Abstract
Background
The diagnosis of mild cognitive impairment (MCI) is clinically unhelpful, as many patients with MCI develop dementia but many do not.
Objective
To identify clinical instruments easily applicable in the clinical routine that might be useful to predict progression to dementia in patients with MCI assessed in the outpatient facility of a memory clinic.
Participants and methods
52 dementia‐free patients (mean (standard deviation) age 70 (6) years; 56% women) with MCI, and 65 healthy controls (age 69 (6) years; 54% women) underwent brain magnetic resonance scan with standardised visual assessment of medial temporal atrophy (MTA) and subcortical cerebrovascular lesions (SVLs). Follow‐up assessment occurred 15.4 (SD 3.4) months after baseline to detect incident dementia and improvement, defined as normal neuropsychological performance on follow‐up.
Results
Patients were classified into three groups according to the presence of memory disturbance only (MCI Mem), other neuropsychological deficits (MCI Oth) or both (MCI Mem+). MCI Mem and Mem+ showed MTA more frequently (31% and 47% v 5% and 14% of controls and MCI Oth, p<0.001). 11 patients developed dementia (annual rate 16.5%) and 7 improved on follow‐up. The only independent predictor of progression was MTA (odds ratio (OR) 7.1, 95% confidence interval (CI) 1.4 to 35.0), whereas predictors of improvement were the absence of memory impairment (OR 18.5, 95% CI 2.0 to 171.3) and normal MRI scan (OR 10.0, 95% CI 1.7 to 60.2).
Conclusion
Neuropsychological patterns identify groups of patients with MCI showing specific clinical features and risk of progression to dementia. MTA clinically rated with a visual scale is the most relevant predictor of progression and improvement.
The concept of mild cognitive impairment (MCI) was originally coined by Petersen et al1 to capture the preclinical stages of Alzheimer's dementia. It is known that the formation of the neuropathological lesions of Alzheimer's disease occurs decades before the first clinical symptoms.2 Memory impairment is typically the initial presentation of Alzheimer's disease, suggesting that memory disturbance in elderly people may be a sign of the transition phase between normal ageing and Alzheimer's disease.1 However, not all patients with MCI deteriorate.3 Available data suggest that the likelihood of a patient with MCI developing Alzheimer's disease in the long term is around 50%, thus making the diagnosis of MCI in itself clinically unhelpful.4
Pathological and clinical data indicate that some indicators of Alzheimer's disease might be used to distinguish those patients with MCI who will progress from those who will not. Some of these indicators, such as hippocampal volumetry,5 τ and aβ proteins in the cerebrospinal fluid,6 and functional defects in the temporoparietal and posterior cingulate cortex,7 require technologically advanced tools and specific competences and are applicable only in specialised centres. Other markers of progression have been proposed, such as the involvement of multiple cognitive domains besides memory,8 or medial temporal lobe atrophy (MTA) on magnetic resonance imaging (MRI).9 These are more easily assessed in a non‐specialised clinical setting and could be implemented in second level centres.
Our study aimed to identify clinical instruments, easily applicable in the clinical routine, that might be useful to predict progression to dementia in patients with MCI.
Methods
Subjects
Patients were taken from a prospective project on the natural history of MCI (Mild Cognitive Impairment in Brescia) carried out in the outpatients' section of our memory clinic (Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Scientific Institute for Research and Care Brescia, Italy). Inclusion criteria were: (a) memory or other cognitive disturbances; (b) Mini‐Mental State Examination (MMSE) score of ⩾24; and (c) spared basic and instrumental activities of daily living or impairment stably due to causes other than cognitive impairment. Exclusion criteria were (a) dementia according to the Diagnostic Statistical Manual for Mental Disorders‐fourth edition (DSM‐IV) criteria; (b) age ⩾90 years; (c) depression or psychosis of juvenile onset; and (d) history or neurological signs of major stroke. The study protocol was approved by the local ethics committee and all participants signed an informed participation consent.
From April 2002 to March 2005, 52 patients were evaluated and had at least a 1‐year follow‐up assessment as of December 2004 (mean (SD) time to follow‐up 15.4 (3.4) months). At baseline all patients underwent: (a) a semistructured interview; (b) a neurological examination; (c) a neuropsychological battery of tests assessing memory, executive and frontal functions, language and visuoconstructional abilities; and (d) an assessment of depressive symptoms (Center for Epidemiologic Studies Depression Scale10).
MCI subgroups
Memory disturbance was defined as scoring below the 10th centile of the age‐specific, sex‐specific and education‐specific distribution in at least one memory test. Cognitive disturbances other than memory deficit were defined as performance below the 10th centile in any non‐memory test. Patients were classified into three groups on the basis of neuropsychological test results: 8 (25%) had isolated memory disturbance (MCI Mem); 17 (33%) did not have memory disturbances but had impairment in at least one non‐memory domain (MCI Oth); and 27 (52%) had impairment in both memory and at least another neuropsychological domain (MCI Mem+).
Progression to dementia was diagnosed according to clinical diagnostic criteria.11,12,13 Changes on clinical assessment between baseline and follow‐up investigations (ΔMMSE (change in MMSE), ΔCDR (change in Clinical Dementia Rating) sum of boxes and ΔiADL (change in instrumental activities of daily living) functions lost were computed as the difference between baseline and follow‐up assessments. Patients were considered to have improved on follow‐up when the neuropsychological deficits were no longer evident.
Controls
Controls were healthy people enrolled in a multicentre multiscanner study on normal brain structure with magnetic resonance imaging (MRI) whose design has been described elsewhere in detail.14 The 65 subjects who were scanned with the same scanner as the patients with MCI and were aged ⩾60 years were selected for the present study.
MRI of the brain
Patients and controls underwent MRI with a standardised protocol.14 To assess MTA and SVLs, a single rater blinded to the clinical data visually assessed digital MRI images, as described elsewhere.15,16,17 MTA was considered to be present when the Scheltens Scale Score was 3 or 4 on at least one side; subcortical vascular disease when the Age‐Related White Matter Changes Scale total score was ⩾6, or when the beginning of confluence of lesions (score 2) was observed in at least one region. Scans were defined as normal when MTA was 0–1 and subcortical vascular disease was absent.
Data analysis
Data were analysed using SPSS V.12. Univariate group differences were analysed by t tests or analysis of variance test for continuous variables and χ2 tests for categorical data.
On follow‐up evaluation, the primary outcome of interest was progression to dementia. The predictive accuracies for dementia of the clinical variables that had different results in progressed and non‐progressed MCI were assessed by univariate logistic regression analyses, with progression to dementia as dependent variable. Variables significantly predicting progression in the univariate model were assessed in a multivariate logistic regression model. The same procedure was followed to assess the predictors of improvement. Diagnostic accuracy was defined as the proportion of patients correctly classified. To confirm the association of the main predictors with disease progression, less restrictive markers of cognitive deterioration were considered; two multiple linear regression models were built, with MMSE or Auditory Verbal Learning delayed recall score at follow‐up as the dependent variable, MMSE or Auditory Verbal Learning delayed recall score at the baseline, MTA, MCI subgroup and normal MRI scan as independent variables.
Results
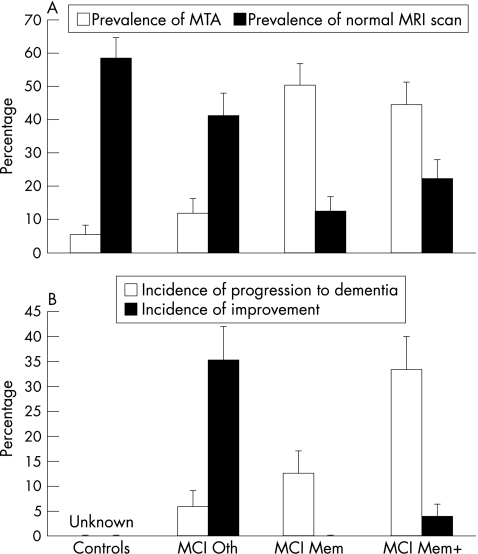
MCI Mem+ patients were older than MCI Oth patients. The mean (SD) age was 69 (6) years in the control group, 66 (5) years in the MCI Oth group, 72 (5) years in the MCI Mem group and 71 (7) years in the MCI Mem+ group (p = 0.03). There were more women in the MCI Oth group than in the other groups. The percentage of women was 54% in the control group, 82% in the MCI Oth group, 25% in the MCI Mem group and 48% in the MCI Mem+ group (p = 0.04). MCI groups with memory disturbance were significantly different from controls on measures of global cognition, more frequently had MTA of moderate to severe degree (p<0.001; see fig 1) and less frequently had normal MRI scan (p<0.01; see fig 1) than both controls and MCI Oth groups. The mean (SD) MMSE was 28.8 (1.2) in the control group, 27.8 (1.6) in the MCI Oth group, 26.6 (2.3) in the MCI Mem group and 27.0 (1.5) in the MCI Mem+ group (p<0.001). The mean (SD) CDR sum of boxes was 0.19 (0.53) in the control group, 0.79 (0.81) in the MCI Oth group, 1.25 (0.53) in the MCI Mem group and 1.11 (0.73) in the MCI Mem+ group (p<0.001).
Figure 1 Prevalence of medial temporal atrophy (MTA) and normal magnetic resonance imaging (MRI) scan and incidence of progression and improvement in controls (n = 65) and patients with mild cognitive impairment with non‐memory deficits (MCI Oth, n = 17), isolated memory deficit (MCI Mem, n = 8) and memory associated with non‐memory deficit (MCI Mem+, n = 27).
Eleven patients progressed to dementia (16.5%/year): three had probable Alzheimer's disease, four had possible Alzheimer's disease with cerebrovascular disease, and four had other forms of dementia (one subcortical vascular dementia and three possible dementia with Lewy bodies). The incidence of progression was highest in the MCI Mem+ group and lowest in the MCI Oth group (fig 1). Patients who had progressed to dementia were not different from those who had not progressed for most variables (table 1), but more often had multiple domain neuropsychological impairment and MTA of moderate to severe degree at the baseline (table 1); these patients showed significantly greater loss in cognitive (ΔMMSE −0.27 (2.09) v −2.54 (2.84), p = 0.005; ΔCDR sum of boxes 0.27 (1.74) v 2.59 (1.20), p<0.001;? and functional (ΔiADLs functions lost 0.13 (0.56) v 1.36 (1.36), p = 0.01) abilities in the follow‐up assessment. In bivariate logistic regression models with progression to dementia as outcome variable, only the presence of a “multiple domain” neuropsychological pattern (being in MCI Mem+ group at baseline) and that of a MTA of moderate to severe degree were associated with a higher rate of progression (table 1). Only MTA survived as an independent predictor of progression in a multivariate logistic regression analysis (diagnostic accuracy 0.75; sensitivity and specificity 0.73 and 0.76, respectively; positive and negative predictive values 0.44 and 0.91, respectively). Linear regression analysis showed that only MTA was also associated with change on global cognition (MMSE score) between baseline and follow‐up (B = −1.44; p = 0.01) but not with change on memory performance (delayed recall of the Auditory Verbal Learning).
Table 1 Predictors of progression in 11 patients with mild cognitive impairment (MCI) who had progressed and 41 patients who had not progressed to dementia, and predictors of improvement in 7 patients with MCI who had improved and 45 patients who had not improved in the follow‐up evaluation.
| Progressed to dementia | Not progressed to dementia | OR (95% CI) | ||
|---|---|---|---|---|
| n = 11 | n = 41 | Bivariate | Multivariate | |
| Sociodemographics | ||||
| Age | 70 (4) | 70 (7) | 1.0 (0.9 to 1.1) | – |
| Sex (females) | 7 (64%) | 22 (54%) | 0.7 (0.2 to 2.6) | – |
| Education | 8 (5) | 7 (4) | 1.1 (0.9 to 1.2) | – |
| Mental features | – | |||
| Duration of cognitive symptoms | 33 (21) | 33 (39) | 1.0 (0.9 to 1.0) | – |
| Mini‐Mental State Examination | 27.1 (1.7) | 27.2 (1.7) | 1.0 (0.6 to 1.4) | – |
| CDR sum of boxes | 1.41 (0.77) | 0.93 (0.70) | 2.5 (0.9 to 6.2) | – |
| Depressive symptoms (CES‐D) | 18.9 (8.5) | 15.6 (7.6) | 1.1 (0.9 to 1.1) | – |
| MCI group* | ||||
| Oth | 1 (6%) | 16 (94%) | Ref | Ref |
| Mem | 1 (13%) | 7 (87%) | 2.3 (0.1 to 42.0) | 1.0 (0.1 to 21.5) |
| Mem+ | 9 (33%) | 18 (67%) | 7.6 (1.0 to 70.2) | 4.6 (0.5 to 45.2) |
| Imaging features | ||||
| Subcortical vascular disease† | 6 (55%) | 12 (29%) | 2.9 (0.7 to 11.4) | – |
| Medial temporal atrophy‡ | 8 (73%) | 10 (24%) | 8.3 (1.8 to 37.3) | 7.1 (1.4 to 35.0) |
| Normal MRI scan§ | 0 (0%) | 14 (34%) | ≈0¶ | – |
| Improved | Not improved | OR (95% CI) | ||
|---|---|---|---|---|
| n = 7 | n = 45 | Bivariate | Multivariate | |
| Sociodemographics | ||||
| Age | 67 (6) | 70 (6) | 0.9 (0.8 to 1.1) | – |
| Sex (females) | 6 (86%) | 23 (51%) | 0.2 (0.1 to 1.6) | – |
| Education | 5.1 (1.5) | 7.7 (4.1) | 0.7 (0.4 to 1.1) | – |
| Mental features | – | |||
| Duration of cognitive symptoms | 22 (15) | 34 (37) | 1.0 (0.9 to 1.0) | – |
| Mini‐Mental State Examination | 28.1 (1.1) | 27.0 (1.7) | 1.8 (0.9 to 3.8) | – |
| CDR sum of boxes | 0.43 (0.45) | 1.12 (0.73) | 0.1 (0.4 to 16.2) | – |
| Depressive symptoms (CES‐D) | 12.4 (4.1) | 16.9 (8.1) | 0.9 (0.8 to 1.1) | – |
| MCI group** | ||||
| Oth | 6 (65%) | 16 (35%) | 18.5 (2.0 to 171.3) | 15.9 (1.1 to 60.5) |
| Mem | 0 (0%) | 8 (100%) | Ref | Ref |
| Mem+ | 1 (4%) | 26 (96%) | ||
| Imaging features | ||||
| Subcortical vascular disease† | 0 (0%) | 18 (40%) | ≈0§§ | – |
| Medial temporal atrophy‡ | 0 (0%) | 18 (40%) | ≈0§§ | – |
| Normal MRI scan§ | 5 (71%) | 9 (20%) | 10.0 (1.7 to 60.2) | 8.3 (1.6 to 162.2) |
ARWMC, Age‐related White Matter Changes; CES‐D, Center for Epidemiologic Studies Depression Scale ; CDR, Clinical Dementia Rating; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; MTA, medial temporal atrophy.
Patients were followed for 14.6 (5.5) and 15.6 (2.6) months (progressed and non‐progressed MCI, p = 0.35), and for 15.6 (2.3) and 15.4 (3.5) months (improved and non‐improved MCI, p = 0.83).
*Progression: in the logistic models, the three MCI groups were analysed as dummy variables. The independent variables in the multivariate model were MCI group and MTA. The presence of memory disturbance only (MCI Mem or MCI Mem+) did not predict progression in the univariate logistic regression model (OR 0.2, 95% CI 0.1 to 7.4).
†Age‐Related White Matter Changes (ARWMC) Scale total score ⩾6 or beginning of confluence of lesions (score 2) in at least one region.
‡% Scheltens' Scale score of 3 or 4.
§ARWMC Scale total score ⩽5; only focal lesions of score 0 or 1 in all regions, and Scheltens' Scale score of 0 or 1 on both sides. OR resulted from univariate (left column) and multivariate (right column, only for the variables significantly predicting progression or improvement in univariate models) logistic regression analyses, with progression to dementia (first part of the table) or improvement (second part of the table) as dependent variable.
¶The ORs and the 95% CIs could not be computed owing to zero numerator.
**Improvement: the two MCI groups showing memory disturbance (MCI Mem and MCI Mem+) were collapsed and analysed as a single group. The independent variables in the multivariate model were the MCI group (with or without memory disturbance) and normal MRI scan.
Seven patients did not show neuropsychological deficits on follow‐up. They were mainly in the MCI Oth group and had normal scans. In bivariate and multivariate logistic regression models with improvement on follow‐up as outcome variable, both these features were associated with clinical improvement (diagnostic accuracy 0.77; sensitivity and specificity 0.86 and 0.76, respectively; positive and negative predictive values 0.35 and 0.97, respectively).
Discussion
Our data underline the importance of a multidisciplinary approach in the assessment of patients with MCI.
Neuropsychological features alone are useful in classifying patients with MCI, but have only limited power in predicting progression to dementia. In our MCI group, impairment of memory and other neuropsychological domains was significantly more represented in the progressing group. This is in agreement with several longitudinal clinical studies reporting that multiple domain neuropsychological impairment was a better predictor of Alzheimer's disease or dementia than memory disturbance alone,8 and suggesting that the closer the cognitive impairment is to the neuropsychological pattern of Alzheimer's disease, the greater is the likelihood of a patient with MCI progressing to dementia.
The significance of MCI without memory impairment remains unclear. Our MCI Oth group was constituted mainly of relatively young women with more depressive symptoms and global cognition intermediate between controls and MCI Mem+, neuroimaging features and a rate of progression similar to that of controls, with improvement over time in one of three cases. These patients could represent either the earliest stage of dementia or the neuropsychological manifestation of a sub‐threshold depressive disturbance.
In the most advanced studies aimed at detecting the biological “fingerprint” of Alzheimer's disease in patients with MCI, accuracy in the prediction of progression increases when biological markers are combined.4 In the present study, when neuropsychological profile and MTA were considered together, only the neuroimaging marker survived as an independent predictor of progression. It should be noted that less than half of our patients developed probable Alzheimer's disease. Although neuropsychological profiles in the preclinical stage of non‐Alzheimer's disease dementias can be heterogeneous, almost all share some degree of involvement of the medial temporal lobe, although this is more marked in Alzheimer's disease and less in other kinds of dementia.18,19 Therefore, MTA in our group could be a better predictor of dementia than memory disturbance.
Conclusion
In conclusion, cognitive impairment of a mild degree in patients referred to a memory clinic can represent the preclinical stage of different dementias. MTA is the single most relevant objective predictor of progression to dementia, although its absence together with the absence of subcortical vascular disease and memory disturbance predict improvement on follow‐up. The small sample size and the short follow‐up time of this study could partly account for the low positive predictive value of our outcome variables for dementia. Observations on larger groups of patients with longer follow‐ups will be needed to confirm the usefulness of such simple clinical markers.
Abbreviations
CDR - Clinical Dementia Rating
MCI - mild cognitive impairment
MCI Mem - MCI with isolated memory deficit
MCI Mem+ - MCI memory associated with non‐memory deficit
MCI Oth - mild cognitive impairment with non‐memory deficits
MMSE - Mini‐Mental State Examination
MTA - medial temporal atrophy
SVL - subcortical cerebrovascular lesion
Footnotes
Competing interests: None declared.
Institutional sites: www.irccs‐fatebenefratelli.it/www.centroAlzheimer.it
References
- 1.Petersen R C, Doody R, Kurz A.et al Current concepts in mild cognitive impairment. Arch Neurol 2001581985–1992. [DOI] [PubMed] [Google Scholar]
- 2.Smith A D. Imaging the progression of Alzheimer pathology through the brain. Proc Natl Acad Sci USA 2002994135–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett D A, Wilson R S, Schneider J A.et al Natural history of mild cognitive impairment in older persons. Neurology 200259198–205. [DOI] [PubMed] [Google Scholar]
- 4.Frisoni G B, Padovani A, Wahlund L O. The predementia diagnosis of Alzheimer disease. Alzheimer Dis Assoc Disord 20041851–55. [DOI] [PubMed] [Google Scholar]
- 5.Jack C R, Jr, Petersen R C, Xu Y C.et al Prediction of AD with MRI‐based hippocampal volume in mild cognitive impairment. Neurology 1999521397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riemenschneider M, Lautenschlager N, Wagenpfeil S.et al Cerebrospinal fluid tau and beta‐amyloid 42 proteins identify Alzheimer disease in subjects with mild cognitive impairment. Arch Neurol 2002591729–1734. [DOI] [PubMed] [Google Scholar]
- 7.Chetelat G, Desgranges B, De La Sayette V.et al Mild cognitive impairment: can FDG‐PET predict who is to rapidly convert to Alzheimer's disease? Neurology 2003601374–1377. [DOI] [PubMed] [Google Scholar]
- 8.Bozoki A, Giordani B, Heidebrink J L.et al Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol 200158411–416. [DOI] [PubMed] [Google Scholar]
- 9.Visser P J, Verhey F R J, Hofman P A M.et al Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry 200272491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radloff L S. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas 19771385–401. [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 12.Erkinjuntti T, Inzitari D, Pantoni L.et al Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl 20005923–30. [DOI] [PubMed] [Google Scholar]
- 13.McKeith I G, Ballard C G, Perry R H.et al Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology 2000541050–1058. [DOI] [PubMed] [Google Scholar]
- 14.Riello R, Sabattoli F, Beltramello A.et al Brain volumes in healthy adults aged 40 years and over: a voxel‐based morphometry study. Aging Clin Exp Res 200517329–336. [DOI] [PubMed] [Google Scholar]
- 15.Bresciani L, Rossi R, Testa C.et al Visual assessment of medial temporal atrophy on MR films in Alzheimer's disease: comparison with volumetry. Aging Clin Exp Res 2005178–13. [DOI] [PubMed] [Google Scholar]
- 16.Scheltens P, Leys D, Barkhof F.et al Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 199255967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahlund L O, Barkhof F, Fazekas F.et al European Task Force on Age‐Related White Matter Changes. A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke 2001321318–1322. [DOI] [PubMed] [Google Scholar]
- 18.Tam C W, Burton E J, McKeith I G.et al Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology 200564861–865. [DOI] [PubMed] [Google Scholar]
- 19.Du A T, Schuff N, Chao L L.et al White matter lesions are associated with cortical atrophy more than entorhinal and hippocampal atrophy. Neurobiol Aging 200526553–559. [DOI] [PubMed] [Google Scholar]