Abstract
Zn-α2-glycoprotein (ZAG) is a 41-kDa soluble protein that is present in most bodily fluids. In addition, ZAG accumulates in fluids from breast cysts and in 40% of breast carcinomas, which suggests that ZAG plays a role in the development of breast diseases. However, the function of ZAG under physiological and cancerous conditions remains unknown. Because ZAG shares 30–40% sequence identity with the heavy chains of class I major histocompatibility complex (MHC) proteins, we compared the biochemical properties of ZAG with those of classical class I MHC molecules. We purified human ZAG from breast cyst fluid and serum and produced a panel of anti-ZAG monoclonal antibodies. Binding assays and acid elution experiments revealed that, in contrast to class I MHC proteins, ZAG does not bind peptides or the class I light chain, β2-microglobulin (β2m). Nevertheless, CD studies indicated that ZAG is thermally stable in the absence of bound peptide or associated β2m, as opposed to class I MHC molecules, which require the presence of both β2m and peptides for stability. These data indicate that the function of ZAG has diverged from the peptide presentation and T-cell interaction functions of class I molecules. To gain insight into the function of ZAG and to compare the three-dimensional structures of ZAG and class I MHC molecules, we produced ZAG crystals that diffract beyond 2.7 Å and have initiated an x-ray structure determination.
Zn-α2-glycoprotein (ZAG) is a soluble protein that was originally isolated from human plasma (1). Its name derives from its tendency to precipitate with zinc salts, its electrophoretic mobility in the region of the α2 globulins, and its 18% carbohydrate content. The function of ZAG is unknown, but several studies have shown that this protein is present in other bodily fluids, including sweat, saliva, cerebrospinal fluid, seminal plasma, milk, ammiotic fluid, and urine (2). In addition, ZAG is found at high concentrations in the fluid from breast cysts and in 40% of breast carcinomas (3–6). These findings, together with the fact that ZAG is induced by glucocorticoids and androgens in breast cancer cell lines (7), suggest that this protein may participate in the development of mammary diseases, including breast cancer.
Amino acid sequence analysis revealed that ZAG is surprisingly similar to class I major histocompatibility complex (MHC) proteins (8). Class I MHC molecules are heterodimers, consisting of a membrane-bound heavy chain noncovalently associated with β2-microglobulin (β2m), a soluble protein that serves as the light chain. Class I molecules bind peptides derived from intracellular proteins and present them to cytotoxic T cells during immune surveillance (9). Crystal structures reveal that they fold into a shape ideally suited for peptide binding. Two long α-helices form the sides of a groove within the first two domains (α1 and α2) of the heavy chain, and a β-pleated sheet forms the bottom. The β2m light chain and the other domain of the heavy chain (α3) lie underneath the α1 and α2 domains, with β2m interacting simultaneously with the side of the α3 domain and the bottom of the α1-α2 domain platform (reviewed in refs. 10 and 11). ZAG is composed of three domains that share 30–40% amino acid sequence identity with the three extracellular domains of class I heavy chains. This level of sequence identity is compatible with structural similarity, as shown by Chothia and Lesk (12).
Analysis of ZAG cDNA and genomic clones provided further data about the relationship between ZAG and class I MHC molecules (13). Similarities in exon organization, intron-exon junctions, and nucleotide sequence indicate that the coding regions of the ZAG gene are homologous to the first four exons of MHC genes, which encode the signal peptide and the three extracellular domains of the heavy chain. However, the ZAG gene does not encode transmembrane or cytoplasmic regions and lacks the regulatory and interferon consensus sequences that are conserved in typical MHC genes (13). In addition, the ZAG gene differs from class I genes in its lack of polymorphism and in its location outside of the MHC (14). The recent identification of cDNAs coding for ZAG in mice and rats (15, 16) shows that the divergence between ZAG and MHC molecules began at least 80 million years ago, before the evolutionary radiation of the placental mammals (17).
This work addresses how this divergence has affected the biochemical and structural characteristics of ZAG. We purified human ZAG from fluid obtained from breast cysts and from serum, produced anti-ZAG mAbs, and compared its properties with those of class I MHC molecules. In addition, as a first step in a three-dimensional structure determination, we produced crystals of ZAG that are suitable for an x-ray diffraction analysis.
MATERIALS AND METHODS
Materials.
Fluid from breast cysts obtained from patients with breast gross cystic disease was kindly provided by F. Vizoso from Hospital de Jove (Gijón, Spain). Human serum was the gift of J. Goin, from Biocell Laboratories. Samples were stored at −20°C. Before use, fats and debris were removed by centrifugation at 10,000 × g and filtration through 0.2 μm filters. Human β2m and anti-β2m polyclonal antiserum were from Sigma. Chromatographic matrices and equipment were from Pharmacia. Antibody isotyping was performed with a kit from GIBCO/BRL. 125I-protein A was from Amersham. Native PAGE was performed using a PhastSystem (Pharmacia).
Purification of ZAG from Breast Cyst Fluid.
Seventy-five milliliters of cyst fluid was loaded on a phenyl Sepharose column equilibrated with 50 mM sodium phosphate (pH 7), 0.75 M (NH4)2SO4. The column was washed with 0.25 M (NH4)2SO4 in sodium phosphate, and ZAG was eluted with 50 mM sodium phosphate (pH 7). Fractions containing ZAG were applied on a nickel-chelating Sepharose column equilibrated with 50 mM sodium phosphate (pH 7.5), 0.5 M NaCl. ZAG was recovered in the flowthrough, while contaminant proteins were retained on the chelating column. ZAG was further purified by chromatography on a Superdex 200 HiLoad FPLC column equilibrated in 20 mM Hepes (pH 7), 150 mM NaCl. Purified ZAG was desalted and concentrated using a Centricon-10 (molecular weight cutoff of 10,000) ultrafiltration device (Amicon) and stored in 20 mM Hepes (pH 7), 0.02% NaN3 at 4°C.
Antibodies.
Rabbit anti-ZAG polyclonal antiserum was produced as described (4). Female BALB/c mice (aged 5 weeks) were primed and twice boosted at 2-week intervals by intraperitoneal injection of 100 μg of purified ZAG in adjuvant. Serum was screened a week after each injection by ELISA as described (18). Three days preceding the fusion, one mouse was boosted with 100 μg of purified ZAG. Splenocytes from the boosted mouse were fused with HL-1 murine myeloma cells, and media from the hybridoma cultures were tested for antibodies against ZAG by ELISA. After subcloning positive clones at clonal density, ascites tumors were produced in pristane-primed BALB/c mice. Eight anti-ZAG mAbs produced by this fusion were subsequently characterized (Table 1). All antibodies are murine IgG1-κ. Conditions for elution of ZAG were determined by ELISA as described (19).
Table 1.
mAbs against ZAG
| Clone name | Western blot | Immuno- precipitation | pH required for elution of ZAG |
|---|---|---|---|
| 1B5 | + | + | 4.0 |
| 1D4 | ++ | ++ | 4.0 |
| 1E2 | ++ | ++ | 3.0 |
| 1H4 | + | ++ | 3.0 |
| 3C5 | ++ | + | 4.5 |
| 3F4 | +/− | ++ | 3.5 |
| 4E3 | − | + | 3.5 |
| 6G2 | ++ | ++ | 3.5 |
Purification of ZAG from Human Serum.
Seventy-five milligrams of the purified mAb 3C5 were coupled to a CNBr-activated Sepharose column according to the manufacturer’s instructions. Two-hundred-fifty-milliliter aliquots of serum were loaded on the 3C5 immunoaffinity column equilibrated with 20 mM of sodium phosphate (pH 7.5), 0.5 M NaCl. After washing with the same buffer, ZAG was eluted with 20 mM sodium acetate (pH 4.5), 0.5 M NaCl into tubes containing Hepes (pH 7). Fractions containing ZAG were desalted and loaded on a Q Sepharose column equilibrated with 20 mM of piperazine (pH 5). For biochemical experiments, ZAG was eluted using 0.1 M NaCl in the piperazine buffer and stored as before. For crystalization, ZAG was eluted using a gradient from 0 to 50 mM NaCl in the piperazine buffer. Under these conditions, ZAG eluted in two peaks. The peak that eluted in higher salt was loaded on a Source phenyl column equilibrated with 50 mM sodium phosphate (pH 6), 0.75 M (NH4)2SO4 and eluted with a gradient from 0.75 M to 0 M (NH4)2SO4 in the same buffer. ZAG was further separated into two peaks that eluted with 0.56 M and 0.41 M (NH4)2SO4, respectively.
β2m Binding Assays.
For chromatographic experiments, 4 μg of ZAG were mixed with 1 to 30 μg of β2m in 25 μl of Tris-buffered saline. After 6 h, the sample was injected on a SMART system Superdex 75 Precision column equilibrated in the same buffer. Absorbance was measured at 280 nm, and fractions were analyzed by SDS/PAGE. For immunoprecipitation assays, 1-ml aliquots of serum were incubated with 25 μl of protein A Sepharose for 30 min. After pelleting, supernatants were incubated for 1 h with 10 μg of polyclonal antiserum against ZAG or β2m, followed by a 30-min incubation with protein A Sepharose. The resins were pelleted and washed with Tris-buffered saline containing 0.1% BSA, 0.1% SDS, 1% Nonidet P-40, and 1% sodium deoxycholate. The immunoprecipitated proteins were separated by SDS/PAGE and blotted onto nitrocellulose filters, which were incubated with antiserum against either ZAG or β2m and developed with 125I-protein A following published protocols (4).
Acid Elutions of ZAG or a Class I MHC Heterodimer (H-2Kd).
Purified ZAG from serum or H-2Kd (a soluble version of the Kd heavy chain complexed with human β2m (20); proteins were analyzed for the presence of bound peptides using established methods (21, 22). Aliquots of 1 mg of protein were diluted into 1 ml of 10% acetic acid, boiled for 5 min, and filtered in a centricon 3 (molecular weight cutoff of 3,000) ultrafiltration device. The filtrates were analyzed on a SMART system μRPC C2/C18 SC column using a 6 ml gradient from 0.06% trifluoroacetic acid in water to 0.05% trifluoroacetic acid in 80% acetonitrile. Absorbance was monitored at 214 nm. The fractions containing peaks were microsequenced using an ABI 477A protein sequencer with a 120A phenylthiohydantoin analyzer as described (4). To detect N-terminally blocked peptides, peaks from a similar experiment were analyzed by matrix-assisted, laser desorption, time-of-flight mass spectrometry using a PerSeptive Biosystems (Framingham, MA) ELITE mass spectrometer.
Thermal Stability Analyses.
An Aviv 62A DS spectropolarimeter equipped with a thermoelectric cell holder and a 1-mm path-length cell was used for CD studies. The heat-induced unfolding of ZAG [1 mg/ml in 5 mM sodium phosphate (pH 7)] was monitored by recording the CD signal at 223 nm while the sample temperature was raised from 25° to 80°C at a rate of 20°C per hour. The transition midpoint (Tm) was calculated by estimating the half-point of the ellipticity change between the pure native and the pure denatured states. Thermal stability measurements were repeated after denaturation in 6 M guanidine hydrochloride, dialysis against denaturant, and refolding by dialysis against 5 mM sodium phosphate (pH 7).
Crystalization of ZAG.
Initial crystalization conditions were obtained from factorial trial screens (23). The best crystals were obtained using serum ZAG eluted with 0.56 M (NH4)2SO4 from the Source phenyl column. Single crystals were grown by vapor diffusion from protein solutions (15 mg/ml) in 20 mM Hepes (pH 7.0), 0.02% NaN3, in 3-μl droplets with 30% (wt/vol) polyethylene glycol 2000 monomethyl ether (Fluka), 0.2 M ammonium acetate, 0.1 M sodium acetate (pH 5). After crystals were formed, the concentration of polyethylene glycol was raised to 35% for cryopreservation. Single crystals were mounted in a thin film of cryopreservation buffer, supported by a rayon loop (24), and quickly cooled by plunging into liquid nitrogen.
RESULTS
Purification of ZAG.
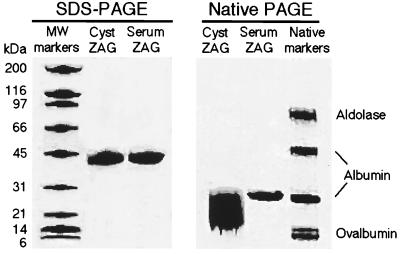
High levels of ZAG are found in the fluid from breast cysts obtained from patients with breast gross cystic disease (4). We developed a purification protocol for isolation of ZAG from breast cyst fluid, which yielded about 20 mg of ZAG per 75 ml of fluid. The purification procedure included three successive chromatographic steps: hydrophobic interaction, metal chelating, and size exclusion chromatography, and resulted in protein that was apparently homogeneous, as judged by SDS/PAGE (Fig. 1). However, subsequent analysis by native PAGE (Fig. 1) revealed heterogeneity in the protein preparation, and multiple crystalization trials failed to produce protein crystals of x-ray diffraction quality. We therefore decided to purify ZAG from another source.
Figure 1.
PAGE analysis of ZAG. Aliquots of ZAG purified from breast cyst fluid or serum were analyzed by SDS/10–15% PAGE and native/8–20% PAGE.
To facilitate purification of ZAG from human serum, mAbs were produced against ZAG purified from breast cyst fluid. The anti-ZAG mAb 3C5 was immobilized on a solid support, and ZAG was purified by immunoaffinity and ion exchange chromatography from serum at yields up to 15 mg/liter. SDS/PAGE (Fig. 1) and mass spectrometry showed that both serum and cyst fluid ZAG have the same molecular mass (39 ± 1 kDa by mass spectrometry; data not shown). However, native PAGE analysis revealed that ZAG isolated from serum was more homogeneous than ZAG isolated from cyst fluid (Fig. 1). Therefore, serum ZAG was used for the subsequent experiments.
β2m Binding Assays.
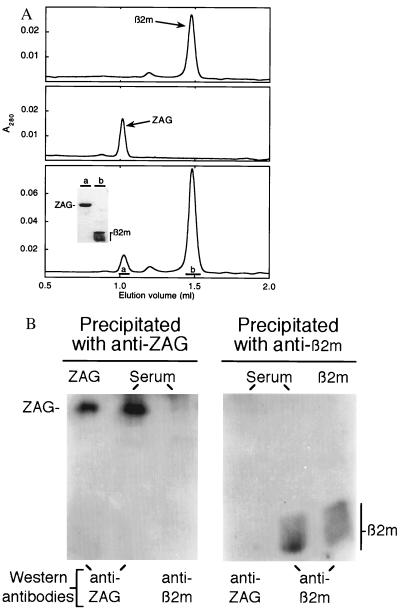
The heavy chains of classical class I MHC proteins are tightly associated with β2m (25). However, purified preparations of ZAG show a single band with an apparent molecular mass of 41 kDa when analyzed by SDS/PAGE, with no sign of a 12-kDa light chain corresponding to β2m (Fig. 1). To ascertain if ZAG binds β2m, different ratios of both proteins were incubated and analyzed by size exclusion chromatography. Regardless of the ZAG/β2m ratio loaded on the column, only peaks containing either ZAG or β2m, but not both proteins, were obtained (Fig. 2A). In addition, β2m binding was not observed using a surface plasmon resonance-based assay in which β2m was passed over a ZAG-coupled biosensor chip (data not shown).
Figure 2.
Chromatographic and immunoprecipitation analyses confirm that ZAG does not bind β2m. (A) β2m (Top), ZAG (Middle), and a mixture of ZAG incubated with β2m (Bottom) were analyzed by size exclusion chromatography. (Inset) SDS/13% PAGE analysis of the peaks in the bottom panel. (B) Aliquots of ZAG, serum, and β2m were immunoprecipitated with either anti-ZAG or anti-β2m antibodies. Precipitated proteins were analyzed by Western blot using antisera against either ZAG or β2m.
The result that purified ZAG does not associate with β2m was confirmed for ZAG in serum using immunoprecipitation assays. Western blot analysis showed that an anti-ZAG antiserum precipitated ZAG from serum, but did not coprecipitate β2m. Conversely, an anti-β2m antiserum precipitated β2m from serum, but did not coprecipitate ZAG (Fig. 2B).
Peptide Elution Assays.
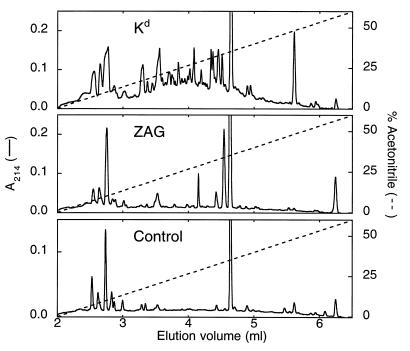
Peptides associated with class I MHC molecules have typically been analyzed by N-terminal sequencing of eluates recovered after ultrafiltration of acid dissociated heterodimers (21, 22). To determine whether ZAG contains bound peptide(s), we compared acid eluates of purified ZAG and the murine class I MHC molecule H-2Kd. Acid eluates were analyzed by reverse phase chromatography (Fig. 3) and N-terminal sequencing. This procedure resulted in identification of multiple peptides in the Kd acid eluate whose sequences corresponded to the previously characterized motif for peptides bound to this class I allele (26). By contrast, most of the peaks in the chromatographic profile of the ZAG acid eluate also were found in eluates extracted from samples without protein, and peptides were not detected in any of the peaks by N-terminal sequencing or by mass spectrometry. Pool sequencing of unpurified acid eluates produced comparable results: peptides were detected in the Kd eluate, but not in the ZAG eluate (data not shown).
Figure 3.
HPLC analysis of acid eluates from Kd, ZAG, and a control with no protein. All peaks in the ZAG eluate were subjected to N-terminal sequencing and mass spectrometry analysis. No peptides were detected.
Thermal Stability of ZAG.
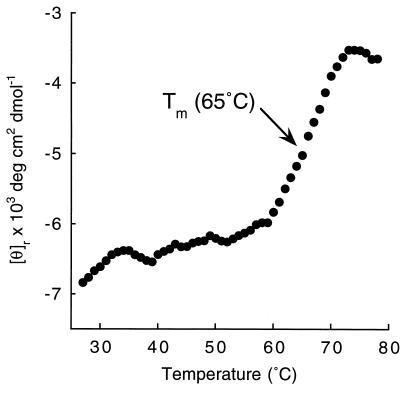
Class I MHC heavy chains show decreased stability in the absence of peptide (20, 27, 28) or β2m (29), whereas ZAG exists as an isolated class I MHC-like heavy chain without bound peptides. To ascertain if ZAG is less stable than class I MHC molecules because of the absence of bound peptide and light chain, we monitored the heat-induced unfolding of ZAG by recording the CD signal at 223 nm while increasing the sample temperature from 25°C to 80°C (Fig. 4). The resulting melting curve shows a Tm of 65°C, which is comparable to previously obtained Tms for peptide-filled class I MHC molecules [H-2Kd: 57°C; (20); HLA-A2: 66°C; (30)]. The Tm obtained from the ZAG melting curve is substantially higher than the Tm of an empty class I MHC molecule (H-2Kd complexed with human or murine β2m: 43–45°C; refs. 20 and 31).
Figure 4.
Thermal denaturation profile of serum ZAG. The CD signal at 223 nm is plotted as molar ellipticity per mean residue after smoothing. Similar profiles were obtained using ZAG isolated from cyst fluid. Thermal denaturation studies were repeated after denaturation and dialysis to remove any potential ZAG ligands without any effect on the denaturation profile.
Crystalization.
ZAG forms crystals of approximate dimensions 0.4 mm × 0.2 mm × 0.1 mm in space group P21212. The unit cell dimensions are a = 106 Å, b = 132 Å, and c = 119 Å. The asymmetric unit of the crystal is estimated to contain two to six molecules based on average volume to mass ratios (Vm) of protein crystals (32), representing solvents contents between 76% (assuming two molecules per asymmetic unit) to 27% (assuming six molecules per asymmetric unit). Crystals were equilibrated in a cryopreservative and subjected to x-ray analysis at −165°C (33). Cryopreserved crystals diffract to 3.2 Å resolution using CuKα radiation from a Rigaku R200 rotating anode x-ray generator. The use of a synchrotron x-ray source improves the resolution limit. Using the A1 beam line at the Cornell High Energy Synchrotron Source (CHESS), data were collected to 2.7 Å resolution from cryopreserved crystals. We are in the process of solving the three-dimensional structure of ZAG using molecular and isomorphous replacement methods.
DISCUSSION
ZAG is a class I MHC homolog produced by secretory epithelial cells that is present in most bodily fluids (2). Because of increased concentrations of ZAG in mammary carcinomas and in cyst fluids from women with gross cystic disease of the breast, it has been suggested that ZAG participates in the development of breast diseases (4). Indeed, ZAG levels are higher in well differentiated breast carcinomas, indicating that ZAG is a biochemical marker of differentiation in breast cancer (5). In this study, we have purified human ZAG from both breast cyst fluids and serum, and compared a series of biochemical characteristics to those of class I MHC molecules. Milligram amounts of ZAG were purified from cyst fluid obtained from women with breast gross cystic disease and used for production of mAbs. These mAbs are suitable for detection of ZAG by immunoprecipitation and Western blot analyses, and may serve as useful reagents for clinical evaluation of ZAG as a biochemical marker of tumors with specific patterns of hormone responsiveness (5, 7). Our inability to produce crystals of ZAG purified from cyst fluid prompted us to isolate ZAG from serum using one of the anti-ZAG mAbs for immunoaffinity chromatography. Additional chromatographic steps resulted in fractionation of ZAG into different forms, one of which yielded well ordered crystals.
ZAG purified from serum and cyst fluid consists of a single chain, by contrast to the related class I MHC molecules, which are heterodimers consisting of a membrane-bound heavy chain associated with β2m, a soluble light chain. To investigate whether ZAG is capable of binding the class I light chain, we incubated purified ZAG with human β2m. No sign of a ZAG/β2m heterodimer was observed by size exclusion chromatography. In addition, immunoprecipitation studies using antibodies against either ZAG or β2m showed that the two proteins do not coprecipitate from human serum. Therefore, unlike most classical and nonclassical class I MHC proteins, ZAG is not associated with the class I light chain, a characteristic it shares with MICA, a divergent member of the class I MHC family (34).
Class I MHC molecules require bound peptide for structural stability (20, 27, 28). Although ZAG is secreted from cells, differing from conventional class I MHC molecules and most MHC homologs which are cell surface proteins, soluble versions of H-2Kd (20) and HLA-G (35) have been shown to bind peptides derived from endogenous proteins. We therefore examined purified ZAG for the presence of bound peptide. Peptides were not detected in acid eluates derived from ZAG, by contrast to results obtained with the Kd eluate. To ascertain if ZAG is stably folded in the absence of bound peptide and β2m, we compared the thermal denaturation profile of ZAG with denaturation profiles of class I molecules. The CD-monitored melting curve of purified ZAG indicated that this protein is significantly more stable than empty class I molecules (20), and as stable as peptide-filled class I MHC heterodimers (20, 30). The structural stability in the absence of peptide or β2m binding indicates that the function of ZAG has diverged from the peptide presentation and T cell interaction functions of class I MHC molecules and seems to rule out the previous suggestion that ZAG functions as a soluble MHC protein to regulate the immune system (8). Several lines of evidence suggest that ZAG is a carrier of a small molecular mass compound. When the protein was originally isolated from serum, it was noted that ZAG was bound to a yellow pigment that could be removed upon denaturation (1), and we have obtained similar results with ZAG isolated from breast cyst fluid (L.M.S. and C.L.-O., unpublished results). Moreover, immunological studies showed that ZAG carries a proteinase-resistant, heat-stable substance whose injection induces glomerulonephritis in experimental animals (36). Taken together, these data suggest that ZAG carries a low molecular mass ligand of a different chemical nature than the peptides bound by MHC molecules, but further studies will be needed to identify the nature of the ZAG ligand(s).
Class I MHC molecules acquire peptide during assembly of their heavy and light chains in the endoplasmic reticulum (9). Because ZAG follows the same biosynthetic pathway as class I molecules, it has an opportunity to bind peptides in the endoplasmic reticulum, yet the biochemical data reported here show that it does not. The neonatal Fc receptor (FcRn) is another class I MHC homolog that does not bind endogenous peptides (37). The FcRn crystal structure reveals that the FcRn counterpart of the MHC peptide binding groove is closed (38), resulting in an MHC-like structure that is stable in the absence of bound peptide (37). Two amino acid changes with respect to MHC molecules are primarily responsible for closure of the FcRn cleft: Arg-164 occludes the FcRn counterpart of pocket A that is used by class I molecules for the peptide N terminus (reviewed in ref. 11), and Pro-162 within the α2 domain helix appears to be responsible for a kink that closes one side of the cleft. Interestingly, ZAG is one of only a few MHC-like molecules that contains a proline at the position of FcRn Pro-162 (Pro-167 in mature ZAG; classical human and murine class I molecules contain mainly valine, a helix-forming residue, at this position). In addition to ZAG and FcRn, proline is found at the analogous position in all CD1 sequences (39). The recent crystal structure of a murine CD1 molecule shows a kink in the α2 domain helix at the position of its proline, and some of the same sort of underlying structural changes that were seen in the FcRn structure (40). At least one form of human CD1 binds lipids instead of peptide antigens (41, 42), and the murine CD1 structure shows a substantially deeper and more hydrophobic binding groove than conventional class I molecules (40). The available structural evidence, therefore, suggests that a proline at this position within the α2 domain helix could be indicative of a structural rearrangement with respect to classical class I MHC molecules, which might be predictive of a function other than peptide binding. X-ray structural analysis of ZAG ultimately should allow its comparison with MHC and MHC-related molecules, and increase understanding of the function of ZAG under physiological and cancerous conditions.
Acknowledgments
We thank Dr. F. Vizoso for breast cyst fluid, Dr. S. Mayo and his laboratory for use of the CD spectrometer, Dr. M. Harrington for use of the PhastSystem, S. Ou and the Caltech monoclonal antibody facility, Dr. Gary Hathaway and the Caltech PPMAL facility, and Dr. A. Chirino for data collection and processing at Cornell High Energy Synchrotron Source. L.M.S. was supported by the Fulbright Visiting Scholar Program.
ABBREVIATIONS
- β2m
β2-microglobulin
- MHC
major histocompatibility complex
- ZAG
Zn-α2-glycoprotein
- Tm
transition midpoint
- neonatal FcRn
Fc receptor
References
- 1.Bürgi W, Schmid K. J Biol Chem. 1961;236:1066–1074. [PubMed] [Google Scholar]
- 2.Tada T, Ohkubo I, Niwa M, Sasaki M, Tateyama H, Eimoto T. J Histochem Cytochem. 1991;39:1221–1226. doi: 10.1177/39.9.1918940. [DOI] [PubMed] [Google Scholar]
- 3.Bundred N J, Miller V R, Walker R A. Histopathology. 1987;11:603–610. doi: 10.1111/j.1365-2559.1987.tb02670.x. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez L M, Vizoso F, Diez-Itza I, López-Otín C. Cancer Res. 1992;52:95–100. [PubMed] [Google Scholar]
- 5.Díez-Itza I, Sánchez L M, Allende M T, Vizoso F, Ruibal A, López-Otín C. Eur J Cancer. 1993;29:1256–1260. doi: 10.1016/0959-8049(93)90068-q. [DOI] [PubMed] [Google Scholar]
- 6.Freije J P, Fueyo A, Uría J, López-Otín C. FEBS Lett. 1991;290:247–249. doi: 10.1016/0014-5793(91)81271-9. [DOI] [PubMed] [Google Scholar]
- 7.López-Boado Y, Diez-Itza I, Tolivia J, López-Otín C. Breast Cancer Res Treat. 1994;29:247–258. doi: 10.1007/BF00666478. [DOI] [PubMed] [Google Scholar]
- 8.Araki T, Gejyo F, Takagaki K, Haupt H, Schwick H G, Bürgi W, Marti T, Schaller J, Rickli E, Brossmer R, Atkinson P H, Putnam F W, Schmid K. Proc Natl Acad Sci USA. 1988;85:679–683. doi: 10.1073/pnas.85.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townsend A, Bodmer H. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 10.Bjorkman P J, Parham P. Annu Rev Biochem. 1990;90:253–88. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- 11.Stern L J, Wiley D C. Structure. 1994;15:245–251. doi: 10.1016/s0969-2126(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 12.Chothia C, Lesk A M. EMBO J. 1986;5:823–826. doi: 10.1002/j.1460-2075.1986.tb04288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freije J P, Fueyo A, Uría J A, Velasco G, Sánchez L M, López-Boado Y S, López-Otín C. Genomics. 1993;18:575–587. doi: 10.1016/s0888-7543(05)80359-3. [DOI] [PubMed] [Google Scholar]
- 14.Pendas A M, Matilla T, Uría J A, Freije J P, Fueyo A, Estivill X, López-Otin C. Cytogenet Cell Genet. 1994;64:263–266. doi: 10.1159/000133708. [DOI] [PubMed] [Google Scholar]
- 15.Fueyo A, Uría J A, Freije J M P, López-Otin C. Gene. 1994;145:245–249. doi: 10.1016/0378-1119(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 16.Ueyama H, Naitoh H, Ohkubo I. J Biochem. 1994;116:677–681. doi: 10.1093/oxfordjournals.jbchem.a124579. [DOI] [PubMed] [Google Scholar]
- 17.Li W-H, Gouy M, Sharp P M, O’Huigin C, Yang Y-W. Proc Natl Acad Sci USA. 1990;87:6703–6707. doi: 10.1073/pnas.87.17.6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghavan M, Chen M Y, Gastinel L N, Bjorkman P J. Immunity. 1994;1:303–315. doi: 10.1016/1074-7613(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 19.Pepper D S. In: Methods in Molecular Biology. Kenney A, Fowell S, editors. Vol. 11. Totowa, NJ: Humana Press; 1992. pp. 135–171. [Google Scholar]
- 20.Fahnestock M L, Tamir I, Narhi L, Bjorkman P J. Science. 1992;258:1658–1662. doi: 10.1126/science.1360705. [DOI] [PubMed] [Google Scholar]
- 21.Van Bleek G M, Nathenson S G. Nature (London) 1990;348:213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- 22.Jardetzky T S, Lane W S, Robinson R A, Madden D R, Wiley D C. Nature (London) 1991;353:325–330. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- 23.Jancarik J, Kim S H. J Appl Cryst. 1991;24:409–411. [Google Scholar]
- 24.Teng T-Y. J Appl Cryst. 1990;23:387–391. [Google Scholar]
- 25.Grey M M, Kubo R T, Colon S M, Poulik M D, Cresswell P, Springer T A, Turner M, Strominger L. J Exp Med. 1973;138:1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk K, Rötzschke O, Stevanovic S, Jung G, Rammensee H G. Nature (London) 1991;351:290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 27.Townsend A, Elliott T, Cerundolo V, Foster L, Barber B, Tse A. Cell. 1990;62:285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- 28.Ljunggren H G, Stam N J, Öhlén C, Neefjes J J, Höglund P, Heemels M T, Bastin J, Schumacher T N M, Townsend A, Kärre K, Ploegh H L. Nature (London) 1990;346:476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 29.Lancet D, Parham P, Strominger J L. Proc Natl Acad Sci USA. 1979;76:3844–3848. doi: 10.1073/pnas.76.8.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouvier M, Wiley D C. Science. 1994;265:398–402. doi: 10.1126/science.8023162. [DOI] [PubMed] [Google Scholar]
- 31.Fahnestock M L, Johnson J L, Feldman R M R, Tsomides T J, Mayer J, Narhi L O, Bjorkman P J. Biochemistry. 1994;33:8149–8158. doi: 10.1021/bi00192a020. [DOI] [PubMed] [Google Scholar]
- 32.Matthews B W. J Mol Biol. 1968;33:491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- 33.Hope H. Annu Rev Biophys Biophys Chem. 1990;19:107–126. doi: 10.1146/annurev.bb.19.060190.000543. [DOI] [PubMed] [Google Scholar]
- 34.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Proc Natl Acad Sci USA. 1996;93:12445–12450. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee N, Malacko A R, Ishitani A, Chen M C, Bajorath J, Marquardt H, Geraghty D E. Immunity. 1995;3:591–600. doi: 10.1016/1074-7613(95)90130-2. [DOI] [PubMed] [Google Scholar]
- 36.Shibata S, Miura K. Nephron. 1982;31:170–176. doi: 10.1159/000182638. [DOI] [PubMed] [Google Scholar]
- 37.Raghavan M, Gastinel L N, Bjorkman P J. Biochemistry. 1993;32:8654–8660. doi: 10.1021/bi00084a037. [DOI] [PubMed] [Google Scholar]
- 38.Burmeister W P, Gastinel L N, Simister N E, Blum M L, Bjorkman P J. Nature (London) 1994;372:336–343. doi: 10.1038/372336a0. [DOI] [PubMed] [Google Scholar]
- 39.Martin L H, Calabi F, Lefevre F-A, Bilsland C A, Milstein C. Proc Natl Acad Sci USA. 1987;84:9189–9193. doi: 10.1073/pnas.84.24.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng, Z. H., Castaño, A. R., Segelke, B., Stura, E. A., Peterson, P. A. & Wilson, I. A. (1997) Science, in press. [DOI] [PubMed]
- 41.Beekman E M, Porcelli S A, Morita C T, Behar S M, Furlong S T, Brenner M B. Nature (London) 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 42.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, Modlin R L. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]