Abstract
Background: Camptocormia, characterised by extreme forward flexion of the thoracolumbar spine and severe stooping in the supine position, seems to be prevalent in Parkinson's disease. Objective: The aim of this study was to identify features of parkinsonian camptocormia and to describe the main clinical characteristics of patients with Parkinson's disease who develop the condition. Methods: An extensive range of clinical, biochemical and imaging data were gathered for 23 patients with Parkinson's disease with camptocormia, notably including magnetic resonance imaging (MRI) of the brain and spine, electromyographic recordings of the paravertebral muscles and muscle biopsies. Results: Camptocormia occurred in severe Parkinson's disease with axial predominance, motor fluctuations and dysautonomic symptoms. The condition was often associated with spondyloarthritic changes and pain. MRI showed paraspinal muscle signal abnormalities in five patients and fatty involution in seven patients. The seven patients had motor unit reductions on the spinal erector electromyogram. The MRI results for the girdle muscles were normal. Cranial MRI showed signal abnormalities for the basal ganglia in three patients. Discussion: Various mechanisms may contribute to the development of parkinsonian camptocormia: dopaminergic depletion in Parkinson's disease induces functional changes in the organisation of the corticospinal and reticulospinal tracts, where dysfunction could contribute to axial rigidity. Furthermore, rigidity of the spinal flexion muscles could lead to under‐use of the spinal extension muscles, which become progressively atrophic. Rigidity may also induce spinal deformations, leading to a neurogenic syndrome via compression of the spinal nerves. Conclusion: The screening and early management of camptocormia in Parkinson's disease is likely to be important for preventing axial disorders and spinal deformations.
Forward flexion of the trunk is a typical symptom of Parkinson's disease and, indeed, was identified by James Parkinson1 when he first described the disease in 1817. Camptocormia (known as “bent spine syndrome”) corresponds to extreme forward flexion of the thoracolumbar spine. The condition—whose name is derived from the Greek words kamptos (“to bend”) and kormos (“trunk”)—was first described by Earle in 18152 and by Brodie in 1837.3 It is characterised by pronounced flexion of the trunk and limited extension in the erect position, with reduced flexion in the supine position. In the most severe cases, patients are literally “doubled over”, causing considerable discomfort and handicap.
The condition's principal organic aetiologies include affections of the central and peripheral nervous system (of which the most common are Parkinson's disease and amyotrophic lateral sclerosis) and vascular, lenticular lesions.4 Other rarer aetiologies5,6 include muscle disorders (muscular dystrophies and myopathies, notably amyloid myopathy7), an adverse effect of valproic acid8 and rheumatological causes (such as spinal stenosis and vertebral infections). Psychiatric causes have also been described, with camptocormia resulting from a psychogenic conversion reaction.9
Although this deformation has long been known in Parkinson's disease, few formal observations have been reported in the literature. Camptocormia in Parkinson's disease was first studied by Djaldetti et al,10 who considered it to be a rare type of dystonia or an extreme form of rigidity. A form of myopathy limited to the trunk extensor muscles was subsequently discussed.11,12 However, such reports have been limited to very small samples and thus incomplete examination of the included patients. The goal of this study was to (i) provide a more comprehensive description of the characteristics of patients with Parkinson's disease who develop camptocormia; (ii) identify the features of parkinsonian camptocormia; and (iii) discuss various hypotheses for the pathogenesis of this condition.
Patients and methods
Patients
The inclusion criteria were Parkinson's disease according to the Gelb criteria13 and a totally or partially reducible forward flexion, because of the frequency of the fixed rachis deviation in our population. We excluded patients with a possible or probable multiple system atrophy according to the Gilman criteria, with another atypical parkinsonian syndrome, with isolated head drop syndrome and non‐reducible spine flexion. During the period from October 2003 to September 2004, we examined about 700 patients with Parkinson's disease in our department and recruited 23 consecutive patients with the diagnosis of Parkinson's disease and the presence of severe, reducible, forward flexion of the thoracolumbar spine, giving a hypothetic prevalence of about 3%, which required confirmation on a larger prospective epidemiological study.
Methods
Clinical examination
The following information was collected: age, sex, medical history; current treatment; dates of onset of Parkinson's disease symptoms and camptocormia; motor fluctuations; autonomic disorders; back pain; variations in trunk flexion during the day and as a function of Parkinson's disease treatment. All patients were evaluated by a neurologist (ACL or DD) using the Unified Parkinson's Disease Rating Scale (UPDRS) part III and axial scores, and the Mini‐Mental State Examination.
The patients were also evaluated by a physiotherapist (AB‐D or VP) with respect to their postural disorders and to discuss whether the use of a corset was desirable. The following data were also gathered: the maximal joint extension (for hips, knees and ankles) and the strength of the paraspinal muscles. The Spinal Mouse electronic measuring device (Aditus System Inc, Loguna Niguel, California, USA) was used to provide geometric data on spine and hip tilt, as it has been designed to measure sagittal back shape and mobility. The Spinal Mouse is run along the spinal column of the patient, with the measuring wheels tracking the contour of the back. A scientifically proven algorithm reliably converts the raw data into several clinically relevant parameters. Here, we used the device for assessment of pelvic tilt (inclination relative to the vertical plane) and then compared the measurements of thoracic kyphosis and lumbar lordosis with those determined by spinal radiography. Statistical analyses were carried out using non‐parametric Spearman's correlation coefficients. Unless otherwise stated, values are presented as the mean (standard deviation (SD)).
Additional investigations
The vertebral axis was studied using cervical and thoracolumbar radiography. The thoracolumbar spinal muscles and the girdle muscles were studied using magnetic resonance imaging (MRI) by the same radiologist (AC) for all patients. Cranial MRI was also carried out, notably to study the basal ganglia.
Electromyographic recording of the thoracolumbar spinal erector muscle was carried out by the same neurophysiologist (J‐FH) for all patients.
Clinical biochemistry investigations included serum creatine kinase, C reactive protein, sedimentation rate, phosphate and calcium, thyroid function and immunological (antinuclear antibodies) tests.
Accessory salivary gland biopsies were carried out to screen for possible amyloid or inflammatory abnormalities. A muscle biopsy was carried out on the erector spinal muscles when an abnormal MRI signal was observed.
Results
Clinical characteristics of patients with Parkinson's disease with camptocormia
Fifteen male and eight female patients were studied. Six patients had a family history of Parkinson's disease, of which three included camptocormia. The patients' mean (SD) age was 68.6 (7.4) and the mean (SD) time since onset of Parkinson's disease symptoms was 10.3 (5.1) years. The Parkinson's disease form, determined by the first symptoms at the onset of the disease, was akinetic‐rigid syndrome in 11 patients and tremor‐associated syndrome in 12 patients. Patients had developed forward flexion of the trunk 5.8 (4) years after the onset of the Parkinson's disease, with the exception of one patient in whom the appearance of camptocormia preceded the diagnosis of Parkinson's disease by 3 years. The form of Parkinson's disease had no influence on the disease duration before the occurrence of camptocormia: of the 11 patients who presented camptocormia ⩽5 years after the onset of Parkinson's disease, four had an akinetic‐rigid syndrome and seven had a tremor‐associated syndrome. Of the 11 patients who developed camptocormia after >5 years of Parkinson's disease, six had an akinetic‐rigid syndrome and five had a tremor‐associated syndrome. Four patients had developed axial akinetic‐rigid syndrome in the year after disease onset.
The drug UPDRS part III score under their usual dopaminergic treatment was 26.7 (10) out of 108 and the axial score was 10.6 (3.2) out of 24. The axial UPDRS:motor UPDRS ratio was 0.40. Thirteen patients (57%) experienced freezing. Fifteen patients (65%) had motor fluctuations that developed 7 (3.6) years after disease onset: of these, 8 patients (34%) had mild to moderate peak‐dose dyskinesia without axial involvement and 4 (17%) had limb dystonia without axial involvement. Twenty two patients (96%) had noticeable autonomic symptoms: seven had orthostatic hypotension and 15 had urinary disorders.
Cognitive function was normal for nine patients (the Mini‐Mental State Examination score was 27.1 (2.4)). Seven patients had mild cognitive impairment, with attention deficit and memory disorders. Six patients had dopaminergic psychosis and one patient had dementia.
All 23 patients received dopaminergic treatment, with an L‐dopa equivalent dose of 939 (516) mg/day (table 1): 6 received L‐dopa only and 15 received L‐dopa in combination with a dopamine agonist. Finally, two patients received a dopamine agonist only. In general, L‐dopa treatment had been introduced 1.9 (2.2) years after the diagnosis of Parkinson's disease. It preceded appearance of camptocormia by 4 (4.3) years in 19 patients and was started 1 year after the appearance of camptocormia in two patients. One patient underwent high‐frequency, chronic, bilateral deep‐brain stimulation of the subthalamic nucleus 4 years after the appearance of camptocormia, without any effect on the condition itself.
Table 1 Characteristics of patients with Parkinson's disease.
| Patient no | Sex | Age (years) | PD duration | Form | Side | Motor UPDRS | Axial subscore | Freezing | Fluctuations | L‐dopa | MMS | L‐dopa equivalent |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 67 | 10 | AR | L | 15 | 9 | 1 | 5 | 2 | 27 | 1900 |
| 2 | F | 74 | 11 | ART | L | 20 | 13 | 0 | — | 2 | 27 | 500 |
| 3 | F | 77 | 12 | ART | L | 41 | 14 | 1 | 6 | 0 | 26 | 800 |
| 4 | M | 57 | 10 | AR | R | 28 | 12 | 1 | 6 | 3 | 30 | 1600 |
| 5 | M | 70 | 7 | AR | R | 50 | 19 | 1 | 1 | 1 | 26 | 1800 |
| 6 | M | 82 | 4 | AR | R | 28 | 10 | 0 | 2 | 1 | — | 750 |
| 7 | F | 76 | 13 | ART | R | 20 | 8 | 0 | 8 | 6 | 27 | 1000 |
| 8 | M | 73 | 18 | ART | L | 34 | 10 | 1 | 12 | 0 | 25 | 1800 |
| 9 | M | 57 | 6 | ART | R | 16 | 6 | 0 | — | — | 27 | 300 |
| 10 | M | 72 | 4 | ART | L | 28 | 10 | 1 | — | 2 | 28 | 150 |
| 11 | M | 60 | 17 | AR | L | 46 | 17 | 1 | 10 | 6 | 28 | 1300 |
| 12 | M | 65 | 14 | AR | L | 31 | 6 | 1 | 5 | 4 | 20 | 800 |
| 13 | F | 67 | 17 | ART | R | 27 | 10 | 1 | 13 | 0 | 28 | 1300 |
| 14 | F | 72 | 19 | ART | L | 15 | 11 | 1 | 5 | 0 | 30 | 850 |
| 15 | M | 61 | 12 | AR | R | 25 | 11 | 1 | 7 | 5 | 30 | 950 |
| 16 | F | 82 | 0 | AR | L | 25 | 8 | 0 | — | — | 29 | 0 |
| 17 | M | 61 | 12 | ART | R | 8 | 7 | 0 | — | 6 | 28 | 1100 |
| 18 | M | 73 | 4 | ART | R | 22 | 11 | 1 | — | 0 | 26 | 1000 |
| 19 | M | 61 | 9 | AR | R | 20 | 10 | 0 | 5 | 0 | 27 | 550 |
| 20 | M | 71 | 6 | AR | L | 23 | 9 | 0 | — | 2 | 30 | 1100 |
| 21 | F | 70 | 15 | AR | R | 26 | 12 | 0 | 13 | 0 | 25 | 800 |
| 22 | M | 60 | 11 | ART | L | 35 | 13 | 1 | 7 | 0 | 29 | 350 |
| 23 | F | 70 | 5 | ART | R | 31 | 8 | 0 | — | 0 | 24 | 900 |
| 15M/8F | 11AR/12ART | 11L/12R | 13/23 | 15/23 | ||||||||
| Mean | 68.6 | 10.3 | 26.7 | 10.6 | 7 | 1.9 | 27.1 | 939 | ||||
| SD | 7.4 | 5.1 | 10 | 3.2 | 3.6 | 2.3 | 2.3 | 516 |
AR, akinetic‐rigid; ART, akinetic‐rigid and tremor; F, female; L, left; M, male; MMS, Mini‐Mental State examination; PD, Parkinson's disease; R, right; UPDRS, Unified Parkinson's Disease Rating Scale.
Durations are presented in years. On‐drug motor UPDRS scores (on 108) and on‐drug axial subscores on 24 (sum of the UPDRS items 18, 27, 28, 29, 30 and 31) are displayed. Freezing of gait was either present (1) or absent (0). “Fluctuations” means the time interval (in years) between PD onset and the appearance of freezing; L‐dopa means the time interval (in years) between PD onset and treatment initiation. L‐dopa equivalent (in mg), equivalent dose of L‐dopa. — indicates that the item is not applicable for the patient in question.
Characteristics of camptocormia
The duration of camptocormia was 4.6 (2.6) years. Eighteen patients developed forward flexion of the trunk over a period >1 year. Four patients reported the rapid appearance of camptocormia in <1 year: in two of these patients, the appearance of the condition seemed to be linked to surgical treatment (abdominal and hip surgery). Finally, one patient reported the acute (overnight) appearance of trunk flexion. Camptocormia fluctuated with dopaminergic treatment in five patients, with an improvement in all the five. In 14 patients, the extent of the stooped posture varied throughout the diurnal cycle and was aggravated by fatigue and stress. No correlation was found between camptocormia duration and L‐dopa treatment duration (table 2, fig 1).
Table 2 Characteristics of camptocormia.
| Patient no | Delay | Duration | Fatigue | Reductibility | Scoliosis | VAS | L‐dopa‐campto | L‐dopa |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 4 | 1 | 1 | 1 | 0 | 4 | 0 |
| 2 | 5 | 6 | 0 | 1 | 1 | 3 | 3 | 0 |
| 3 | 0 | 9 | 1 | 1 | 1 | 8 | 0 | 0 |
| 4 | 3 | 6 | 0 | 1 | 0 | 5 | 0 | 0 |
| 5 | 6 | 1 | 1 | 2 | 0 | 0 | 5 | 1 |
| 6 | 2 | 2 | 1 | 2 | 0 | 0 | −1 | 1 |
| 7 | 9 | 4 | 1 | 1 | 0 | 0 | 3 | 0 |
| 8 | 14 | 4 | 1 | 2 | 1 | 3 | 14 | 1 |
| 9 | 4 | 2 | 1 | 2 | 1 | 2 | — | — |
| 10 | 1 | 3 | 1 | 2 | 1 | ND | −1 | 0 |
| 11 | 13 | 4 | 1 | 1 | 0 | 1 | 7 | 0 |
| 12 | 4 | 10 | 1 | 1 | 1 | 5 | 0 | 1 |
| 13 | 13 | 4 | 0 | 2 | 0 | 7 | 13 | 0 |
| 14 | 11 | 8 | 0 | 1 | 1 | 5 | 11 | 0 |
| 15 | 6 | 5 | 0 | 1 | 1 | 4 | 2 | 1 |
| 16 | — | 3 | 0 | 2 | 1 | 4 | — | — |
| 17 | 7 | 5 | 0 | 1 | 1 | 4 | 1 | 0 |
| 18 | 2 | 2 | 0 | 1 | 0 | 7 | 2 | 0 |
| 19 | 6 | 3 | 1 | 1 | 1 | 5 | 6 | 0 |
| 20 | 5 | 1 | 1 | 1 | 0 | 0 | 3 | 0 |
| 21 | 6 | 9 | 1 | 1 | 1 | 5 | 6 | 0 |
| 22 | 4 | 7 | 1 | 2 | 0 | 6 | 4 | 0 |
| 23 | 1 | 4 | 0 | 1 | 1 | 8 | 1 | 0 |
| 14/23 | 8/23 | 14/23 | 5/21 | |||||
| Mean | 5.8 | 4.6 | 3.7 | 4.0 | ||||
| SD | 4.0 | 2.6 | 2.7 | 4.3 |
All durations are in years. “Interval” indicates the time interval between Parkinson's disease onset and the occurrence of camptocormia. “Duration” indicates the duration of camptocormia. “L‐dopa‐campto” means the time interval between the introduction of L‐dopa and the onset of camptocormia. “L‐dopa” qualifies the influence of L‐dopa on the degree of camptocormia (1, yes; 0, no). “Fatigue” indicates whether fatigue had an influence on the intensity of camptocormia (1, yes; 0, no). Reducibility is rated as 1 (partial) or 2 (total). Scoliosis is rated as 1 (present) or 0 (absent). ND, not determined; VAS, a correction Visual Analogue Scale from 0 (no pain) to 10 (maximal pain); “—”, not applicable for the patient in question.
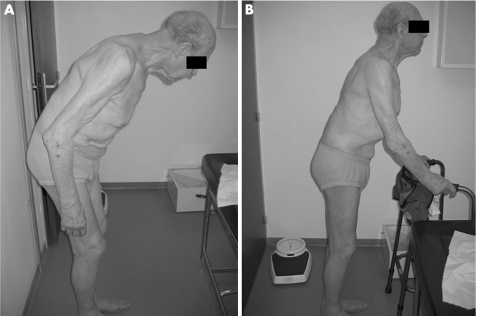
Figure 1 Camptocormia is characterised by (A) an extreme shape of the forward flexion of the thoraco‐lumbar spine in the erect position, (B) which is totally or partially reducible. Informed consent was obtained for publication of this figure.
The range of trunk flexions determined clinically and using the Spinal Mouse varied from 40° in milder cases to 90° in the most severe ones. In 8 of 23 patients, the condition completely disappeared in the supine position. In the other 15 patients, the spine was prevented from completely extending passively or when in the supine position because of permanent spinal deformation and axial rotation. Camptocormia was associated with scoliosis in 14 patients and was pain free in 5 patients. Eighteen patients described low back pain aggravated by sitting, standing and walking. The pain score on a Visual Analogue Scale (where 0 and 10 corresponded to “no pain” and “maximal pain”, respectively) was 3.7 (2.7).
All patients (n = 15) displayed the same clinical pattern of postural disorders, with anteversion of the pelvis (9/15), axial front hypertonia with fixed flexion of the hips (10/15), knees (all 15) and ankles (all 15) and, finally, paresis of the paraspinal muscles (all 15).
Table 3 shows the measurements determined by Spinal Mouse for the 15 patients compared with those determined by spinal radiography: a high degree of intertechnique correlation was found for both thoracic kyphosis (r = 0.884; p = 0.001) and lumbar lordosis (r = 0.892; p = 0.001).
Table 3 Measurements (in degrees) determined by the Spinal Mouse for 15 patients (pelvic tilt and of maximal extension of the hip, knees and ankle joints).
| Patient no Normal | Pelvic tilt 0; +30 | Hips 0; −10 | Knees +5; +10 | Ankles +20; +30 |
|---|---|---|---|---|
| 2 | 48 | +17.5 | −1.5 | −25 |
| 4 | 19 | 0 | −7.5 | +7.5 |
| 6 | 34 | +7.5 | −12.5 | 0 |
| 11 | 75 | +40 | −20 | −5 |
| 12 | 46 | +2.5 | −10 | 0 |
| 13 | 33 | +10 | −17.5 | +2.5 |
| 14 | 14 | −2.5 | −5 | +10 |
| 15 | 31 | +35 | −7.5 | 0 |
| 16 | 20 | 0 | +2.5 | −2.5 |
| 17 | 50 | −7.5 | −10 | +7.5 |
| 18 | 13 | −12.5 | −7.5 | 0 |
| 19 | 40 | +5 | −15 | −20 |
| 20 | 29 | +12.5 | −15 | +2.5 |
| 21 | 64 | +7.5 | 0 | 0 |
| 23 | 40 | +17.5 | −10 | −10 |
Twelve patients wore a corset: the eight flexible leather corsets were well tolerated (and resulted in a decrease in back pain in six wearers) and a rigid corset was well tolerated in one of the two patients who chose this device. Neither of the two moulded, supportive seats was well tolerated.
Table 4 gives the details of the additional investigations carried out.
Table 4 Results of additional investigations.
| Examinations | No of patients |
|---|---|
| Spinal radiographs | 23 |
| Degenerative injuries | 15 |
| Vertebral settling | 6 |
| Listhesis | 5 |
| Scoliosis | 15 |
| Muscle MRI | 17 |
| Normal | 5 |
| Fatty involution | 7 |
| Muscle signal abnormalities | 5 |
| Cranial MRI | 22 |
| Normal | 6 |
| Overall atrophy | 6 |
| Leucopathy | 7 |
| Abnormal signal of basal ganglia | 3 |
| Detection electromyography | 23 |
| Normal | 14 |
| Motor unit reduction | 5 |
| Myopathic | 3 |
| Neuropathic | 1 |
MRI, magnetic resonance imaging.
Radiology
Cervical or thoracolumbar radiographs showed that all patients with a vertebral pathology, as a settling or a slide, had degenerative injuries and osteoarthropathy. Of the 14 patients with scoliosis, seven also had vertebral settling or slide but none of these injuries were at the most extreme point of scoliosis; furthermore, eight patients presented with an akinetic‐rigid syndrome of Parkinson's disease. The scoliosis‐affected side of the body was not correlated with the predominant side of the Parkinson's disease.
MRI of the thoracolumbar paraspinal muscles showed muscle signal abnormalities in five patients: two were non‐specific and three showed high signal intensity on the T2‐weighted, fat‐suppressive (short inversion time inversion recovery (STIR)) sequence (in the left side for one of the three patients and bilaterally for the other two). The results for the girdle muscles were normal in all patients.
Cranial MRI showed abnormal signals for the basal ganglia in three patients: one displayed a small lacunar infarct in the left putamen and the remaining two had a lacunar infarct in the head of the left and right caudate nuclei, respectively. We noted that the patients with rapid‐onset camptocormia had normal cranial MRI results.
Electromyography
When investigated using thoracolumbar spinal erector muscle electromyography, all the patients with a motor unit reduction were found to have involution of the muscle fibres (seen in the paraspinal muscle MRI). No other correlations were found. Surface electromyography of the left lumbar paraspinal muscles was carried out for the patient with a vascular lesion in the putamen, but no dystonia‐related paradoxical activities were apparent.
Clinical biochemistry
No noticeable abnormalities were found in any of the laboratory tests.
Histology
The 18 accessory salivary gland biopsy specimens did not show any sign of amyloid abnormalities or inflammatory disease (sarcoidosis, Sjögren disease).
Muscle biopsy was carried out in the five patients who had signal abnormalities on their thoracolumbar spine MRI. For patient 17, the STIR sequence showed hyperintensity of the bilateral spinal muscles from T12 to L5, increased signal intensity after gadolinium injection and a fatty involution. The corresponding muscle biopsy showed an inflammatory infiltrate, with a few rimmed vacuoles but no characteristic tubulofilamentous inclusions visible on electron microscopy, thus excluding inclusion‐body myositis. Our diagnosis was focal, paravertebral myositis. For patient 21, the STIR sequence showed a large fatty infiltration in the paraspinal muscles, with bilateral signal hyperintensities. The muscle biopsy showed type‐II fibre atrophy and no signs of myositis. For patient 19, the STIR sequence showed hyperintensity at the edge of the right paraspinal muscles, with an increase in signal intensity after gadolinium injection and mild fatty infiltration. The muscle biopsy showed only fibrosis and fatty infiltration. The biopsy for the last two patients with MRI signal abnormalities for the paravertebral muscles showed fibrosis and fatty infiltration.
Discussion
Clinical characteristics of patients with Parkinson's disease with camptocormia
Our study population with Parkinson's disease with camptocormia was relatively old (median age = 70 years), with a long disease duration (11 years). The UPDRS axial subscore (10/24) was high when compared with the overall UPDRS motor score (26/108, with a ratio of 0.43 for the most extreme value), suggesting a predominance of axial involvement in Parkinson's disease with camptocormia. Four patients had axial disorders from the very onset of Parkinson's disease. Most patients had motor fluctuations and freezing, and all frequently displayed autonomic symptoms. Hence, there may be a specific clinical pattern of Parkinson's disease associated with camptocormia, as characterised by predominantly male patients, old age, a long disease history, prominent axial disorders, motor fluctuations and autonomic symptoms.
In a study of eight patients, Djaldetti et al10 described camptocormia in Parkinson's disease as an extreme form of trunk rigidity. Their patient population was quite similar to that of the present study, with a mean age of 66 years, mean disease duration of 13.1 years and freezing of gait and motor fluctuations in 50% (4/8). All Djaldetti et al's patients had an akinetic‐rigid syndrome of Parkinson's disease, but this was not confirmed in our larger sample. Moreover, in our population, dyskinesia never affected the axial part of the body and axial dystonia was never noted.
Features of parkinsonian camptocormia
In our study, camptocormia appeared 5.8 (4) years after disease onset and this duration was not related to the treatment duration. The extent of the bent posture varied over the course of the day and improved with L‐dopa treatment in only 24% of patients. Camptocormia was also characterised by the high prevalence of lumbar or thoracolumbar scoliosis (in 61% of patients, preventing complete, passive extension) and by mild‐to‐moderate low back pain in 77% of the patients. In the population described by Djaldetti et al, the time interval between onset of Parkinson's disease and the appearance of a severely bent posture ranged from 0 to 14 years. However, these authors also reported (1) a worsening of the patients' bent posture by fatigue and (2) the lack of a relationship between the onset of L‐dopa treatment and appearance of camptocormia.10 For the patients reported by Wunderlich et al11 and Schäbitz et al,12 parkinsonian camptocormia appeared after 4–15 years of Parkinson's disease and, once again, was not greatly modified by treatment for Parkinson's disease.
Possible physiopathological mechanisms of parkinsonian camptocormia
Few literature reports10,11,14 of the focal, paraspinal myopathy reported here suggest that the condition is rare but possibly not fortuitous in parkinsonian camptocormia. We found only five patients with abnormal muscle signals potentially related to focal, paraspinal myopathy. Paraspinal muscle MRI signal abnormalities require cautious interpretation and do not necessarily imply inflammatory myopathy—especially since our biopsies did not show any specific histological features. The presence of a few rimmed vacuoles is not specific for inclusion‐body myositis and can indicate a non‐specific myositic process or muscle injury resulting from neuropathy.14
A combination of camptocormia and Parkinson's disease can also suggest mitochondrial disease involving the paraspinal muscles,12 as mitochondrial DNA mutations have been considered to be a physiopathological mechanism in Parkinson's disease.15 Parkinsonian camptocormia could also be related to synucleinopathy, as Lewy bodies have been described in an advanced form of Parkinson's disease outside the central nervous system, in the neuronal cell body of the paravertebral sympathetic plexus.16,17 However, in Parkinson's disease, the spreading of mitochondriopathy and synucleopathy from the central nervous system to the muscle afferent neurones or paravertebral muscles has never been described.
Furthermore, another hypothesis for explaining parkinsonian camptocormia is dystonia of the trunk with excessive activation of the abdominal wall muscles.10,18,19 Six of our 23 patients had familial antecedents of Parkinson's disease (including three with camptocormia), and a mutation in the parkin gene has been described with severe trunk dystonia (mimicking camptocormia).20 However, all our patients were elderly and lacked familial antecedents of early‐onset parkinsonism and had no axial dystonia. Moreover, the dystonia hypothesis cannot explain the resistance‐free, passive reduction of camptocormia in the supine position.
Most of our patients had radiological signs of spondyloarthrotic changes, including lumbar or thoracolumbar scoliosis, which might be a potential risk factor for developing camptocormia.10 In Parkinson's disease, scoliosis might be secondary to postural imbalance, favoured by asymmetrical axial muscle tone.21,22 Back pain could result from vertebral degeneration, as well as from paraspinal muscle hypertonia.23 Parkinsonian camptocormia could be the consequence of axial rigidity of the flexion muscles, with a weakness of the erector spinal muscles—possibly due to dysfunction of the basal ganglia controlling the reticulospinal pathway. The main projections of the reticulospinal pathway are located in the axial muscles, and they control the dynamic and static posture. Dysfunction of the basal ganglia could also cause freezing and autonomic disturbances,24 which were highly prevalent in our population. Camptocormia could be due to an imbalance between excessive central motor drive to the ventral and dorsal trunk musculatures (leading to excessive activation of the abdominal wall muscles) and reduced motor drive to the paraspinal muscles (which would favour secondary muscle atrophy and injury).12 Indeed, we found that the most frequent MRI abnormality was a fatty involution in the paraspinal muscle region. Lack of use of the erector muscles (due to an extreme form of rigidity of the spinal flexion muscles) could induce this type of fatty involution. Subsequently, vertebral deformations could appear and might, in some patients, induce spinal nerve injuries via direct compression or stretching of the back branch, which in turn could increase muscle involution.25,26 The rare cases of focal myopathy could therefore stem from a mechanical pathogenesis with posture impairment, as proposed by Serratrice et al5 and by Wunderlich et al.11 Early physical treatment could minimise the complications of this condition.
Acknowledgements
We thank Dr D Fraser (Biotech Communication, Damery, France) for proofreading the manuscript.
Abbreviations
MRI - magnetic resonance imaging
STIR - short inversion time inversion recovery
UPDRS - Unified Parkinson's Disease Rating Scale
Footnotes
Competing interests: None declared.
Informed consent was obtained for publication of fig 1.
References
- 1.Parkinson J.An essay of the shaking palsy. London: Whittingham and Rowland, 1817
- 2.Earle H. Reply to the review of Mr Bayton's essay on the cure of crooked spine. Edinburgh Med Surg J 18151135–51. [PMC free article] [PubMed] [Google Scholar]
- 3.Brodie B C. Pathological and surgical observations of the disease of the joints. In: Longman, Hurst, Rees, Orme, Brown, eds. London 1818 1822376 Reprinted by the Classics of Medicine Library, Birmingham, Alabama, USA, 1989
- 4.Nieves A V, Miyasaki J M, Lang A E. Acute onset dystonic camptocormia caused by lenticular lesions. Mov Disord 20011617–180. [DOI] [PubMed] [Google Scholar]
- 5.Serratrice G, Pouget J, Pelissier J F. Bent spine syndrome. J Neurol Neurosurg Psychiatry 19966051–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laroche M, Delisle M B, Aziza R.et al Is camptocormia a primary muscular disease? Spine 1995201011–1016. [DOI] [PubMed] [Google Scholar]
- 7.Delcey V, Hachulla E, Michon‐Pastorel U.et al La camptocormie: un signe de myopathie axiale. A propos de 7 observations. Rev Med Interne 200223144–154. [DOI] [PubMed] [Google Scholar]
- 8.Kiuru S, Iivanainen M. Camptocormia, a new side effect of sodium valproate. Epilepsy Res 19871254–257. [DOI] [PubMed] [Google Scholar]
- 9.Souques M, Rosanoff‐Saloff M. La camptocormie. Rev Neurol 191630937–939. [Google Scholar]
- 10.Djaldetti R, Mosberg‐Galili R, Sroka H.et al Camptocormia (bent spine) in patients with Parkinson's disease: characterization and possible pathogenesis of an unusual phenomenon. Mov Disord 199914443–447. [DOI] [PubMed] [Google Scholar]
- 11.Wunderlich S, Csoti I, Reiners K.et al Camptocormia in Parkinson's disease mimicked by focal myositis of the paraspinal muscles. Mov Disord 200217598–600. [DOI] [PubMed] [Google Scholar]
- 12.Schäbitz W R, Glatz K, Schuhan C.et al Severe forward flexion of the trunk in Parkinson's disease: focal myopathy of the paraspinal muscles mimicking camptocormia. Mov Disord 200318408–414. [DOI] [PubMed] [Google Scholar]
- 13.Gelb D J, Oliver E, Gilman S. Diagnostic criteria for Parkinson's Disease. Arch Neurol 19995633–39. [DOI] [PubMed] [Google Scholar]
- 14.Charpentier P, Dauphin A, Stojkovic T.et al Maladie de Parkinson, camptocormie et myosite focale paraspinale. Rev Neurol 2005161459–463. [DOI] [PubMed] [Google Scholar]
- 15.De Coo I F, Renier W O, Ruitenbeek W.et al A 4‐base pair deletion in the mitochondrial cytochrome b gene associated with parkinsonism/MELAS overlap syndrome. Ann Neurol 199945130–133. [DOI] [PubMed] [Google Scholar]
- 16.Forno L S, Norville R L. Ultrastructure of Lewy bodies in the stellate ganglion. Acta Neuropathol (Berlin) 197634183–197. [DOI] [PubMed] [Google Scholar]
- 17.Gibb W R, Scott T, Lees A J. Neuronal inclusions of Parkinson's disease. Mov Disord 199162–11. [DOI] [PubMed] [Google Scholar]
- 18.Friedman J H. Episodic camptocormia in PD. Mov Disord 200161201. [DOI] [PubMed] [Google Scholar]
- 19.Slawek J, Derejko M, Lass P. Camptocormia as a form of dystonia in Parkinson's disease. Eur J Neurol 200310107–108. [DOI] [PubMed] [Google Scholar]
- 20.Inzelberg R, Hattori N, Nisipeanu P.et al Camptocormia, axial dystonia, and parkinsonism: phenotypic heterogeneity of a parkin mutation. Neurology 2003601393–1394. [DOI] [PubMed] [Google Scholar]
- 21.De Sèze M, Lavignolle B, Mazaux J M.et al Déviations rachidiennes et maladie de Parkinson. Rev Rhum. In press
- 22.Marsden C D, Duvoisin R C, Jenner P.et al Relationship between animal models and clinical parkinsonism. Adv Neurol 19759165–175. [PubMed] [Google Scholar]
- 23.Pelissier J, Pérennou D, Enjalbert M.et al Rachis et maladie de Parkinson. In: Simon L, Hérisson C, Biot B, eds. Le rachis vieillissant. Paris: Masson, 1992333–339.
- 24.Delwaide P J, Pepi J L, De Pasqua V.et al Projections from basal ganglia to tegmentum: a subcortical route for explaining the pathophysiology of Parkinson's disease signs? J Neurol 2000247(Suppl 2)II75–II81. [DOI] [PubMed] [Google Scholar]
- 25.Penisson‐Besnier I, Menei P, Dubas F.et al Démonstration histologique du processus de dénervation des muscles paravertébraux dans un cas de cyphose lombaire réductible (camptocormie). Rev Rhum 199461868–870. [PubMed] [Google Scholar]
- 26.Delisle M B, Laroche M, Dupont H.et al Morphological analyses of paraspinal muscles: camporiso, of progressive lumbar kyphosis (camptocormia) and narrowing of lumbar canal by disc protrusions. Neuromusc Disord 19935/6579–582. [DOI] [PubMed] [Google Scholar]