Abstract
Campylobacter jejuni is one of the most common causes of acute enteritis in the developed world. The consumption of contaminated poultry, where C. jejuni is believed to be a commensal organism, is a major risk factor. However, the dynamics of this colonization process in commercially reared chickens is still poorly understood. Quantification of these dynamics of infection at an individual level is vital to understand transmission within populations and formulate new control strategies. There are multiple potential routes of introduction of C. jejuni into a commercial flock. Introduction is followed by a rapid increase in environmental levels of C. jejuni and the level of colonization of individual broilers. Recent experimental and epidemiological evidence suggest that the celerity of this process could be masking a complex pattern of colonization and extinction of bacterial strains within individual hosts. Despite the rapidity of colonization, experimental transmission studies exhibit a highly variable and unexplained delay time in the initial stages of the process. We review past models of transmission of C. jejuni in broilers and consider simple modifications, motivated by the plausible biological mechanisms of clearance and latency, which could account for this delay. We show how simple mathematical models can be used to guide the focus of experimental studies by providing testable predications based on our hypotheses. We conclude by suggesting that competition experiments could be used to further understand the dynamics and mechanisms underlying the colonization process. The population models for such competition processes have been extensively studied in other ecological and evolutionary contexts. However, C. jejuni can potentially adapt phenotypically through phase variation in gene expression, leading to unification of ecological and evolutionary time-scales. For a theoretician, the colonization dynamics of C. jejuni offer an experimental system to explore these ‘phylodynamics’, the synthesis of population dynamics and evolutionary biology.
Keywords: Campylobacter jejuni, stochasticity, transmission, colonization, persistence, population genetics
1. Introduction
Campylobacter jejuni is one of the leading causes of bacterial food poisoning in the world today. Spread by the faeco-oral route, C. jejuni can colonize the intestinal mucosa of most warm-blooded animals (Newell & Fearnley 2003). In commercial broiler chickens, this colonization is commensal, with C. jejuni found at highest levels in the mucosal crypts of the caeca and to a lesser extent in the small intestine (Beery et al. 1988). However, in humans, colonization can lead to acute enteritis, often associated with invasion of the intestinal epithelial cells. Symptoms usually present after an incubation period of 1–7 days and range in severity from protracted watery diarrhoea to bloody diarrhoea with fever and abdominal cramps (Black et al. 1988). Infections are typically self-limiting, without the need for medical intervention; however, severe sequelae can result. Significantly, campylobacterosis has been linked to the, albeit rare, autoimmune disorders Miller Fisher syndrome and Guillain-Barré syndrome (Altekruse et al. 1999). A risk factor for human disease, at least in the developed world, is the consumption of contaminated poultry products. It is estimated that 80% of raw chicken sold in UK is contaminated with C. jejuni (Corry & Atabay 2001). This contaminated meat may be responsible for between 20 and 40% of reported infections (Havelaar et al. 2007). While the commensal colonization of chickens by C. jejuni has been well documented, comparatively little is known about the dynamics of this process.
The use of theoretical models in veterinary epidemiology has a relatively shallow history, their utility still viewed with some scepticism by the professional establishment (Wingfield et al. 2006). Epidemiological studies of C. jejuni have almost exclusively focused on static risk assessment models based on questionnaires. The results of these studies are often inconclusive and contradictory. The quantitative assessment of control strategies requires a firm grasp not only on the routes of transmission but also the mechanistic processes that underlie them. New microbiological methods of genetic typing and tagging of bacterial strains offer new insights into colonization at the level of the individual (Hendrixson & DiRita 2004; Grant et al. 2005). Scaling these individual level effects to the level of the population, in this case the broiler house, is a non-trivial task. Mechanistic models of transmission can translate experimentally derived hypotheses into quantitative predictions for the patterns of prevalence at the population level.
Few studies, indeed only two that we are aware of, have attempted to quantify rates of transmission and fit mechanistic models to the spread of C. jejuni in a broiler house. Estimation of transmission rates is a notoriously difficult task, sensitive to the choice of (theoretical and animal) model assumptions. We review these two key studies before going on to consider how new experimental evidence concerning the within-host dynamics of infection may modify their conclusions. We argue that understanding the colonization dynamics of C. jejuni is the key to understanding transmission. In §7, we consider how the analysis of this experimental system is complicated by the exceptional genetic plasticity of C. jejuni. This leads to a convergence of time-scales between evolutionary and ecological interactions, which complicate the analysis of experimental data. Models which synthesize evolutionary processes and population dynamics, the so-called phylodynamic models, are relatively new (Grenfell et al. 2004).
This review follows a top-down description of the epidemiology and ecology of C. jejuni in broilers. An understanding of epidemiological dynamics depends on both field data and those from experimental studies. We begin by reviewing the results of epidemiological field studies and the natural course of colonization of commercial flocks. We then introduce the basic model framework which has been used to estimate rates of transmission within flocks before delving deeper into the biology of colonization at the individual (and strain) level and the implications that this could have in turn for patterns of prevalence at the level of the population.
2. Colonization of the flock
Many epidemiological studies have been carried out to quantify the possible sources of introduction of C. jejuni into a flock. Although the conclusions of individual studies vary widely, a recent systematic review has provided the first clear indications of consensus (Adkin et al. 2006). Horizontal transmission is the most probable mechanism (Sahin et al. 2002) with possible sources including wild birds (Craven et al. 2000) and other farm animals (Gregory et al. 1997), for which insects (Ekdahl et al. 2005), contaminated groundwater (Pearson et al. 1993) and farm workers (Berndtson et al. 1996a) could be important vectors. Although the potential for vertical transmission from breeder hens to broilers exists (Newell & Fearnley 2003), in practice this is thought to be at best a rare occurrence (Shanker et al. 1986; van de Giessen et al. 1992; Chuma et al. 1997; Petersen et al. 2001) with no significant risk to commercial flocks (Callicott et al. 2006).
Although many flocks appear to be dominated by a single strain (Ring et al. 2005), two competing variants (Jacobs-Reitsma et al. 1995; Berndtson et al. 1996b) and even greater multiplicities of strains (Thomas et al. 1997; Hiett et al. 2002) have been isolated from the same flock. We would naturally expect the rate of introduction (from the environment external to a facility) to vary greatly between facilities depending on the methods of housing, bio-security policies and the prevalence of C. jejuni in the surrounding environment. The best illustration of this is the reported higher prevalence of C. jejuni found in organic flocks compared with conventionally farmed flocks (Van Overbeke et al. 2006).
Prevalence within infected commercial flocks is strongly age dependent with chickens less than two to three weeks old rarely being colonized naturally (Stern et al. 2001a). The reasons for this so-called lag phase (Newell & Fearnley 2003) are unclear but could include the effect of maternally derived antibodies (Cawthraw et al. 1994; Sahin et al. 2003) or age-related differences in the intestinal environment and natural gut flora (van der Wielen et al. 2000). Understanding the lag phase of colonization could be the key to the development of new control measures. Several studies have demonstrated that the prevalence of C. jejuni in chickens (Wallace et al. 1997), other farm animals (Stanley et al. 1998a,b) and incidence in humans exhibits a marked seasonal variation with the highest levels during the summer months (Nylen et al. 2002; Meldrum et al. 2004). The peak months of prevalence in chickens and incidence in humans have a dependence on latitude and a close correlation with temperature (Louis et al. 2004; Patrick et al. 2004) suggesting that a common factor, extrinsic to the host populations, may be responsible.
After infection, chickens rapidly (in less than a day) exhibit high levels of C. jejuni in the caecal contents (Shanker et al. 1990). Faecal shedding to the internal environment within the housing facility (and then ingestion) is likely to be the route of bird-to-bird transmission rather than direct contact.
3. Dynamics of transmission
Quantitative predictions of the effectiveness of control strategies are impossible without a mechanistic understanding of the processes underlying transmission (Anderson & May 1979). Given the public health and commercial importance of C. jejuni as a zoonotic pathogen, it is somewhat surprising how little is known concerning the routes and dynamics of colonization in chickens. Few studies have attempted to quantify transmission rates (Shanker et al. 1990; Stern et al. 2001b) and only two studies which we are aware of in the literature have attempted to estimate (Van Gerwe et al. 2005) and describe (Hartnett et al. 2001) colonization dynamics within a commercial broiler house. Both studies chose, in the face of the multiple possible routes of introduction and transmission previously discussed, to describe colonization as a simple epidemic and attempt to estimate a single epidemiological parameter, the most basic parameter determining the rate of spread of an infectious agent—the transmission rate. Despite this their estimates differed by an order of magnitude (β=0.1–0.3, Hartnett et al. 2001; β=1.04, Van Gerwe et al. 2005). In an attempt to understand this disparity, we need to consider the assumptions made by these two key studies, in particular their chosen definition of the transmission rate and the implications for future studies.
3.1 Definition of transmission rates
The general definition of transmission rates used in the literature varies substantially according to the principal modes of transmission and life history of the disease in question. For example, the demography of the host population and the within-host dynamics of infection must be taken into account when interpreting the meaning of a particular transmission rate. Both published studies for C. jejuni in broilers chose to use variants of the standard transmission term where the rate of new infections is proportional to the number of susceptible individuals (S) and the fraction of the population (N) which is infectious (I) multiplied by a (constant) transmission rate (β, with units of contacts per day). Although details of their implementations vary, both models essentially describe the epidemic process as a unidirectional flow from susceptibility (S) to infectiousness (I), equivalent to the simple stochastic SI model. The dynamical structure is most clearly illustrated by the deterministic implementation which can be summarized in the pair of ordinary differential equations:
| (3.1) |
By describing the population only in terms of the numbers which are susceptible and infectious, we are making an implicit set of assumptions. We assume that all individuals in the population are identical (have equal susceptibility to infection) and there is only one infectious agent (one strain) being transmitted. The rate of spread depends on a constant ‘transmission rate’ and the probability that an infectious individual will meet a susceptible individual if the population is ‘well mixed’, so-called ‘homogenous’ mixing.
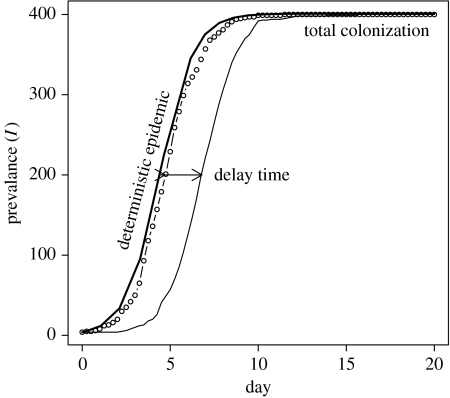
Solutions of the stochastic model closely follow the shape of solutions of the deterministic skeleton above, with the addition of a variable delay time in the upswing of the epidemic (figure 1). This delay is the product of uncertainty in the chain of transmission at the start of the epidemic due to low numbers: so-called demographic stochasticity (Renshaw 1991). For a fixed transmission rate, this delay time is therefore determined by the initial number of infectives (I(0)) rather than the total population size (N). For a given epidemic curve, derived experimentally or through simulation of the stochastic model, the delay time can be defined as the difference in the time to reach the midpoint of the epidemic (I/N=0.5) from that expected from the deterministic SI model (Van Gerwe et al. 2005).
Figure 1.
Stochastic SI model. Two stochastic replicates of SI model (with N=400 and I(0)=4) demonstrating variation in delay times and similarity of epidemic phases. Note that, although increasingly unlikely, the delay time could be arbitrarily large with no extinction of the pathogen (β=1.04 contacts per day; parameters from Van Gerwe et al. 2005).
An important distinction should be made between this delay time and the lag phase phenomena described earlier (length up to three weeks). The lag phase is not observed in experimental studies, presumably due to the differences between the artificial inoculation and the natural course of acquiring C. jejuni. These include the inocula used, experimental models typically use relatively high doses of a single bacterial strain; the differences in the physiological state of the bacteria, i.e. broth culture versus bacteria from the environment and the presence of naturally acquired gut flora in commercial flocks. In a commercial flock, chicks will also be exposed to a wide spectrum of bacteria leading to the rapid establishment of a natural gut flora (van der Wielen et al. 2000). Experimental models often use 1-day-old chicks with inocula typically containing large numbers of a single bacterial species.
3.2 Scaling and interpretation of transmission rates
This choice (equation (3.1)) of definition of the transmission rate, so-called frequency-dependent mass action (De Jong et al. 1995), assumes that there is homogenous mixing within the population and the number of contacts between birds leading to transmission of C. jejuni is independent of the size of the flock. Great care must be taken in applying this model to C. jejuni transmission in broilers, particularly as the population sizes in the experimental studies from which we are able to estimate β are orders of magnitude smaller than commercial flocks. Hartnett's study was based on experimental data from individually housed birds and small groups (Shanker et al. 1990), while Van Gerwe used data from a larger study of 400 birds at the same density as commercial barns (Jacobs-Reitsma 1998). This alone could explain the order of magnitude difference in the quoted transmission rates estimated by these two authors. Resolving how transmission scales with flock size is an outstanding and pressing issue for understanding C. jejuni population dynamics. Given the practicalities of control, animal welfare, time and cost limiting the size of experimental studies, it is only likely to be properly addressed through well-designed field studies with regular (and extensive) sampling.
Applying the simple epidemic model, which implicitly assumes direct transmission, to C. jejuni is conceptually problematic given the probable importance of indirect, environmental transmission and transmission due to coprophagy. Both C. jejuni models justify this modelling assumption using the study of chicken movement patterns by Preston & Murphy (1989). They described persistent social clustering of chickens, which Van Gerwe et al. (2005) interpreted as evidence of homogenous mixing. The argument being that since the chickens were continually moving the clusters were well mixed spatially. Hartnett et al. (2001) proposed the opposing position that clustering would restrict transmission to a single cluster in the first phase of the colonization process. Hartnett et al. (2001) therefore used a two-stage model which only simplified to the homogenous mixing SI model above (equation (3.1)) after an initial delay time of approximately 3 days. The approach was justified by arguing that there is a change in route of transmission after the primary infection events. Transmission in the first stage is restricted to direct transmission within a cluster, giving way to more widespread transmission throughout the flock, via contamination of the environment.
Hartnett essentially assumes the existence of a delay time, which is backed up by Van Gerwe et al.'s analysis of experimental data. While the argument for a change in transmission routes is seductive, there is no quantitative evidence to support this hypothesis and the simple stochastic SI model produces the same qualitative delay (figure 1) without the complex contact structure assumed by Hartnett. However, what is particularly interesting is that the length of delay time estimated by Van Gerwe et al. (2005) between 3 and 7 days was far greater than that expected from the stochastic SI model (mean delay of 0.9 days).
If this delay is indeed due to the requirement for a build-up of environmental contamination, then simpler models (than Hartnett's) could take this into account by modelling the environmental contamination directly (Anderson & May 1981). This would necessitate more information not only on the levels of viable bacteria in the environment but also on how the bacteria survive in this, presumably, more hostile environment. Delving deeper into the biology of colonization at the individual level suggests that there may also be a genetic basis for the delay, to which we will return later in §6. However, there are also simpler, infection life-history traits which could be experimentally derived and may have important implications not only for explaining this delay time but also for the determination of transmission rates.
4. Colonization of the individual broiler
The definition and estimation of the most basic measure of colonization, the minimum infectious dose (Chen et al. 2006) or ID50,1 is complicated for C. jejuni by the potential of recycling of infectious material due to coprophagy. However, doses as low as 40 colony forming units (Cawthraw et al. 1996) have been shown experimentally to successfully colonize 1-day-old chicks, although the infectious dose varies between strains of C. jejuni (Shanker et al. 1988, 1990; Stern et al. 1988; Ringoir & Korolik 2003), physiological status of the strain, and age and breeds of chicken (Newell & Fearnley 2003). Colonization can be defined by the presence of detectable levels of C. jejuni in fresh faecal samples or directly from caecal contents post-mortem. There is necessarily a period of establishment during which the bird is infected (inoculated) but not infectious (excreting). This deterministic delay is not accounted for by the simple SI model, one factor which may explain the disparity with experimental estimates of the delay time which we will return to in §5.
The route of introduction into the chicken is also potentially important, although only with respect to artificial inoculation. Shanker observed an order of magnitude of lower ID50 for cloacal challenge when compared with the more regular oral introduction (Shanker et al. 1988). This may be the result of the direct (shorter) connection of the cloacal opening to the primary source of colonization of C. jejuni, the caeca, through the rectum. The implication is that large numbers of bacteria may be lost on the journey through the digestive system after oral inoculation. Essentially, this implies that the success of colonization is not only determined solely by competitive pressures within the caeca, but also by the probability of survival within the gut. This could be an important clue to the nature of the within-host colonization dynamics. Young et al. (1999) failed to achieve colonization via cloacal challenge; however, it is unclear whether this was due to differences in methodology or challenge strain.
Signature tagged mutagenesis studies (Hensel et al. 1995), where multiple individually tagged mutants are screened for colonization potential through experimental infection have proven highly successful for many pathogens (Mazurkiewicz et al. 2006). Application of the technique to C. jejuni has been problematic, with high-frequency loss of (individually) colonization proficient mutants in a 1-day chick model (Hendrixson & DiRita 2004) and older birds with a standardized gut flora (Grant et al. 2005). The implication of these studies was that different strains of campylobacter would compete for resources, which has recently been demonstrated for wild-type strains (Konkel et al. 2007).
It would be natural to assume that competition between bacteria could be an important mechanism leading to clearance of a given strain. Such competition models have a long history in ecology (Volterra & Brelot 1931; Lotka 1956) and have recently started to be applied to model the competition dynamics of bacterial flora with respect to food safety (Dens et al. 1999; Vereecken et al. 2000). However, these deterministic approaches do not account for the importance of chance, variability and mutation (Kimura 1962) which may be of particular importance for the ecology of C. jejuni.
The experiments of Grant et al. (2005) found evidence, not only for displacement of mutants but also frequent transmission between birds housed together. Competitive processes could be a mechanism leading to clearance. If displacement and transmission occur fast enough, we may not observe the effects of clearance in the population. However, the transmission rates necessary to explain a given epidemic curve could be quite different.
Clearance has been described in the field although few studies have considered within flock prevalence, the perception being that introduction necessarily leads to rapid flock-wide colonization (Berndtson et al. 1996a; Gregory et al. 1997; Evans & Sayers 2000; Shreeve et al. 2000). However, lower levels of prevalence have been reported (Heuer et al. 2001) although this may be only the result of late colonization and there is known to be a wide range of colonization abilities between strains (Ringoir & Korolik 2003).
To our knowledge, only one study provides evidence of clearance occurring with wild-type strains (Achen et al. 1998). An attempt was made to minimize coprophagy by placing birds on wire mesh. Although not a unique housing method, this attempt may have been more successful due to the virulent pathology of the campylobacter infection leading to continual diarrhoea. The unusual pathology is itself a warning that the results may not be generally applicable. The study suggests that maintenance of C. jejuni within an individual host is dependent on recycling of bacteria through environmental contamination and coprophagy. This hypothesis is further supported by a similar study (Willis et al. 2002) which showed a lower prevalence of campylobacter in experimentally infected birds housed in cages when compared with those on a solid floor. Let us consider the impact that clearance would have on transmission rates, if indeed it does occur, by considering an elaboration of the simple SI model considered previously.
5. From the individual to the flock: implications of clearance and latency on transmission rates
In this section, we consider how two simple life-history traits of C. jejuni, a latent period and clearance (in this model recovery without immunity to re-infection), could affect the stochastic delay time observed experimentally (§3.1) at the population level. Although we could formulate a single model, we choose for simplicity of argument to consider these two mechanisms separately.
We can incorporate clearance straightforwardly by adding a flow from the infective class I to the susceptible class S at rate α to the SI model to form a SIS model,
| (5.1) |
which introduces a new non-trivial equilibrium fraction of pathogen-free hosts α/β (figure 2). The existence of an equilibrium level of prevalence (rather than total colonization) is not enough to reject the clearance mechanism outright. Although the experimental studies reported total colonization, the sampling rate used to determine prevalence was reduced for the latter time points (based on the assumption that colonization would be total). A level of prevalence high enough that routine sampling (at a fixed rate) would not find uninfected birds would still be consistent with the data.
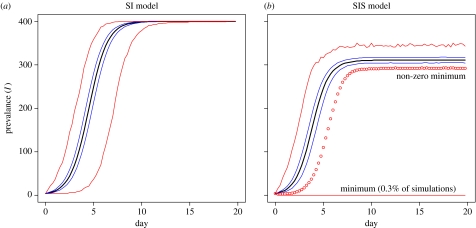
Figure 2.
Effect of clearance on epidemic curve. (a) SI model (β=1.04 contacts per day, N=400, I(0)=4, parameters from Van Gerwe et al. 2005). (b) SIS model (β=1.5 contacts per day, clearance rate α=1/3 per day). The transmission rate of the SIS model was adjusted to match the (median) attack of the epidemic phase. Despite the high clearance rate, there is little change to the delay time in the SIS model and a major (easily detectable) reduction in prevalence. For both models, the envelopes of the ‘five number’ summaries of 10 000 replicates of the stochastic model are plotted. Bold black lines indicate the median, blue lines the upper and lower quartiles and red lines the maximum and minimum. For the SIS model, 0.3% of simulations result in no epidemic taking place, lower values of I(0) would result in a higher probability of this occurring.
The inclusion of a latent period requires the definition of a new exposed (E) class, into which newly infected individuals enter, progressing to infectious status at a constant rate ν. This construction makes a very specific assumption that the time spent in latency is exponentially distributed. The exact distribution of the latent period distribution is known to affect both the estimation of transmission rates and the amount of stochastic variability we would expect (Wearing et al. 2005). Exponentially distributed latent periods should lead to the greatest amount of variability (longest delay time) and provides the simplest mathematical description. The deterministic skeleton of this SEI model is
| (5.2) |
The dynamics of the original SI model are recovered for small α→0 and large ν→∞. In order to compare different epidemic models fairly, we must demand that they produce the same qualitative epidemic dynamics. Increasing values of the clearance rate (α) and latent period (T=1/ν) lead to slower epidemics. While this could increase the previously defined delay time arbitrarily, to allow a fair comparison with Van Gerwe et al. (2005), we must demand that the newly formulated models are still consistent with the original data. Without access to the data, we make the (indirect) requirement that our SIS and SEI models match the maximum rate of increase of I at the midpoint of the epidemic of the SI model with Van Gerwe's parameters.
This of course, is only a matter of curve fitting. What is more interesting is that the new model structures can lead to increased variability in the critical early period of the epidemic even when they represent the same rate of epidemic spread. In practice, given that the initial conditions of the experiments were chosen to minimize the effects of demographic stochasticity (four inoculated chicks, I(0)=4 in the SI and SIS models, E(0)=4 in the SEI model), neither clearance nor inclusion of a latent period alone could increase the delay time to the levels observed experimentally. Owing to the rapidity of transmission, clearance would only lead to an increase in the delay time of at the most half-a-day, even at a high rate of clearance, which would have an easily observable impact on prevalence at the end of the experiment (figure 2). However, there is important structural difference between the SI and SIS models—the SI model colonization always takes place, while the SIS model admits the possibility that colonization of the flock will fail.
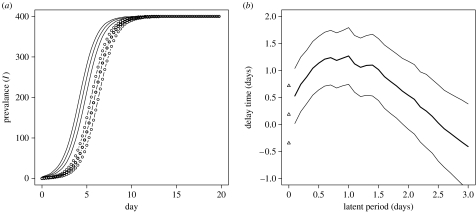
Short latent periods do have the potential to significantly increase the length of the stochastic delay time (figure 3). However, this increase in the delay time is limited to a maximum of approximately 1.2 days for a latent period of approximately 1 day. Van Gerwe et al. (2005) reported that the initially inoculated birds were all C. jejuni positive within 1–2 days. This simple analysis of a theoretical model demonstrates that a latent period in a reasonable biological range would have a significant (measurable) effect at the population level. It should also be noted that barring the delay time, both models with and without a latent period could be equally well supported by the data. However, the transmission rate required in a model with a 1-day latent period has to be twice that of the SI model (β=2.13 compared with β=1.04 contacts per day) to match the same epidemic dynamics, which could have important implications for the estimated efficacy of control measures. This analysis also demonstrates that there are theoretical bounds on the extent to which a latent period could increase the delay time of C. jejuni transmission and still remain consistent with the experimental data.
Figure 3.
Effect of latent period on delay time. (a) Inclusion of a latent period of 1 day (open circles) leads to a significant increase in the delay time as compared with the simple epidemic (SI) model (solid lines). The median (bold) and envelopes of the upper and lower quartiles from 10 000 replicates of the stochastic model are plotted. (b) Delay time, measured as the difference in the time to reach the midpoint of the epidemic between the stochastic model (with latent period) and the deterministic SI model, plotted against latent period. Correcting the transmission rate in order to match the maximum growth rate of the simple epidemic (SI) model places a limit on the extent to which the delay time can be increased and still be consistent with the experimental data. Bold line represents the mean delay time, fine lines the envelope created by the standard deviations about the mean calculated over 10 000 stochastic replicates. Triangles indicate the mean and envelope of delay time for the stochastic SI model (without latent period).
This is the beauty of developing theoretical models in tandem with experimental studies. The relative importance of different biological mechanisms can be assessed quantitatively based on our biological hypotheses and can focus attention on processes that may not have previously been of interest. In this section, we choose to present the impact that clearance and latency could have on the previously observed and unexplained delay time in experimental transmission studies. In this case, neither mechanism could generate (mean) delay times in excess of 1 day and therefore could not account for all of the variability seen in the experimental study (Van Gerwe et al. 2005). However, we have demonstrated the potential impact of a period of latency. In stark comparison to the estimation of transmission rates, which we argue can only be achieved through field studies, such life-history traits are best explored through controlled experiments in model systems. The dynamics of the internal processes by which individuals become infectious, crudely considered here as a simple latent period, will have knock on effects for the estimation of transmission between individuals.
6. Phase variation—dynamics of gene expression
The internal dynamics of infection are not necessarily limited to the course of colonization by a single bacterial strain. Multiple co-infections within flocks and individual birds have been reported using serotyping (van de Giessen et al. 1992; Jacobs-Reitsma et al. 1995) and genotyping (Stern et al. 1997; Rivoal et al. 1999) methods. The presence of multiple strain types within a flock is presumably a consequence of multiple horizontal introductions from the environment. However, it is also possible that genetic drift is occurring during the short duration of colonization (Wassenaar et al. 1998; Hook et al. 2005). This hypothesis is further supported by the increase in colonization potential (lowering of ID50) observed for strains passaged through hosts (Cawthraw et al. 1996; Ringoir & Korolik 2003; Jones et al. 2004). This evolutionary change occurs over an exceedingly short period (of the order of 3 days). However, C. jejuni does possess a mechanism by which to change its gene expression patterns rapidly through the mechanism of phase variation, the effects of which on colonization potential are well described (Carrillo et al. 2004; Gaynor et al. 2004).
6.1 Mechanisms of phase variation in C. jejuni
Several hyper-variable regions of the C. jejuni genome have been identified, corresponding primarily to genes involved in expression of the flagella, the capsule and lipooligosaccharide (Parkhill et al. 2000). These hyper-variable sequences feature short runs of the same nucleotide (homo-polymeric tracts). Such repeats lead to a high probability of slip stranded misalignments occurring during DNA replication. This can stop a gene being expressed, or activate a previously suppressed gene with the addition or loss of a single nucleotide (Li & Graur 1991). In some cases, this can serve as a molecular on/off switch for gene expression, which occurs more rapidly than random mutation, and is heritable. If selective pressures are favourable, certain variants could rapidly ascend through the population.
What effects would we expect phase-variation to have on colonization dynamics and the competition between different strains and what models could be used to describe this process?
6.2 Exploring phase variation through competition experiments
From a modelling perspective, if we wish to understand the complex community dynamics of a bacterial flora, it is important to understand the null model first—the dynamics of a single variant of C. jejuni in colonizing a host. In vitro competition experiments, where we can control more variables, critically the size of population bottlenecks applied to the system (Wahl & Gerrish 2001), can be used to inform the analysis of the in vivo studies, as well as to investigate the basic biology of the organism. Bottlenecks not only increase the probability of stochastic extinction, but also accelerate the evolutionary process by selecting for the most abundant (and fittest) genotypes (Gog et al. 2003). This selective pressure can make competition experiments in vivo a highly sensitive method of determining if adaptation has occurred. Over a long growth phase, even small changes in one variant's growth rate can lead to a large competitive advantage and a corresponding change in the ratio of the two variants. The relative difference in growth rates can therefore be determined to a much greater accuracy than individual growth rates, estimates of which are limited in accuracy by the number of time points that can be collected. This could also potentially form the basis for more sensitive methods of estimation of phase variation rates, which are not well defined for C. jejuni.
6.3 Phase variation and transmission experiments
The experimental determination of phase variation rates is a key requirement for the further development of models of colonization for C. jejuni. Standard methods for the estimation of mutation rates are based on some assumptions which are not valid for phase variation, particularly equal growth rate/fitness for each type, no back mutation and an initial condition of one genotype/phenotype (Saunders et al. 2003). The main difficulty is in controlling ‘jackpot’ cultures (mutation early in the experiment leading to a much higher proportion of mutants at the end of the experiment).
If cultured strains are going through adaptive changes between in vitro culture and in vivo colonization, phase variation could be an excellent explanation for the delay time in the transmission experiments discussed above. Selection for growth in vitro, either during inoculum preparation or repeated laboratory passage, could lead to a reduction in the diversity of variants. We might expect that a broader distribution of gene expressions in a population would be an advantage for successful colonization. Indeed using similar arguments, modelling studies, not specifically developed for C. jejuni, have shown that growth in hostile, fluctuating conditions can be aided by a more broad diversity of gene expressions (Thattai & van Oudenaarden 2004; Kussell & Leibler 2005). This is a potential danger of using animal models to estimate the transmission rates for real populations. The perceived fitness of strains passaged in vivo may just be an artefact of the emasculating effect of the culture process weakening colonization ability (Wiles et al. 2006).
7. Conclusions
In this review, we have given a broad overview, over a range of scales from the individual bacterium up to the commercial flock, of the natural history of C. jejuni in broiler chickens. Several key questions concerning colonization remain, which could be of great importance in reducing prevalence in commercial flocks.
The most pressing is the origin of the lag phase and to a lesser extent the delay time from experimental transmission experiments. We know from experimental studies that 1-day-old chicks are capable of being colonized by C. jejuni and yet this does not happen in commercial flocks until the chicks are around two to three weeks old. Is this due to the success of bio-security in keeping C. jejuni from entering flocks or do chicks remain C. jejuni free over this period even with a constant low level of exposure? If so, it is imperative to understand if this is a function of dose, competitive interactions with other commensal bacteria or changes in the physiological (or immunological) status of the host.
Addressing these questions experimentally would ideally involve the development of more sophisticated experimental models which more closely mimic the natural infection process (Wiles et al. 2006). However, they may be best addressed through a combination of carefully designed field studies and smaller scale experiments concentrating on determining life-history traits. The estimation of transmission rates from model experimental systems will only ever be of limited utility. The necessarily reduced size of experimental populations tells us nothing concerning all the important scaling relationships of transmission rates between flocks of different sizes. Determination of these most fundamental epidemiological parameters is the crucial first step in obtaining mechanistic models of transmission. Detailed studies of the natural progression of C. jejuni in real flocks with a high frequency of sampling is likely to be the only successful way of doing so.
In contrast to the estimation of transmission rates, issues pertinent to the life history of infection and model structure are best addressed in controlled experimental studies. Multiple infection experiments revealed complex population and competition dynamics within a single host (Hendrixson & DiRita 2004; Grant et al. 2005; Konkel et al. 2007). Understanding these dynamics will require a joint effort between modellers and experimentalists. We argue strongly that the collaboration between modellers and experimentalists should start at the beginning, when designing experimental studies, rather than with consultation at the end in the more conventional pattern of events. This symbiotic process can be beneficial to all sides, modelling feeding into experimental design and consequently leading to further model refinements.
We considered how the inclusion of two such life-history traits, latency and clearance, which are not well described for C. jejuni, would affect estimates of transmission rates and patterns of prevalence in the standard transmission model. In particular, we explored mechanisms which could lead to the exceptionally long stochastic delay time estimated by Van Gerwe et al. (2005) from transmission experiments.
There is only very limited evidence that clearance of C. jejuni from hosts occurs in broiler flocks. Even if it did occur at a reasonably high rate, recycling of bacteria through the environment could lead to continual re-seeding of C. jejuni into the intestinal tract. However, the potential importance of this indirect form of transmission brings into doubt the validity of using simple epidemic models for C. jejuni transmission. Models which explicitly account for the levels of environmental contamination (more typically used for macro-parasites) would be more appropriate. This would require more detailed information on the survival of C. jejuni in the environment and critically the mechanisms by which natural infections occur. The accumulation of environmental contamination is perhaps the simplest explanation for the delay time observed by Van Gerwe et al. (2005) and assumed by Hartnett et al. (2001).
We demonstrated that a latent period, the consequence of the time required for a broiler to start shedding C. jejuni at high dose after infection (inoculation), could at best increase the delay time approximately by 1 day. With a biologically plausible latent period, the estimated transmission rate could be up to twice that estimated by the SI model. However, the compartmental SEI model, which we considered makes a simplistic (exponential) assumption about the distribution of latent times within a flock. We might expect the period of latency, effectively the time-scale of colonization of an individual, to depend on variations in host susceptibility and the magnitude of the infectious dose. The theoretical importance of such details have already been well established (Wearing et al. 2005) and provides further motivation for more refined experimental explorations of the natural infection process. Nevertheless, the simple models explored in this review illustrate the importance of understanding the detailed, within-host dynamics of infection in formulating accurate models of within-flock transmission.
We conclude by briefly describing the remarkable genetic plasticity of C. jejuni through the mechanism of phase variation. Understanding the ecology and epidemiology of C. jejuni will require the unification of work from disciplines over a large hierarchy of scales. Modelling can form a bridge between these scales. Finally, understanding C. jejuni colonization dynamics will require the unification of ecological and evolutionary processes, synthesizing disparate modelling concepts from ecology, genetics and evolutionary biology.
Acknowledgments
This work was supported by a BBSRC project grant to D.J.M. and J.R.G. J.R.G. is supported by the Royal Society. The authors are members of the Cambridge Infectious Disease Consortium (CIDC).
Footnotes
One contribution of 20 to a Theme Issue ‘Cross-scale influences on epidemiological dynamics: from genes to ecosystems’.
Defined as the minimum dose at which 50% of inoculated hosts become infected.
References
- Achen M, Morishita T.Y, Ley E.C. Shedding and colonization of Campylobacter jejuni in broilers from day-of-hatch to slaughter age. Avian Dis. 1998;42:732–737. doi: 10.2307/1592708. [DOI] [PubMed] [Google Scholar]
- Adkin A, Hartnett E, Jordan L, Newell D, Davison H. Use of a systematic review to assist the development of campylobacter control strategies in broilers. J. Appl. Microbiol. 2006;100:306–315. doi: 10.1111/j.1365-2672.2005.02781.x. [DOI] [PubMed] [Google Scholar]
- Altekruse S, Stern N, Fields P, Swerdlow D. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 1999;5:28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R, May R. Population biology of infectious diseases: part I. Nature. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- Anderson R, May R. The population dynamics of microparasites and their invertebrate hosts. Phil. Trans. R. Soc. B. 1981;291:451–524. doi: 10.1098/rstb.1981.0005. [DOI] [Google Scholar]
- Beery J, Hugdahl M, Doyle M. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 1988;54:2365–2370. doi: 10.1128/aem.54.10.2365-2370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndtson E, Danielsson-Tham M, Engvall A. Campylobacter incidence on a chicken farm and the spread of campylobacter during the slaughter process. Int. J. Food Microbiol. 1996a;32:35–47. doi: 10.1016/0168-1605(96)01102-6. [DOI] [PubMed] [Google Scholar]
- Berndtson E, Emanuelson U, Engvall A, Danielsson-Tham M. A 1-year epidemiological study of campylobacters in 18 Swedish chicken farms. Prev. Vet. Med. 1996b;26:167–185. doi: 10.1016/0167-5877(95)01008-4. [DOI] [Google Scholar]
- Black R, Levine M, Clements M, Hughes T, Blaser M. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- Callicott K.A, et al. Lack of evidence for vertical transmission of Campylobacter spp. in chickens. Appl. Environ. Microbiol. 2006;72:5794–5798. doi: 10.1128/AEM.02991-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo C.D, et al. Genome-wide expression analyses of Campylobacter jejuni NCTC11168 reveals coordinate regulation of motility and virulence by flhA. J. Biol. Chem. 2004;279:20 327–20 338. doi: 10.1074/jbc.M401134200. [DOI] [PubMed] [Google Scholar]
- Cawthraw S, Ayling R, Nuijten P, Wassenaar T, Newell D. Isotype, specificity, and kinetics of systemic and mucosal antibodies to Campylobacter jejuni antigens, including flagellin, during experimental oral infections of chickens. Avian Dis. 1994;38:341–349. doi: 10.2307/1591960. [DOI] [PubMed] [Google Scholar]
- Cawthraw S, Wassenaar T, Ayling R, Newell D. Increased colonization potential of Campylobacter jejuni strain 81116 after passage through chickens and its implication on the rate of transmission within flocks. Epidemiol. Infect. 1996;117:213–215. doi: 10.1017/s0950268800001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Geys H, Cawthraw S, Havelaar A, Teunis P. Dose response for infectivity of several strains of Campylobacter jejuni in chickens. Risk Anal. 2006;26:1613–1621. doi: 10.1111/j.1539-6924.2006.00850.x. [DOI] [PubMed] [Google Scholar]
- Chuma T, Makino K, Okamoto K, Yugi H. Analysis of distribution of Campylobacter jejuni and Campylobacter coli in broilers by using restriction fragment length polymorphism of flagellin gene. J. Vet. Med. Sci. 1997;59:1011–1015. doi: 10.1292/jvms.59.1011. [DOI] [PubMed] [Google Scholar]
- Corry J, Atabay H. Poultry as a source of campylobacter and related organisms. J. Appl. Microbiol. 2001;90:96–114. doi: 10.1046/j.1365-2672.2001.01358.x. [DOI] [PubMed] [Google Scholar]
- Craven S.E, Stern N.J, Line E, Bailey J.S, Cox N.A, Fedorka-Cray P. Determination of the incidence of Salmonella spp., Campylobacter jejuni, and Clostridium perfringens in wild birds near broiler chicken houses by sampling intestinal droppings. Avian Dis. 2000;44:715–720. doi: 10.2307/1593118. [DOI] [PubMed] [Google Scholar]
- De Jong M.C.M, Diekmann O, Heesterbeek J.A.P. Epidemic models: their structure and relation to data. In: Mollison D, editor. How does infection-transmission depend on population size? Publications of the Newton Institute. ch. 5. Cambridge University Press; Cambridge, UK: 1995. pp. 84–94. [Google Scholar]
- Dens E.J, Vereecken K.M, Van Impe J.F. A prototype model structure for mixed microbial populations in homogeneous food products. J. Theor. Biol. 1999;201:159–170. doi: 10.1006/jtbi.1999.1021. [DOI] [PubMed] [Google Scholar]
- Ekdahl K, Normann B, Andersson Y. Could flies explain the elusive epidemiology of campylobacteriosis? BMC Infect. Dis. 2005;5:11. doi: 10.1186/1471-2334-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S.J, Sayers A.R. A longitudinal study of campylobacter infection of broiler flocks in Great Britain. Prev. Vet. Med. 2000;46:209–223. doi: 10.1016/S0167-5877(00)00143-4. [DOI] [PubMed] [Google Scholar]
- Gaynor E.C, Cawthraw S, Manning G, MacKichan J.K, Falkow S, Newell D.G. The genome-sequenced variant of Campylobacter jejuni NCTC 11168 and the original clonal clinical isolate differ markedly in colonization, gene expression, and virulence-associated phenotypes. J. Bacteriol. 2004;186:503–517. doi: 10.1128/JB.186.2.503-517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gog J.R, Rimmelzwaan G.F, Osterhaus A.D.M.E, Grenfell B.T. Population dynamics of rapid fixation in cytotoxic T lymphocyte escape mutants of influenza A. Proc. Natl Acad. Sci. USA. 2003;100:11 143–11 147. doi: 10.1073/pnas.1830296100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A.J, Coward C, Jones M.A, Woodall C.A, Barrow P.A, Maskell D.J. Signature-tagged transposon mutagenesis studies demonstrate the dynamic nature of cecal colonization of 2-week-old chickens by Campylobacter jejuni. Appl. Environ. Microbiol. 2005;71:8031–8041. doi: 10.1128/AEM.71.12.8031-8041.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E, Barnhart H, Dreesen D.W, Stern N.J, Corn J.L. Epidemiological study of Campylobacter spp. in broilers: source, time of colonization, and prevalence. Avian Dis. 1997;41:890–898. doi: 10.2307/1592343. [DOI] [PubMed] [Google Scholar]
- Grenfell B.T, Pybus O.G, Gog J.R, Wood J.L.N, Daly J.M, Mumford J.A, Holmes E.C. Unifying the epidemiological and evolutionary dynamics of pathogens. Science. 2004;303:327–332. doi: 10.1126/science.1090727. [DOI] [PubMed] [Google Scholar]
- Hartnett E, Kelly L, Newell D, Wooldridge M, Gettinby G. A quantitative risk assessment for the occurrence of campylobacter in chickens at the point of slaughter. Epidemiol. Infect. 2001;127:195–206. doi: 10.1017/s0950268801005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelaar, A. et al 2007 Effectiveness and efficiency of controlling campylobacter on broiler chicken meat. Risk Anal OnlineEarly article. ( 10.1111/j.1539-6924.2006.00835.x) [DOI] [PubMed]
- Hendrixson D, DiRita V.J. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol. Microbiol. 2004;52:471–484. doi: 10.1111/j.1365-2958.2004.03988.x. [DOI] [PubMed] [Google Scholar]
- Hensel M, Shea J.E, Gleeson C, Jones M.D, Dalton E, Holden D.W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- Heuer O, Pedersen K, Andersen J.S, Madsen M. Prevalence and antimicrobial susceptibility of thermophilic campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol. 2001;33:269–274. doi: 10.1046/j.1472-765X.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- Hiett K.L, Stern N.J, Fedorka-Cray P, Cox N.A, Musgrove M.T, Ladely S. Molecular subtype analyses of Campylobacter spp. from Arkansas and California poultry operations. Appl. Environ. Microbiol. 2002;68:6220–6236. doi: 10.1128/AEM.68.12.6220-6236.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook H, Fattah M, Ericsson H, Vagsholm I, Danielsson-Tham M. Genotype dynamics of Campylobacter jejuni in a broiler flock. Vet. Microbiol. 2005;106:109–117. doi: 10.1016/j.vetmic.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Jacobs-Reitsma W. Experimental horizontal spread of campylobacter amongst one-day-old broilers. In: Lastovica A.J, Newell D.G, Lastovica E.E, editors. Campylobacter, Helicobacter and related organisms. 1st edn. University of Cape Town; Cape Town, South Africa: 1998. pp. 377–378. [Google Scholar]
- Jacobs-Reitsma W, van de Giessen A, Bolder N, Mulder R. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol. Infect. 1995;114:413–421. doi: 10.1017/s0950268800052122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M.A, Marston K.L, Woodall C.A, Maskell D.J, Linton D, Karlyshev A.V, Dorrell N, Wren B.W, Barrow P.A. Adaptation of Campylobacter jejuni NCTC11168 to high-level colonization of the avian gastrointestinal tract. Infect. Immun. 2004;72:3769–3776. doi: 10.1128/IAI.72.7.3769-3776.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. On the probability of fixation of mutant genes in a population. Genetics. 1962;47:713–719. doi: 10.1093/genetics/47.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel M.E, Christensen J.E, Dhillon A.S, Lane A.B, Hare-Sanford R, Schaberg D.M, Larson C.L. Campylobacter jejuni strains compete for colonization in broiler chicks. Appl. Environ. Microbiol. 2007;73:2297–2305. doi: 10.1128/AEM.02193-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussell E, Leibler S. Phenotypic diversity, population growth, and information in fluctuating environments. Science. 2005;309:2075–2078. doi: 10.1126/science.1114383. [DOI] [PubMed] [Google Scholar]
- Li W, Graur D. Sinauer Associates; Sunderland, MA: 1991. Fundamentals of molecular evolution. [Google Scholar]
- Lotka A. Dover Publications, Inc; New York, NY: 1956. Elements of mathematical biology. [Google Scholar]
- Louis V.R, Gillespie I.A, O'Brien S.J, Russek-Cohen E, Pearson A.D, Colwell R.R. Temperature-driven campylobacter seasonality in England and Wales. Appl. Environ. Microbiol. 2004;71:85–92. doi: 10.1128/AEM.71.1.85-92.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurkiewicz P, Tang C.M, Boone C, Holden D.W. Signature-tagged mutagenesis: barcoding mutants for genome-wide screens. Nat. Rev. Genet. 2006;7:929–939. doi: 10.1038/nrg1984. [DOI] [PubMed] [Google Scholar]
- Meldrum R.J, Griffiths J.K, Smith R.M.M, Evans M.R. The seasonality of human Campylobacter infection and Campylobacter isolates from fresh, retail chicken in Wales. Epidemiol. Infect. 2004;133:49–52. doi: 10.1017/S0950268804003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D.G, Fearnley C. Sources of campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003;69:4343–4351. doi: 10.1128/AEM.69.8.4343-4351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylen G, et al. The seasonal distribution of Campylobacter infection in nine European countries and New Zealand. Epidemiol. Infect. 2002;128:383–390. doi: 10.1017/S0950268802006830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- Patrick M.E, Christiansen L.E, Waino M, Ethelberg S, Madsen H, Wegener H.C. Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Appl. Environ. Microbiol. 2004;70:7474–7480. doi: 10.1128/AEM.70.12.7474-7480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson A.D, Greenwood M, Healing T.D, Rollins D, Shahamat M, Donaldson J, Colwell R.R. Colonization of broiler chickens by waterborne Campylobacter jejuni. Appl. Environ. Microbiol. 1993;59:987–996. doi: 10.1128/aem.59.4.987-996.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen L, Nielsen E.M, On S.L.W. Serotype and genotype diversity and hatchery transmission of Campylobacter jejuni in commercial poultry flocks. Vet. Microbiol. 2001;82:141–154. doi: 10.1016/S0378-1135(01)00382-0. [DOI] [PubMed] [Google Scholar]
- Preston A, Murphy L. Movement of broiler chickens reared in commercial conditions. Br. Poult. Sci. 1989;30:519–532. doi: 10.1080/00071668908417176. [DOI] [PubMed] [Google Scholar]
- Renshaw E. Cambridge University Press; Cambridge, UK: 1991. Modelling biological populations in space and time. [Google Scholar]
- Ring M, Zychowska M.A, Stephan R. Dynamics of Campylobacter spp. spread investigated in 14 broiler flocks in Switzerland. Avian Dis. 2005;49:390–396. doi: 10.1637/7319-010305R1.1. [DOI] [PubMed] [Google Scholar]
- Ringoir D.D, Korolik V. Colonisation phenotype and colonisation potential differences in Campylobacter jejuni strains in chickens before and after passage in vivo. Vet. Microbiol. 2003;92:225–235. doi: 10.1016/S0378-1135(02)00378-4. [DOI] [PubMed] [Google Scholar]
- Rivoal K, Denis M, Salvat G, Colin P, Ermel G. Molecular characterization of the diversity of Campylobacter spp. isolates collected from a poultry slaughterhouse: analysis of cross-contamination. Lett. Appl. Microbiol. 1999;29:370–374. doi: 10.1046/j.1472-765X.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Sahin O, Morishita T, Zhang Q. Campylobacter colonization in poultry: sources of infection and modes of transmission. Anim. Health Res. Rev. 2002;3:95–105. doi: 10.1079/AHRR200244. [DOI] [PubMed] [Google Scholar]
- Sahin O, Luo N, Huang S, Zhang Q. Effect of campylobacter-specific maternal antibodies on Campylobacter jejuni colonization in young chickens. Appl. Environ. Microbiol. 2003;69:5372–5379. doi: 10.1128/AEM.69.9.5372-5379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders N.J, Moxon E.R, Gravenor M.B. Mutation rates: estimating phase variation rates when fitness differences are present and their impact on population structure. Microbiology. 2003;149:485–495. doi: 10.1099/mic.0.25807-0. [DOI] [PubMed] [Google Scholar]
- Shanker S, Lee A, Sorrell T. Campylobacter jejuni in broilers: the role of vertical transmission. J. Hyg. 1986;96:153–159. doi: 10.1017/s002217240006592x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker S, Lee A, Sorrell T. Experimental colonization of broiler chicks with Campylobacter jejuni. Epidemiol. Infect. 1988;100:27–34. doi: 10.1017/s0950268800065523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanker S, Lee A, Sorrell T. Horizontal transmission of Campylobacter jejuni amongst broiler chicks: experimental studies. Epidemiol. Infect. 1990;104:101–110. doi: 10.1017/s0950268800054571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreeve J.E, Toszeghy M, Pattison M, Newell D.G. Sequential spread of campylobacter infection in a multipen broiler house. Avian Dis. 2000;44:983–988. doi: 10.2307/1593076. [DOI] [PubMed] [Google Scholar]
- Stanley K, Wallace J, Currie J, Diggle P, Jones K. Seasonal variation of thermophilic campylobacters in lambs at slaughter. J. Appl. Microbiol. 1998a;84:1111–1116. doi: 10.1046/j.1365-2672.1998.00450.x. [DOI] [PubMed] [Google Scholar]
- Stanley K.N, Wallace J.S, Currie J.E, Diggle P.J, Jones K. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J. Appl. Microbiol. 1998b;85:472–480. doi: 10.1046/j.1365-2672.1998.853511.x. [DOI] [PubMed] [Google Scholar]
- Stern N.J, Bailey J.S, Blankenship L.C, Cox N.A, McHan F. Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 1988;80:330–334. doi: 10.2307/1590822. [DOI] [PubMed] [Google Scholar]
- Stern N.J, Myszewski M.A, Barnhart H.M, Dreesen D.W. Flagellin A gene restriction fragment length polymorphism patterns of Campylobacter spp. isolates from broiler production sources. Avian Dis. 1997;32:899–905. doi: 10.2307/1592344. [DOI] [PubMed] [Google Scholar]
- Stern N, Cox N, Bailey J, Berrang M, Musgrove M. Comparison of mucosal competitive exclusion and competitive exclusion treatment to reduce Salmonella and Campylobacter spp. colonization in broiler chickens. Poult. Sci. 2001a;41:156–160. doi: 10.1093/ps/80.2.156. [DOI] [PubMed] [Google Scholar]
- Stern N, Cox N, Musgrove M. Incidence and levels of campylobacter in broilers after exposure to an inoculated seeder bird. J. Appl. Poult. Res. 2001b;10:315–318. [Google Scholar]
- Thattai M, van Oudenaarden A. Stochastic gene expression in fluctuating environments. Genetics. 2004;167:523–530. doi: 10.1534/genetics.167.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L, Long K, Good R, Panaccio M, Widders P. Genotypic diversity among Campylobacter jejuni isolates in a commercial broiler flock. Appl. Environ. Microbiol. 1997;63:1874–1877. doi: 10.1128/aem.63.5.1874-1877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Giessen A, Mazurier S.I, Jacobs-Reitsma W, Jansen W, Berkers P, Ritmeester W, Wernars K. Study on the epidemiology and control of Campylobacter jejuni in poultry broiler flocks. Appl. Environ. Microbiol. 1992;58:1913–1917. doi: 10.1128/aem.58.6.1913-1917.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wielen P.W.J.J, Biesterveld S, Notermans S, Hofstra H, Urlings B.A.P, van Knapen F. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000;66:2536–2540. doi: 10.1128/AEM.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gerwe T.J.W.M, Bouma A, Jacobs-Reitsma W.F, van den Broek J, Klinkenberg D, Stegeman J.A, Heesterbeek J.A.P. Quantifying transmission of Campylobacter spp. among broilers. Appl. Environ. Microbiol. 2005;71:5765–5770. doi: 10.1128/AEM.71.10.5765-5770.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overbeke I, Duchateau L, De Zutter L, Albers G, Ducatelle R. A comparison survey of organic and conventional broiler chickens for infectious agents affecting health and food safety. Avian Dis. 2006;50:196–200. doi: 10.1637/7448-093005R.1. [DOI] [PubMed] [Google Scholar]
- Vereecken K.M, Dens E.J, Van Impe J.F. Predictive modeling of mixed microbial populations in food products: evaluation of two-species models. J. Theor. Biol. 2000;205:53–72. doi: 10.1006/jtbi.2000.2046. [DOI] [PubMed] [Google Scholar]
- Volterra V, Brelot M. Gauthier-Villars; Paris, France: 1931. Leçons sur la théorie mathématique de la lutte pour la vie. [Google Scholar]
- Wahl L.M, Gerrish P.J. The probability that beneficial mutations are lost in populations with periodic bottlenecks. Evolution. 2001;55:2606–2610. doi: 10.1554/0014-3820(2001)055%5B2606:TPTBMA%5D2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wallace J, Stanley K, Currie J, Diggle P, Jones K. Seasonality of thermophilic campylobacter populations in chickens. J. Appl. Microbiol. 1997;82:219–224. doi: 10.1046/j.1365-2672.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- Wassenaar T.M, Geilhausen B, Newell D.G. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl. Environ. Microbiol. 1998;64:1816–1821. doi: 10.1128/aem.64.5.1816-1821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearing H, Rohani P, Keeling M.J. Appropriate models for the management of infectious diseases. PLoS Med. 2005;2:e174. doi: 10.1371/journal.pmed.0020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles S. Opinion: Modelling infectious disease: time to think outside the box? Nat. Rev. Microbiol. 2006;4:307–312. doi: 10.1038/nrmicro1386. [DOI] [PubMed] [Google Scholar]
- Willis W, Murray C, Talbott C. Campylobacter isolation trends of cage versus floor broiler chickens: a one-year study. Poult. Sci. 2002;81:629–631. doi: 10.1093/ps/81.5.629. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Miller H, Honhold N. FMD control strategies. Vet. Rec. 2006;158:706–707. doi: 10.1136/vr.158.20.706-a. [DOI] [PubMed] [Google Scholar]
- Young C.R, Ziprin R.L, Hume M, Stanker L. Dose response and organ invasion of day-of-hatch leghorn chicks by different isolates of Campylobacter jejuni. Avian Dis. 1999;43:763–767. doi: 10.2307/1592745. [DOI] [PubMed] [Google Scholar]