subpopulation of NAc neurons receives converging input from both the hippocampus and the BLA, and each of these regions can mediate distinct patterns of behaviour via interactions with the ventral striatum. The above-mentioned findings indicate that DA release facilitated by activation of inputs from the hippocampus or BLA serves to augment the influence that a particular input exerts over the firing of NAc neurons. However, under other circumstances, DA can also inhibit firing evoked by either hippocampal or BLA afferents.27,28,60 Given these differential actions of DA, we were particularly interested in assessing whether hippocampal-evoked increases in mesoaccumbens DA efflux could differentially gate firing of NAc neurons driven by hippocampal and BLA inputs converging on the same cell. To address this, we recorded from a separate population of NAc neurons that responded to stimulation of both the hippocampus and BLA. In these cells, we again observed that brief, higher-frequency activation of the hippocampal-NAc pathway potentiated hippocampal-evoked firing of NAc neurons and that this was associated with a transient increase in tonic DA levels. However, in these same neurons, we observed that firing evoked by the BLA was substantially suppressed (Fig. 3C). Moreover, both the potentiation of hippocampal-evoked firing and the suppression of BLA-evoked activity were dependent on stimulation of D1 receptors. Blockade of these receptors with R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepine-7-ol (SCH 23390) abolished both phenomena, whereas D2 receptor blockade was without effect (Fig. 3C). It is important to note that converging inputs from the hippocampus and BLA tend to form synapses onto different regions of individual medium spiny neurons.18 Thus it is unlikely that these differential effects on hippocampal and BLA-evoked firing are mediated by direct synaptic interactions between these 2 glutamatergic inputs. Rather, increases in extrasynaptic DA concentrations facilitated by hippocampal inputs may diffuse to other regions of the same NAc neurons where BLA–NAc synapses are situated. How DA exerts these opposing actions over these different inputs to the same cell is unclear. However, it is reasonable to propose that activation of postsynaptic D1 receptors may facilitate activity evoked by hippocampal inputs, whereas DA may act on presynaptic DA heteroreceptors on glutamatergic terminals to suppress firing evoked by the BLA (Fig. 4).
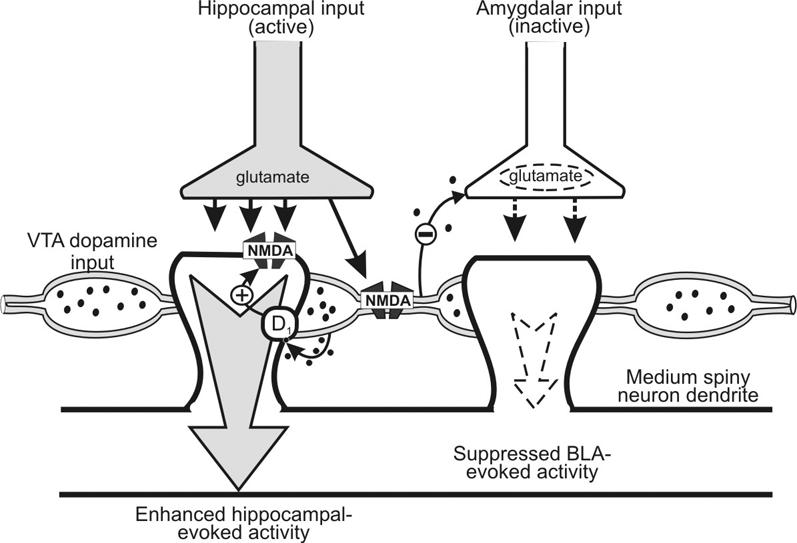
Fig. 4: Diagram of hippocampal, basolateral amygdala (BLA) and dopamine (DA) inputs synapsing on separate dendrites of an individual medium spiny neuron in the nucleus accumbens (NAc), illustrating the processes that may mediate the differential effects of DA on NAc neuron firing. On the left spine, glutamatergic inputs from the hippocampus can activate both the postsynaptic NAc dendrite and facilitate the release of DA by activating N-methyl-D-aspartate (NMDA) receptors localized on the intravaricose segments of DA axons. Activation of postsynaptic D1 receptors can enhance postsynaptic NMDA receptor function, leading to an enhancement of hippocampal-evoked activity. DA release facilitated by hippocampal afferents may also diffuse to inhibit BLA–NAc synapses on other parts of the neuron (right spine). The size of the arrows represents the relative strength of the response evoked by each input after DA modulation of neural activity. Adapted from Floresco and colleagues.17
One issue that deserved further consideration was why DA should have these opposing effects on hippocampal and BLA inputs to the NAc. One explanation for the differential modulation by DA over hippocampal-and BLA-evoked activity is that, in these experiments, there was a selective increase in the activity in the hippocampal-NAc pathway when DA was released while, at the same time, inputs from the BLA were quiescent. It follows therefore that the inhibitory effects of DA on BLA-evoked spiking activity should be reversed if BLA inputs are active at the time of DA release. Indeed, coincidental tetanic stimulation of both the fimbria and the BLA resulted in potentiation of both hippocampal-and BLA-evoked spiking activity in the same neuron (Fig. 3C).
These data indicate that DA transmission in the NAc plays a critical role in an input selection mechanism that permits certain inputs to have preferential but temporary influence over neural activity in the NAc. Higher-frequency activity in glutamatergic limbic afferents to the NAc facilitates the release of mesoaccumbens DA that is time-locked to the depolarization of NAc neurons, which serves to facilitate subsequent evoked neural activity. Further, a cooperative interaction between D1 and NMDA receptors plays a key role in this potentiation. In addition, DA release facilitated by high-frequency hippocampal inputs also exerts an activity-dependent inhibition of BLA inputs converging on the same NAc neuron. Therefore, the modulation of mesoaccumbens DA release by hippocampal afferents provides an intrinsic, activity-dependent, coincidence-detection mechanism. These data highlight the importance of the timing of DA release coincidental to the arrival of higher-frequency glutamatergic inputs to NAc neurons. DA can potentiate active glutamatergic inputs while inhibiting those inputs that are inactive when D1 receptor stimulation occurs. These findings are in keeping with contemporary theory regarding the function of DA transmission in the basal ganglia, which postulates that D1 receptor activity serves to strengthen the most salient inputs while at the same time inhibiting weaker inputs.33,37,64,65 The DA-mediated amplification and inhibition of separate excitatory inputs synapsing on the same NAc neurons would facilitate the ability of this nucleus to regulate response selection or behavioural switching.11,33,34,66,67 Indeed, the following has been proposed:
DA activity promotes the likelihood of switching between alternative sources of information ... [and] may increase the probability of a new input to a given brain region influencing the output and/or reducing the input being shut off from influencing the output.33
Accordingly, activity-dependent modulation by DA of glutamatergic limbic afferents to the ventral striatum may represent a cellular mechanism for input selection that governs the smooth coordination of behaviour by permitting information processed by one limbic region to have preferred access to the motor systems via the NAc and thereby temporarily exerting control over the type and intensity of adaptive behavioural responses.
Dopaminergic regulation of behaviour mediated by hippocampal-NAc circuits
These neurophysiological data allow for certain predictions to be made about the role of DA in mediating behaviours governed by limbic-striatal circuitries. Considering that D1 receptors interacting with NMDA glutamate receptors serve to augment the influence that certain inputs exert over NAc neuronal activity, it follows that blockade of either of these receptors should disrupt behaviours mediated by these circuits. Further, in light of the fact that DA also serves to gate the access that certain inputs have to the motor system via the NAc, it would be expected that D1 receptor activity would be particularly important in ambiguous situations where there is uncertainty about the motivational significance of environmental stimuli. These types of situations may occur during the initial stages of associative learning or when the particular stimuli that are associated with reward change over time. In keeping with these predictions, psychopharmacological studies have shown that blockade of either D1 or NMDA receptors in the NAc impairs the acquisition of instrumental or Pavlovian associations,68,69 the detection of changes in the spatial arrangement of objects in an environment,70 response allocation71 and shifting between behavioural strategies.72,73
To explore these issues further, we conducted a series of experiments to investigate the importance of DA in mediated behaviour dependent on hippocampal–ventral striatal circuitry.74 In these studies, we used a variant of the radial arm maze task, which assesses exploratory foraging abilities. In this “random foraging” task, 4 out of 8 arms on a radial maze are randomly baited each day so that the animal cannot predict the location of food from trial to trial. Rodents quickly adapt an optimal foraging strategy, entering novel arms not visited previously during the daily trial in a nonsequential manner and avoiding arms that were previously entered, until they have retrieved the 4 pieces of food. We have shown previously that this type of exploratory foraging is critically dependent on a neural circuit linking the vSub to the NAc. Asymmetric disconnection between these 2 regions severely disrupts the use of an efficient search strategy and increases the number of arm re-entry errors.75 In our studies that investigated the role of DA in this behaviour, animals were well trained to the point that they made no more than 1 re-entry error per daily trial for 4 consecutive days before any pharmacologic manipulations were administered.
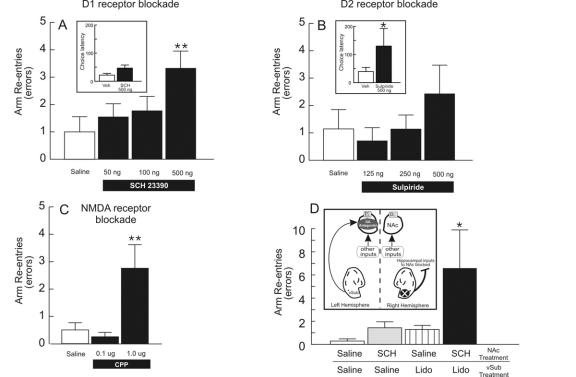
According to our electrophysiological data, blockade of DA D1 receptors in the NAc would be expected to impair the accuracy of this type of exploratory locomotion mediated by hippocampal-NAc circuits, whereas D2 receptor blockade should not affect search behaviour but might alter other aspects of performance. Consistent with these hypotheses, we observed that infusions of a D1 receptor antagonist into the NAc increased the number of errors committed by rats performing this task (Fig. 5A). In contrast, similar infusions of a D2 receptor blocker did not affect search behaviour (Fig. 5B). However, D2 receptor blockade was not without effect because these manipulations did increase the latencies to enter arms to a substantially greater degree than D1 receptor antagonism (Fig. 5A, B insets). In keeping with our neurochemical and electrophysiological data implicating interactions between DA and NMDA receptors in the NAc, local infusions of the NMDA antagonist 3-(2-carboxypiperazine-4-yl)propyl-1-phosphate (CPP) also disrupted search behaviour in a manner similar to that observed following D1 receptor blockade (unpublished observations, Fig. 5C). From these data, it is apparent that both D1 and NMDA receptor activity in the NAc mediates accuracy of approach behaviour in situations where an organism must monitor multiple stimuli that may or may not be associated with reward. On the other hand, D2 receptors may play a more prominent role in motivational aspects of task performance. Indeed, more recent findings have shown a similar dissociation in the role of D1 and D2 receptors in the NAc in mediating attentional performance.76
Fig. 5: D1 and N-methyl-D-aspartate (NMDA) receptor activity in the nucleus accumbens (NAc) mediate exploratory foraging behaviour dependent on hippocampal–ventral striatal circuitry. Panels A, B and D adapted from Floresco and Phillips.74 (A) Intra-NAc infusion of the D1 antagonist R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepine-7-ol (SCH 23390) disrupts the efficient search for food during a “random foraging” task on a radial arm maze but does not exert as pronounced an effect on choice latency (inset). (B) Blockade of D2 receptors in the NAc with sulpiride does not affect the accuracy of search behaviour but induces a dramatic increase in choice latency (inset). (C) NMDA receptor blockade in the NAc with 3-(2-carboxypiperazine-4-yl)propyl-1-phosphate (CPP) also impaired search behaviour in a manner similar to D1 receptor antagonism. (D) D1 receptors serve to selectivity modulate hippocampal inputs to the NAc during exploratory foraging. Neither unilateral inactivation of the ventral subiculum (vSub) by lidocaine (Lido, striped bar) infusions nor unilateral D1 receptor blockade in the NAc (SCH, grey bar) disrupted search behaviour. However, simultaneous administration of both of these asymmetric manipulations caused a substantial disruption in efficient search, wherein rats were more likely to re-enter arms visited previously during the trial. The inset diagrams the asymmetric infusion procedure used in this experiment, consisting of a unilateral infusion of a D1 receptor antagonist into the NAc and a contralateral infusion of lidocaine into the vSub (X). Following this procedure, no inputs would be modulated by D1 receptor activity in the left NAc. Conversely, in the right NAc all inputs except hippocampal afferents would be modulated by dopamine (DA). Thus this procedure would prevent DA modulation of hippocampal inputs to the NAc in both hemispheres.
These behavioural observations, in combination with our electrophysiological data, suggest that the impairments in exploratory foraging induced by D1 receptor blockade in the NAc are possibly attributable to a disruption in the ability of hippocampal inputs to augment their influence over NAc neuronal activity and thereby exert a controlling influence over ongoing behaviour. In a separate experiment, we tested this hypothesis directly with the use of an asymmetric infusion protocol (Fig. 5D, inset). This manipulation combined a unilateral inactivation of the vSub in one hemisphere with a contralateral infusion of a D1 antagonist in the NAc. The rationale for this procedure makes 2 assumtions: the first is that a unilateral infusion of the D1 antagonist would ensure that none of the afferents to the NAc in that hemisphere (including those from the vSub) would be modulated by DA; the second is that, in the contralateral hemisphere in which the vSub is inactivated, all afferents except those from the hippocampus would be modulated by DA. Thus this asymmetric infusion procedure would ensure a selective disruption of dopaminergic modulation of hippocampal inputs to the NAc in both hemispheres. Accordingly, this manipulation also disrupted search behaviour on this task in a manner similar to bilateral blockade of D1 receptors in the NAc (Fig. 4D). These findings confirmed that one function of mesoaccumbens DA transmission is to selectively facilitate activity in hippocampal-NAc circuits that mediate exploratory locomotion.
The use of this exploratory “random foraging” task proved useful in delineating the role of mesoaccumbens DA transmission in a behaviour dependent on intact serial transmission between hippocampal–ventral striatal circuits. However, these behavioural protocols do not reflect accurately the conditions that an organism may encounter in more natural environments, where response priorities can change on a moment-to-moment basis in response to different biologically relevant stimuli. Indeed, the normal behavioural repertoire of all mammals allows for the monitoring of numerous stimuli that may have biological relevance, which would necessitate the reallocation of response priorities from one pattern of behaviour to another at a moment's notice. Nevertheless, when viewed collectively, the data reviewed here provide insight into the underlying neural mechanisms by which DA may facilitate behavioural switching.
The expression of a particular pattern of behaviour, such as exploration of a novel environment, may be mediated by discrete patterns of activity in an ensemble of NAc neurons that are driven by similar patterns of activity in the hippocampal formation.49 Bursts of activity in this circuit may occur in response to orienting to a particular stimulus in the environment, which results in the initiation of an approach response or in continued exploration of the environment. Enhancements in tonic levels of mesoaccumbens DA, either by glutamate-dependent mechanisms localized within the NAc or by activation of DA cell bodies in the ventral tegmental area, would facilitate exploration by ensuring that hippocampal inputs activate preferentially a group of neurons that mediate approach to novel stimuli while simultaneously inhibiting firing of separate neuronal ensembles mediating other types of behaviour. However, the presence of a different class of stimuli (e.g., a threat stimulus, such as a predator) can rapidly change response priorities, leading to the expression of qualitatively different patterns of behaviour. Here, exploratory activities are discontinued, and instead, the organism begins to assess possible routes of escape. Once the animal orients toward a set of cues associated with safety, it may engage in an appropriate approach response toward that goal.
The underlying neural activity that mediates switching from exploratory to escape behaviour is likely distributed over several different cortical and limbic regions that may include the BLA.47,77 Intrinsic processing mechanisms in the BLA interpret sensory information regarding the aversive stimulus and activate neural circuits that mediate an appropriate withdrawal response. Higher-frequency inputs from the BLA that terminate in the NAc could promote the release of mesoaccumbens DA,43 thereby facilitating the reorganization of ensemble activity and augmenting BLA-evoked activity while potentially inhibiting inputs from the hippocampus, via D1 and D2 receptor-dependent mechanisms.11,26,27,61 The overall effect would be that the inputs regulating ensemble activity in the NAc would be switched from the hippocampus to the BLA. As such, behaviour would no longer be directed toward novel stimuli but, instead, would be directed toward safety. Once the animal orients toward a particular cue that predicts safety, synchronous firing of BLA neurons could activate a particular ensemble of NAc neurons responsible for the initiation of an appropriate avoidance response. Note that both the potentiating and suppressive effects of DA on limbic-evoked neural activity are relatively short-lived; after a period of avoidance behaviour and once the threat is no longer apparent, activity in the hippocampus may initiate a similar DA-mediated cascade of cellular events. In this case, ensemble activity of NAc neurons could again be reorganized, switching from BLA to hippocampal-driven patterns of firing, which would change patterns of behaviour from avoidance to exploration.
Transformation of NAc neural activity into behaviour
The question remains as to how distinct patterns of ensemble activity of NAc neurons, evoked by separate glutamatergic inputs and modulated by DA, are transformed into adaptive patterns of motor output. The NAc sends a dense GABAergic projection to the ventral pallidum, although neuropeptides such as enkephalin and substance P have also been colocalized in this projection.2,3,78–80 The density of this striatal-pallidal projection implies that this pathway may contribute to subsequent stages of limbic-motor integration, whereby signals from the NAc driven by excitatory inputs may be directed to motor effector sites in the hindbrain. This notion is supported by the findings that behaviours mediated by interactions between the hippocampus, BLA or prefrontal cortex and the NAc are disrupted following lesions to the ventral pallidum.75,81–83
In contrast to the medium spiny neurons of the NAc, neurons in the ventral pallidum display relatively high rates of spontaneous activity. As such, patterns of excitation in a particular ensemble of GABAergic NAc projection neurons, driven by excitatory inputs, would be expected to induce topographically organized patterns of inhibition in a similar group of pallidal neurons.84,85 Ventral pallidal outputs are also GABAergic and project to several subcortical regions, including the medial-dorsal thalamus, the subthalamic nucleus, the VTA and, in particular, motor effector sites in the mesencephalon that in turn send direct projections to the spinal cord.2,3,85–87 Thus, in addition to contributing to thalamo-cortical loops88 and regulating DA neuron population activity,38 separate projections from the ventral pallidum to these mesencephalic motor regions (such as the pedunculopontine nucleus) may be a direct pathway by which neural activity in the NAc can be translated into overt behaviours.
The high rates of spontaneous activity of pallidal neurons maintain a tonic inhibitory influence over the neurons in these mesencephalic motor regions. Accordingly, brief periods of inhibition of pallidal output neurons would exert a disinhibitory increase in firing of these motor sites, resulting in ambulatory locomotion.2,84,87,89,90 Thus it is reasonable to propose that that patterns of motor activity associated with firing of NAc neurons are manifested by patterns of inhibition and disinhibition in trans-synaptic pathways linking the ventral pallidum and motor effector sites in the mesencephalon. Specifically, increased firing of NAc neurons that is driven by corticolimbic glutamatergic afferents and time-locked to points when animals orient toward environmental stimuli would be expected to decrease firing of pallidal neurons. This, in turn, would be expected to result in a disinhibitory increase in firing of neurons in certain mesencephalic subnuclei. These brief periods of increased neural activity could initiate forward locomotion through connections with motor pattern generators in the brainstem. Thus activation of these polysynaptic subcortical pathways, at points that are time-locked to periods when an animal must choose to approach a particular environmental cue, may activate motor effector sites that can bias the animal to approach or withdraw from a particular stimulus.
Note that disruptions of neural activity in either the NAc, the ventral pallidum or certain mesencephalic subnuclei do not abolish all forms of motor activity. Instead, these subcortical sites play a more selective role in goal-directed locomotion, where animals must discriminate between, and approach, particular stimuli that may have motivational relevance.82,83,90–92 It is unlikely that the relay of signals from the NAc to the ventral pallidum and mesencephalic locomotor region represent simple motor commands that regulate all types of locomotion. It is more likely that these circuits feed into motor systems, biasing the direction of behavioural output. A sequence of complex goal-directed behaviours would be mediated by multiple periods of topographically organized patterns of activity and inactivity in these subcortical circuits, which would be expressed as a distinct sequence of motor responses.
With respect to ventral striatal function, it is apparent that manipulations of the NAc do not disrupt all types of behavioural output directed toward motivationally relevant stimuli. In situations where stimulus-response-outcome associations are highly predictable (i.e., the particular motivationally relevant stimuli in an environment remain stable over time), approach toward these stimuli is mediated through cortico-striatal circuits incorporating the dorsal striatum.93 In contrast, the ventral striatum plays a more prominent role in guiding behaviour when there is uncertainty about which stimuli may be associated with reward or when there are changes in the incentive value of these stimuli.64 For example, dopaminergic or glutamatergic manipulations of the NAc impair the initial learning of instrumental or Pavlovian approach responses (when the motivational relevance about environmental stimuli is unclear) but do not alter behaviour once these responses have been acquired.68,69 Similarly, as we have shown, these manipulations also impair foraging behaviour in well-trained animals because the specific spatial locations that may contain food change from trial to trial.74 It is these ambiguous situations that give rise to competing responses regarding which stimuli to attend to and approach, and this is when the NAc may have the greatest influence over behavioural output. Here, distinct patterns of activity in separate NAc neuronal ensembles, driven by anatomically and functionally distinct glutamatergic afferents, can bias the direction of behaviour toward particular stimuli, via descending projections that activate the motor system. In this context, DA transmission acts as the mediator of these competing inputs, amplifying the ability of a particular cognitive system to drive behaviour in a particular direction while at the same time attenuating the influence that other systems exert over ongoing behaviour.
Summary and conclusions
It is well recognized that the NAc serves as an interface between different corticolimbic information-processing systems and, as such, plays a critical role in processes that determine response priorities.2 The findings reviewed here add important insight into the heterogeneous nature of mesoaccumbens DA transmission and the multiple actions that extrasynaptic DA activity exerts over both the neurophysiological interactions that occur in limbic-striatial circuits and behaviours mediated by these pathways.
On the basis of the evidence reviewed here, it would appear unlikely that neural activity within the NAc is involved directly in establishing goals and priorities for the organism; these functions appear to be performed by systems within the temporal and frontal cortices. In contrast, the NAc serves simply as a sophisticated relay system, the output of which is dictated by a biasing action of DA over different corticolimbic afferents. Appropriate response strategies for specific environmental contingencies may be determined by several different processes, including pre-existing stimulus–reward associations, such as those formed in the amygdala81; spatial and contextual information derived from the hippocampus75; or the flexible processing of different behavioural strategies mediated by the prefrontal cortex.94 The capacity of these different networks to bias the internal functioning of the NAc may determine which alternative response strategy will gain direct access to the motor system via efferents from the NAc. In this sense, one may view the function of the NAc as to facilitate the transformation of cognitive processes into meaningful patterns of behaviour without being involved in cognition per se.
Acknowledgments
I thank my collaborators who made essential contributions to the work presented here (C.D. Blaha, A.A. Grace, P. Taepavarapruk, A.R. West and C.R. Yang).
Footnotes
2006 CCNP Young Investigator Award
Dr. Floresco is a Michael Smith Foundation for Health Research Scholar, a Canadian Institutes of Health Research New Investigator and the recipient of a Young Investigator award from the National Alliance for Research on Schizophrenia and Depression.
Competing interests: None declared.
Correspondence to: Dr. Stan B. Floresco, Department of Psychology and Brain Research Centre, University of British Columbia, 2136 West Mall, Vancouver BC V6T 1Z4; floresco@psych.ubc.ca
References
- 1.Groenewegen HJ, Berendse HW, Meredith GE, et al. Functional anatomy of the ventral, limbic system innervated striatum. In: Willner P, Scheel-Krüger J, editors. The mesolimbic dopamine system: from motivation to action. New York: John Wiley and Sons; 1991. p. 19-59.
- 2.Mogenson GJ, Brudzynski SM, Wu M, et al. From motivation to action: a review of dopaminergic regulation of limbic→nucleus accumbens→ventral pallidum→pedunculopontine nucleus circuitries involved with limbic-motor integration. In: Kalivas PW, Barnes CD, editors. Limbic motor circuits and neuropsychiatry. Boca Raton (FL): CRC Press; 1993. p. 193-263.
- 3.Zahm DS, Heimer L. Two transpallidal pathways originating in the nucleus accumbens. J Comp Neurol 1990;302:437-46. [DOI] [PubMed]
- 4.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 1980;14:69-97. [DOI] [PubMed]
- 5.Zaborszky L, Alheid GF, Beinfeld MC, et al. Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience 1985;14:427-53. [DOI] [PubMed]
- 6.Zahm DS, Heimer L. Ventral striatopallidal parts of the basal ganglia in the rat: I. Neurochemical compartmentationas reflected by the distributions of neurotensin and substance P immunoreactivity. J Comp Neurol 1988;272:516-35. [DOI] [PubMed]
- 7.Groenewegen HJ, Mulder AB, Beijer AVJ, et al. Hippocampal and amygdaloid interactions in the nucleus accumbens. Psychobiology 1999;27:149-64.
- 8.Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, et al. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgarus leucoagglutinin. Neuroscience 1987;23:103-20. [DOI] [PubMed]
- 9.Brog JS, Salyapongse A, Deutch A, et al. The pattern of afferent innervation of the core and shell in the “accumbens” part of the ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 1993;338:255-78. [DOI] [PubMed]
- 10.Shinonaga Y, Takada M, Mizuno N. Topographical organization of collateral projections from the basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neuroscience 1994;58:389-97. [DOI] [PubMed]
- 11.Pennartz CMA, Groenewegen HJ, Lopes Da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol 1994;42:719-61. [DOI] [PubMed]
- 12.O'Donnell P, Greene J, Pabello N, et al. Modulation of cell firing in the nucleus accumbens. Ann N Y Acad Sci 1999;877:157-75. [DOI] [PubMed]
- 13.O'Donnell P. Ensemble coding in the nucleus accumbens. Psychobiology 1999;27:187-97.
- 14.Callaway CW, Hakan RL, Henriksen SJ. Distribution of amygdala input to the nucleus accumbens septi: an electrophysiological investigation. J Neural Transm Gen Sect 1991;83:215-25. [DOI] [PubMed]
- 15.O'Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 1995;15:3622-39. [DOI] [PMC free article] [PubMed]
- 16.Mulder AB, Hodenpijl MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci 1998;18:5095-102. [DOI] [PMC free article] [PubMed]
- 17.Floresco SB, Blaha CD, Yang CR, et al. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci 2001;21:2851-60. [DOI] [PMC free article] [PubMed]
- 18.French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 2003;119:19-31. [DOI] [PubMed]
- 19.Sherry DF, Schacter DL. The evolution of multiple memory systems. Psychol Rev 1987;94:439-54.
- 20.Burns LH, Annett L, Kelley AE, et al. Effects of lesions to amygdala, ventral subiculum, medial prefrontal cortex and nucleus accumbens on the reaction to novelty: implications for limbic-striatal interactions. Behav Neurosci 1996;110:60-73. [DOI] [PubMed]
- 21.White NM, McDonald RJ. Acquisition of a spatial conditioned place preference is impaired by amygdala lesions and improved by fornix lesions. Behav Brain Res 1993;55:269-81. [DOI] [PubMed]
- 22.Nauta WJH, Smith GP, Faul RLM, et al. Efferent connections and nigral afferents of the nucleus accumbens speti in the rat. Neuroscience 1978;3:385-401. [DOI] [PubMed]
- 23.Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res 1990;527:266-79. [DOI] [PubMed]
- 24.Totterdell S, Smith AD. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat 1989;2:285-98. [PubMed]
- 25.Johnson LR, Aylward RLM, Hussain Z, et al. Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience 1994;61:851-65. [DOI] [PubMed]
- 26.Yang CR, Mogenson GJ. Electrophysiological responses of neurones in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Res 1984;324:69-84. [DOI] [PubMed]
- 27.Yang CR, Mogenson GJ. Dopamine enhances terminal excitability of hippocampal-accumbens neurons via D2 receptor: role of dopamine in presynaptic inhibition. J Neurosci 1986;6:2470-8. [DOI] [PMC free article] [PubMed]
- 28.Pennartz CMA, Dolleman-vender Weel MJ, Kitai ST, et al. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol 1992;67:1325-33. [DOI] [PubMed]
- 29.Gonon F, Sundstrom L. Excitatory effects of dopamine released by impulse flow in the rat nucleus accumbens in vivo. Neuroscience 1996; 75:13-8. [DOI] [PubMed]
- 30.Harvey J, Lacey MG. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci 1997;17:5271-80. [DOI] [PMC free article] [PubMed]
- 31.Hernández-López S, Bargas J, Surmeier DJ, et al. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J Neurosci 1997;17:3334-42. [DOI] [PMC free article] [PubMed]
- 32.Cepeda C, Levine MS. Dopamine and N-Methyl-D-Aspartate receptor interactions in the neostriatum. Dev Neurosci 1998;20:1-18. [DOI] [PubMed]
- 33.Oades RD. The role of noradrenaline in tuning and dopamine in switching between signals in the CNS. Neurosci Biobehav Rev 1985;9:261-82. [DOI] [PubMed]
- 34.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward seeking. Brain Res Brain Res Rev 1999;31:6-41. [DOI] [PubMed]
- 35.Redgrave P, Prescott TJ, Gurney K. Is the short-latency dopamine response too short to signal reward error? Trends Neurosci 1999;22:146-51. [DOI] [PubMed]
- 36.Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci 2000;23:473-500. [DOI] [PubMed]
- 37.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 1998;80:1-27. [DOI] [PubMed]
- 38.Floresco SB, West AR, Ash B, et al. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 2003;6:968-73. [DOI] [PubMed]
- 39.Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci 2001;21:4915-22. [DOI] [PMC free article] [PubMed]
- 40.Meiergerd SM, Patterson TA, Schenk JO. D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J Neurochem 1993;61:764-7. [DOI] [PubMed]
- 41.Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology (Berl) 2000;151:242-51. [DOI] [PubMed]
- 42.Blaha CD, Yang CR, Floresco SB, et al. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci 1997;9:902-11. [DOI] [PubMed]
- 43.Floresco SB, Yang CR, Phillps AG, et al. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anesthetized rat. Eur J Neurosci 1998;10:1241-51. [DOI] [PubMed]
- 44.Howland JG, MacKenzie EM, Yim TT, et al. Electrical stimulation of the hippocampus disrupts prepulse inhibition in rats: frequency-and site-dependent effects. Behav Brain Res 2004;152:187-97. [DOI] [PubMed]
- 45.Howland JG, Taepavarapruk P, Phillips AG. Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats. J Neurosci 2002;22:1137-45. [DOI] [PMC free article] [PubMed]
- 46.Leonetti M, Desvignes C, Bougault I, et al. 2-Chloro-N-[(S)-phenyl [(2S)-piperidin-2-yl] methyl]-3-trifluoromethyl benzamide, monohydrochloride, an inhibitor of the glycine transporter type 1, increases evoked-dopamine release in the rat nucleus accumbens in vivo via an enhanced glutamatergic neurotransmission. Neuroscience 2006;137:555-64. [DOI] [PubMed]
- 47.Ono T, Nishijo H, Uwano T. Amygdala role in conditioned associative learning. Prog Neurobiol 1995;46:401-22. [DOI] [PubMed]
- 48.Pratt WE, Mizumori SJY. Characteristics of basolateral amygdala neuronal firing on a spatial memory task involving differential reward. Behav Neurosci 1998;112:554-70. [DOI] [PubMed]
- 49.Martin PD, Ono T. Effects of reward anticipation, reward presentation, and spatial parameters on the firing of single neurons recorded in the subiculum and nucleus accumbens of freely moving rats. Behav Brain Res 2000;116:23-38. [DOI] [PubMed]
- 50.Gracy KN, Pickel VM. Ultrastructural immunocytochemical localization of the N-methyl-D-aspartate receptor and tyrosine hydroxylase in the shell of the rat nucleus accumbens. Brain Res 1996;739:169-81. [DOI] [PubMed]
- 51.Legault M, Rompre PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci 2000;20:1635-42. [DOI] [PMC free article] [PubMed]
- 52.Legault M, Wise RA. Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse 1999;31:241-9. [DOI] [PubMed]
- 53.Uchimura N, Higashi H, Hishi S. Hyperpolarizing and depolarizing actions of dopamine via D-1 and D-2 receptors on nucleus accumbens neurons. Brain Res 1986;375:368-72. [DOI] [PubMed]
- 54.O'Donnell P, Grace AA. Dopaminergic reduction of excitability in nucleus accumbens neurons recorded in vitro. Neuropsychopharmacology 1996;15:87-97. [DOI] [PubMed]
- 55.Hu XT, Wang RY. Comparison of effects of D-1 and D-2 dopamine receptor agonists on neurons in the rat caudate putamen: an electrophysiological study. J Neurosci 1988;8:4340-8. [DOI] [PMC free article] [PubMed]
- 56.Surmeier DJ, Kitai ST. D1 and D2 dopamine receptor modulation of sodium and potassium currents in rat neostriatal neurons. Prog Brain Res 1993;99:309-24. [DOI] [PubMed]
- 57.Perez MF, White FJ, Hu XT. Dopamine D(2) receptor modulation of K(+) channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. J Neurophysiol 2006;96:2217-28. [DOI] [PubMed]
- 58.Kiyatkin EA, Rebec GV. Dopaminergic modulation of glutamate induced excitations of neurons in the neostriatum and nucleus accumbens of awake, unrestrained rats. J Neurophysiol 1996;75:142-53. [DOI] [PubMed]
- 59.Chergui K, Lacey MG. Modulation by dopamine D1-like receptors of synaptic transmission and NMDA receptors in rat nucleus accumbens is attenuated by the protein kinase C inhibitor Ro 32-0432. Neuropharmacology 1999;38:223-31. [DOI] [PubMed]
- 60.Yim CY, Mogenson GJ. Response of nucleus accumbens neurons to amygdala stimulation and its modification by dopamine. Brain Res 1982;239:401-15. [DOI] [PubMed]
- 61.DeFrance JF, Sikes RW, Chronister RB. Dopamine action in the nucleus accumbens. J Neurophysiol 1985;54:1568-77. [DOI] [PubMed]
- 62.Wickens JR, Begg AJ, Arbuthnott GW. Dopamine reverses the depression of rat corticostriatal synapses which normally follows high-frequency stimulation of cortex in vitro. Neuroscience 1996;70:1-5. [DOI] [PubMed]
- 63.Floresco SB, Blaha CD, Yang CR, et al. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J Neurosci 2001;21:6370-6. [DOI] [PMC free article] [PubMed]
- 64.Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521-50. [DOI] [PubMed]
- 65.Nicola SM, Surmeier DJ, Malenka RC. Dopaminergic modulation of the neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 2000;23:185-215. [DOI] [PubMed]
- 66.van den Bos R, Charria Ortiz GA, Bergmans AC, et al. Evidence that dopamine in the nucleus accumbens is involved in the ability of rats to switch to cue-directed behaviors. Behav Brain Res 1991;42:107-14. [DOI] [PubMed]
- 67.Salamone JD, Cousins MS, Bucher S. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev 1997;21:341-59. [DOI] [PubMed]
- 68.Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci 2000;20: 7737-42. [DOI] [PMC free article] [PubMed]
- 69.Dalley JW, Laane K, Theobald DE, et al. Time-limited modulation of appetitive Pavlovian memory by D1 and NMDA receptors in the nucleus accumbens. Proc Natl Acad Sci U S A 2005;102:6189-94. [DOI] [PMC free article] [PubMed]
- 70.Ferretti V, Florian C, Costantini VJ, et al. Co-activation of glutamate and dopamine receptors within the nucleus accumbens is required for spatial memory consolidation in mice. Psychopharmacology (Berl) 2005;179:108-16. [DOI] [PubMed]
- 71.Nowend KL, Arizzi M, Carlson BB, et al. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav 2001;69:373-82. [DOI] [PubMed]
- 72.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 2005;8:805-12. [DOI] [PubMed]
- 73.Floresco SB, Ghods-Sharifi S, Vexelman C, et al. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci 2006;26:2449-57. [DOI] [PMC free article] [PubMed]
- 74.Floresco SB, Phillips AG. Dopamine and hippocampal input to the nucleus accumbens play an essential role in the search for food in an unpredictable environment. Psychobiology 1999;27:277-86.
- 75.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci 1997;17:1880-90. [DOI] [PMC free article] [PubMed]
- 76.Pezze MA, Dalley JW, Robbins TW. Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology 2007;32:273-83. [DOI] [PMC free article] [PubMed]
- 77.LeDoux JE. Emotion: clues from the brain. Annu Rev Psychol 1995;46:209-35. [DOI] [PubMed]
- 78.Walaas I, Fonnum F. The distribution and origin of glutamate decarbozylase and choline acetyltransferase in ventral pallidum and other basal forbrain regions. Brain Res 1979;177:325-36. [DOI] [PubMed]
- 79.Jones DL, Mogenson GJ. Nucleus accumbens to globus pallidus GABA projection: electrophysiological and iontophoretic investigations. Brain Res 1980;188:93-105. [DOI] [PubMed]
- 80.Zahm DS, Zaborsky L, Alones VE, et al. Evidence for the coexistance of glutamate decarboxylase and met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res 1985;325: 317-21. [DOI] [PubMed]
- 81.Everitt BJ, Morris KA, O'Brien A, et al. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience 1991;42:1-18. [DOI] [PubMed]
- 82.McAlonan GM, Robbins TW, Everitt BJ. Effects of medial dorsal thalamic and ventral pallidal lesions on the acquision of a conditioned place preference: Further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience 1993;52:605-20. [DOI] [PubMed]
- 83.Floresco SB, Braaksma DN, Phillips AG. Involvement of the ventral pallidum in working memory tasks with or without a delay. Ann N Y Acad Sci 1999;877:711-6. [DOI] [PubMed]
- 84.Yang CR, Mogenson GJ. Hippocampal signal transmission to the mesencephalic locomotor regions and its regulation by dopamine D-2 receptors in the nucleus accumbens: an electrophysiological and behavioral study. Neuroscience 1987;23:1041-55. [DOI] [PubMed]
- 85.Tsai CT, Mogenson GJ, Wu M, et al. A comparison of the effects of electrical stimulation of the amygdala and hippocampus on subpallidal output neurons to the pedunculopontine nucleus. Brain Res 1989;494:22-9. [DOI] [PubMed]
- 86.Swanson LW, Mogenson GJ, Gerfen CR, et al. Evidence for a projection from the lateral preoptic area and substantia innominata to the ‚mesencephalic locomotor region' in the rat. Brain Res 1984;295:161-78. [DOI] [PubMed]
- 87.Skinner RD, Garcia-Rill E. Mesolimbic interactions with mesopontine modulation of locomotion. In: Kalivas PW, Barnes CD, editors. Limbic motor circuits and neuropsychiatry. Boca Raton (FL): CRC Press; 1993. p. 155-91.
- 88.O'Donnell P, Lavin A, Enquist LW, et al. Interconnected parallel circuits between rat nucleus accumbens and thalamus revealed by retrograde transynaptic transport of pseudorabies virus. J Neurosci 1997;17:2143-67. [DOI] [PMC free article] [PubMed]
- 89.Milner KL, Mogenson GJ. Electrical and chemical activation of the mesencephalic and subthalamic locomotor regions in freely moving rats. Brain Res 1988;452:273-85. [DOI] [PubMed]
- 90.Mogenson GJ, Wu M. Disruption of food hoarding behavior by injections of procaine into the mediodorsal thalmus, GABA into subpallidal region and haloperidol into accumbens. Brain Res Bull 1988;20:247-51. [DOI] [PubMed]
- 91.Seamans JK, Phillips AG. Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci 1994;108:456-68. [DOI] [PubMed]
- 92.Keating GL, Winn P. Examination of the role of the pedunculopontine tegmental nucleus in radial maze tasks with or without a delay. Neuroscience 2002;112:687-96. [DOI] [PubMed]
- 93.Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci 2002;25:563-93. [DOI] [PubMed]
- 94.Block AE, Dhanji H, Thompson-Tardif SF, et al. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex 2007;17:1625-36. [DOI] [PubMed]