Abstract
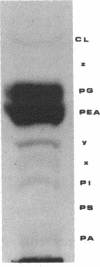
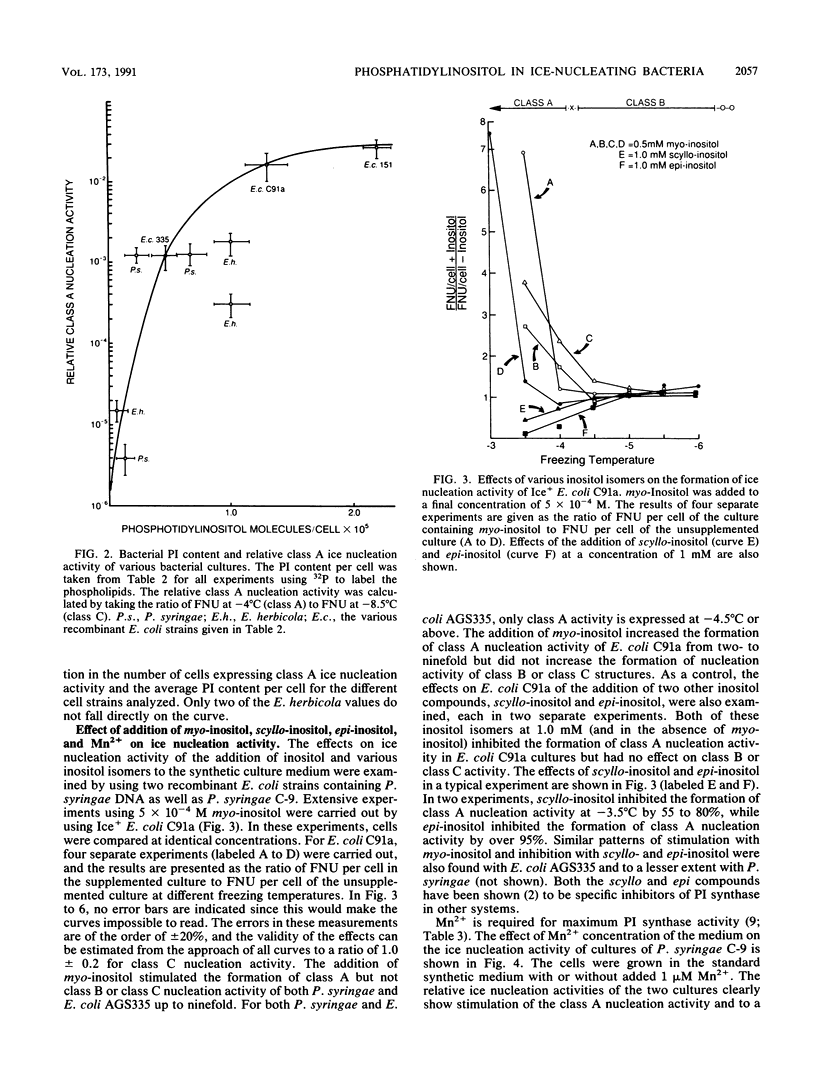
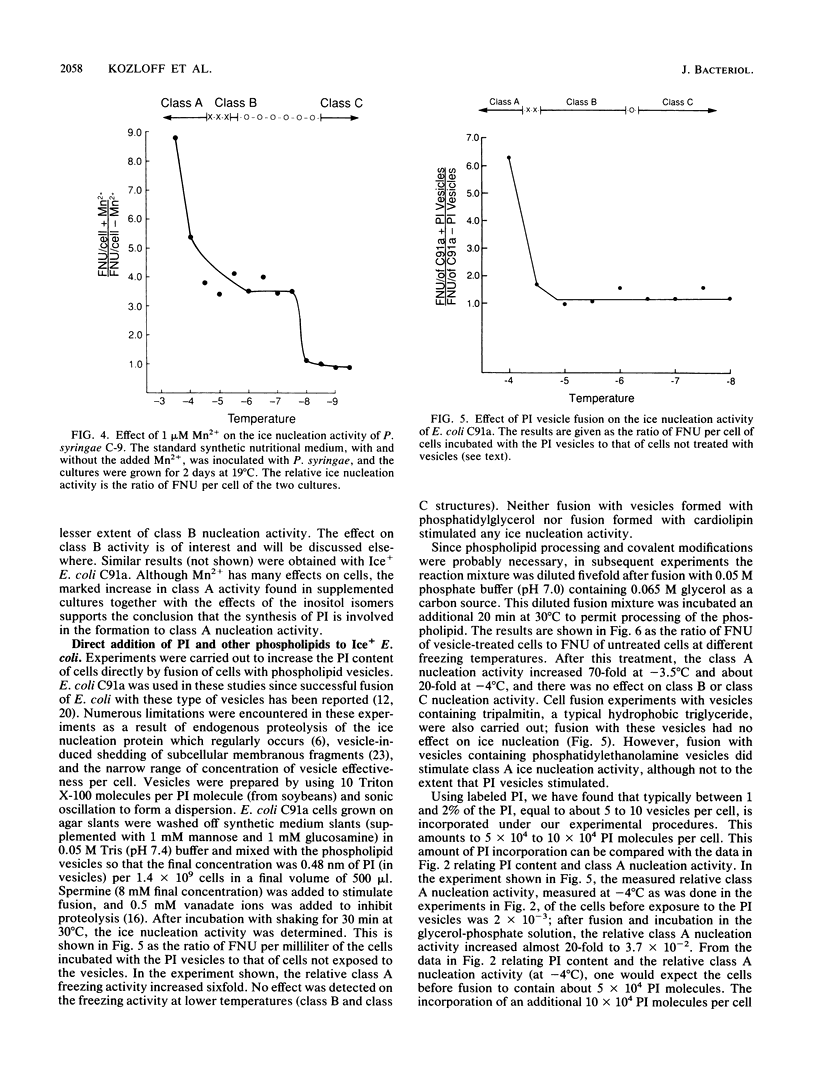
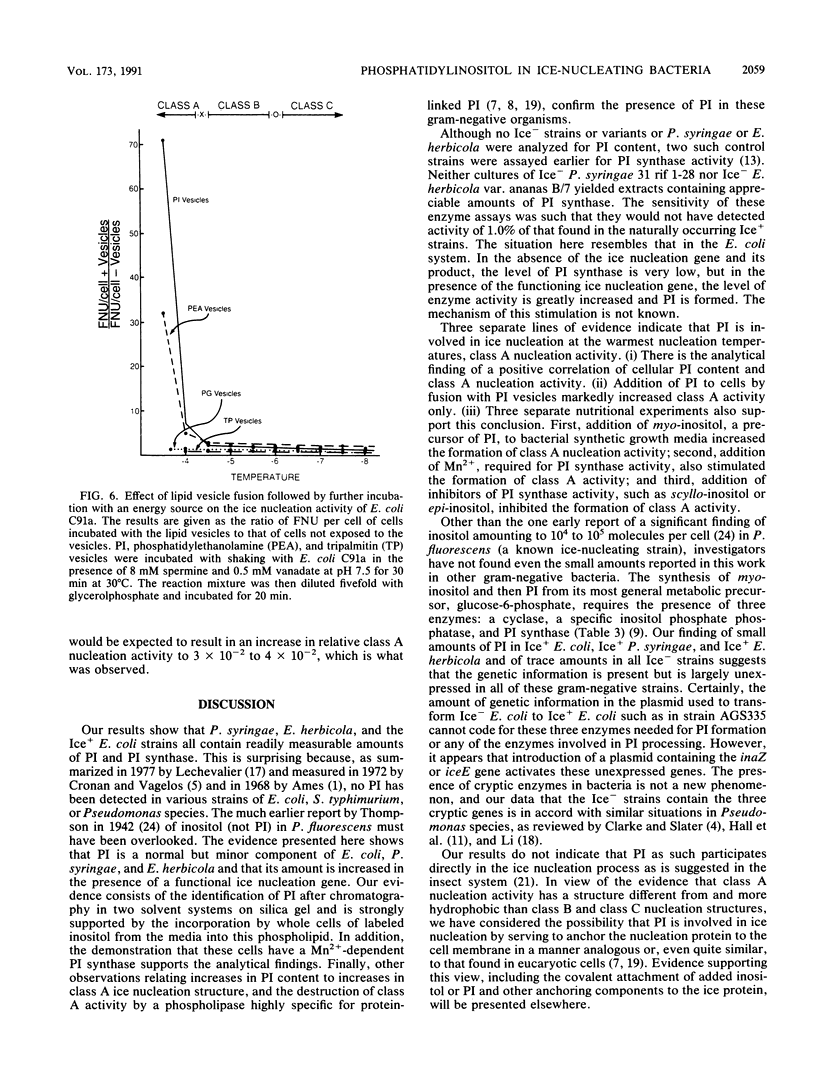
The nature of the phospholipids of the various bacteria that have ice nucleation activity in supercooled water has been determined. The seven bacteria studied included Pseudomonas syringae, Erwinia herbicola, three Escherichia coli K-12 strains that are phenotypically Ice+ because they contain plasmids with different amounts of either P. syringae or E. herbicola cloned DNA, and two E. coli K-12 strains without cloned ice gene DNA. All five Ice+ bacterial strains contained small amounts (0.1 to 1.0% of the total phospholipids) of phosphatidylinositol (PI), a phospholipid not previously detected in E. coli, Pseudomonas, or Erwinia species. The Ice- E. coli strains also contained trace level of PI that amounted to 2 to 30% of the level found in the Ice+ E. coli strains. Extracts of Ice+ strains contained low but measurable activities of PI synthase, while the activities in Ice- strains amounted to only 8 to 12% or less of that found in extracts of Ice+ bacteria. The functioning of the ice gene apparently increased both the PI synthase activity and the PI content of Ice+ strains from low endogenous levels. The relative ice nucleation activity at -4 degrees C or above (class A nucleation activity) of all Ice+ strains was found to be proportional to their PI content. The addition of myo-inositol (5 x 10(-4) M) to synthetic culture media increased the class A nucleation activity of both Ice+ E. coli strains and P. syringae up to sevenfold but had no stimulating effect on ice nucleation at lower temperatures (class B and class C nucleation activities). If these cells after fusion with PI vesicles were incubated with an energy source, the class A nucleation activity increased 70-fold over that present before fusion. These results indicate that PI plays an important role in ice nucleation at warm temperatures and is a likely precursor or component of the class A structure.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins J. A., Agranoff B. W. Distribution and properties of CDP-diglyceride:inositol transferase from brain. J Neurochem. 1969 Apr;16(4):513–527. doi: 10.1111/j.1471-4159.1969.tb06850.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Deininger C. A., Mueller G. M., Wolber P. K. Immunological characterization of ice nucleation proteins from Pseudomonas syringae, Pseudomonas fluorescens, and Erwinia herbicola. J Bacteriol. 1988 Feb;170(2):669–675. doi: 10.1128/jb.170.2.669-675.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering T. L., Masterson W. J., Hart G. W., Englund P. T. Biosynthesis of glycosyl phosphatidylinositol membrane anchors. J Biol Chem. 1990 Jan 15;265(2):611–614. [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Fischl A. S., Carman G. M. Phosphatidylinositol biosynthesis in Saccharomyces cerevisiae: purification and properties of microsome-associated phosphatidylinositol synthase. J Bacteriol. 1983 Apr;154(1):304–311. doi: 10.1128/jb.154.1.304-311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A. G., Lindow S. E. Phospholipid requirement for expression of ice nuclei in Pseudomonas syringae and in vitro. J Biol Chem. 1988 Jul 5;263(19):9333–9338. [PubMed] [Google Scholar]
- Hall B. G., Yokoyama S., Calhoun D. H. Role of cryptic genes in microbial evolution. Mol Biol Evol. 1983 Dec;1(1):109–124. doi: 10.1093/oxfordjournals.molbev.a040300. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Interaction of Salmonella typhimurium with phospholipid vesicles. Incorporation of exogenous lipids into intact cells. J Biol Chem. 1977 Oct 25;252(20):7398–7404. [PubMed] [Google Scholar]
- Kozloff L. M., Lute M., Westaway D. Phosphatidylinositol as a Component of the Ice Nucleating Site of Pseudomonas syringae and Erwinia herbiola. Science. 1984 Nov 16;226(4676):845–846. doi: 10.1126/science.226.4676.845. [DOI] [PubMed] [Google Scholar]
- Kozloff L. M., Schofield M. A., Lute M. Ice nucleating activity of Pseudomonas syringae and Erwinia herbicola. J Bacteriol. 1983 Jan;153(1):222–231. doi: 10.1128/jb.153.1.222-231.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Lewis V., Berry D. Effect of growth temperature on the lipids, outer membrane proteins, and lipopolysaccharides of Pseudomonas aeruginosa PAO. J Bacteriol. 1987 May;169(5):1960–1966. doi: 10.1128/jb.169.5.1960-1966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimore F. S., Waxman L., Goldberg A. L. Studies of the ATP-dependent proteolytic enzyme, protease La, from Escherichia coli. J Biol Chem. 1982 Apr 25;257(8):4187–4195. [PubMed] [Google Scholar]
- Lechevalier M. P. Lipids in bacterial taxonomy - a taxonomist's view. CRC Crit Rev Microbiol. 1977;5(2):109–210. doi: 10.3109/10408417709102311. [DOI] [PubMed] [Google Scholar]
- Low M. G. Glycosyl-phosphatidylinositol: a versatile anchor for cell surface proteins. FASEB J. 1989 Mar;3(5):1600–1608. doi: 10.1096/fasebj.3.5.2522071. [DOI] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P. Transfer of phosphoethanolamine residues from phosphatidylethanolamine to the membrane-derived oligosaccharides of Escherichia coli. J Bacteriol. 1987 Feb;169(2):682–686. doi: 10.1128/jb.169.2.682-686.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orser C., Staskawicz B. J., Panopoulos N. J., Dahlbeck D., Lindow S. E. Cloning and expression of bacterial ice nucleation genes in Escherichia coli. J Bacteriol. 1985 Oct;164(1):359–366. doi: 10.1128/jb.164.1.359-366.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps P., Giddings T. H., Prochoda M., Fall R. Release of cell-free ice nuclei by Erwinia herbicola. J Bacteriol. 1986 Aug;167(2):496–502. doi: 10.1128/jb.167.2.496-502.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. A., Arellano F., Kozloff L. M. Three separate classes of bacterial ice nucleation structures. J Bacteriol. 1990 May;172(5):2521–2526. doi: 10.1128/jb.172.5.2521-2526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber P. K., Deininger C. A., Southworth M. W., Vandekerckhove J., van Montagu M., Warren G. J. Identification and purification of a bacterial ice-nucleation protein. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7256–7260. doi: 10.1073/pnas.83.19.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]