Abstract
Background
Balance impairment is a frequently encountered problem in patients with Parkinson's disease. A profound balance disorder, however, is an atypical feature.
Methods
Tandem gait performance (10 consecutive tandem steps) was judged in 36 consecutive patients with Parkinson's disease and 49 consecutive patients with atypical parkinsonism.
Results
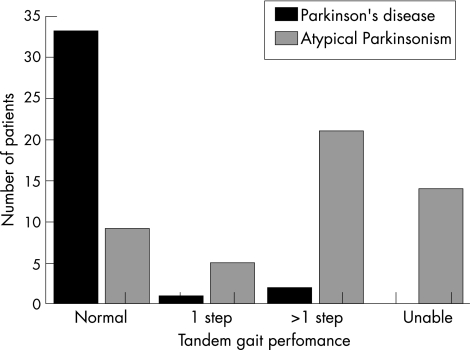
Only 9 (18%) patients with atypical parkinsonism had a fully normal tandem gait (not a single side step) as opposed to 33 (92%) patients with Parkinson's disease. Analysis for the subgroup of patients with a disease duration of <3 years yielded the same diagnostic accuracy.
Conclusions
Tandem gait performance has a good diagnostic ability to differentiate patients with atypical parkinsonism from those with Parkinson's disease, and might be used as an additional “red flag” to assist existing clinical tests in identifying atypical parkinsonism.
Differentiating Parkinson's disease from atypical parkinsonism can be challenging, particularly in the early stages of the disease.1 This differentiation is relevant, because Parkinson's disease has a much better overall prognosis and responds better to symptomatic treatment. Improved diagnostic accuracy will also facilitate recruitment of appropriate patients into future clinical trials.
Several clinical tests have been suggested to identify atypical parkinsonism,2,3,4 including a therapeutic trial on dopamine replacement, but these cannot remove all diagnostic uncertainty. In addition, several ancillary studies are available, including magnetic resonance imaging (MRI), nuclear imaging of the dopaminergic system or sphincter, and electromyography. These investigations are generally expensive, invasive and time consuming, and although helpful, have not been widely accepted for use in the routine diagnostic investigation of parkinsonian disorders.1,5 It would be attractive to have simple clinical tests that can assist in the early differential diagnosis of parkinsonism.
Patients with Parkinson's disease usually have a normal stance width during gait, whereas those with atypical parkinsonism often have a broad stance width.6 This suggests that patients with atypical parkinsonism have greater instability in the mediolateral plane, which is best probed using the tandem gait test. Profound balance impairment, especially when this develops early in the disease course, is an atypical parkinsonian feature. However, an abnormal tandem gait is never explicitly mentioned as a diagnostic test in patients with parkinsonism. Here, we explored whether tandem gait performance is useful as a simple clinical diagnostic test to differentiate patients with Parkinson's disease from those with atypical parkinsonism.
Patients and Methods
Patients
We conducted a prospective study to establish features that would help us differentiate Parkinson's disease from atypical parkinsonism. Eighty five consecutive patients referred to the Parkinson Centre Nijmegen, Nijmegen, The Netherlands, were diagnosed by movement disorder specialists (BRB and RAJE) according to established diagnostic criteria.1,7 Our cohort included 36 patients with Parkinson's disease, 24 with the parkinsonian variant of multiple system atrophy, 4 with progressive supranuclear palsy (PSP), 2 with dementia with Lewy bodies, 3 with corticobasal degeneration, 14 with vascular parkinsonism and 2 with clear atypical parkinsonism that did not fulfil any of the established diagnostic criteria. Clinical diagnosis was based on a detailed and standardised protocol that included comprehensive clinical testing and additional ancillary investigations in the framework of our study (routinely a cerebral MRI, 123IBZM‐single photon emission computed tomography (SPECT) analysis, electromyogram of the anal sphincter muscle, orthostatic blood pressure changes and occasionally 99mTc‐hexamethyl propyleneamine oxime‐SPECT or dopamine transporter imaging). All patients received at least two outpatient evaluations, and diagnosis included evaluation of treatment response to an adequately dosed trial of drugs to treat Parkinson's disease.
Patients were clustered into two groups: those with Parkinson's disease (n = 36) and those with atypical parkinsonism (n = 49). The relatively high proportion of patients with atypical parkinsonism and the relatively young age of our patients with Parkinson's disease reflect the tertiary referral bias that is encountered in dedicated movement disorder clinics such as ours.
Procedure
All patients underwent a detailed neurological examination by a single assessor (WFA) who was blinded to the diagnosis made by the movement disorder specialists (table 1). For the tandem gait test, patients were instructed to take 10 consecutive tandem steps along a straight line without walking aids and support, with eyes open. Performance was scored as follows: score 0, no side steps; score 1, single side step; score 2, multiple side steps; score 3, unable to take >4 consecutive steps in a straight line, with eyes open. If tandem gait was abnormal during the first attempt, patients were allowed a second trial, and the best performance was scored. Ethics commission approval, as well as written informed consent, was obtained.
Table 1 Clinical characteristics and tandem gait performance.
| Parkinson's disease (n = 36) | Atypical parkinsonism (n = 49) | p Value | ||
|---|---|---|---|---|
| Median (25–75% centiles) | ||||
| Age | 57.5 (49–64) | 69 (59–74) | <0.001 | |
| Disease duration (years) | 2.5 (2–4.7) | 3.3 (1.8–5) | NS | |
| Schwab and England* | 80 (70–90) | 90 (75–90) | NS | |
| UPDRS‐III | 29 (21.5–33.5) | 32 (25–49.5) | NS | |
| PIGD | 2.5 (2.0–4.0) | 5.0 (3.0–7.0) | <0.001 | |
| n (frequency; %) | χ2 | |||
| Men:women | 23: 13 | 24: 25 | NS† | |
| Anti‐parkinsonian drugs | 15 (42) | 15 (31) | NS† | |
| Psychoactive drugs | 3 (9) | 9 (18) | NS‡ | |
| History of falls | 2 (6) | 23 (47) | <0.001 | |
| Walking aids | 1 (3%) | 10 (20) | 0.02‡ | |
| Patients per age group with an abnormal tandem gait (score>0; %) | ||||
| Unadjusted for the total group | 8 | 82 | ||
| Age cohort of 50–70 years§ | 8 | 75 | ||
| For 50‐year‐old patients¶ | 7 | 59 | ||
| For 70‐year‐old patients¶ | 12 | 88 | ||
NS, not significant; PIGD, Postural Instability and Gait Disability (items 27–30 of the UPDRS‐III); UPDRS‐III, Unified Parkinson's Disease Rating Scale part three.
*Schwab and England is a daily activity scale and is part of the total UPDRS.
†Fisher's exact test.
‡Pearson's χ2 test.
§This age‐matched cohort included all the patients in this age range (25 patients with Parkinson's disease and 28 patients with atypical parkinsonism).
¶Age‐dependent percentages were calculated using a generalised linear model with binomial distribution and additive link function.
Statistical analysis
Clinical characteristics were compared between patients with Parkinson's disease and those with atypical parkinsonism using the Mann–Whitney U test, Pearson's χ2 test or Fisher's exact test. Sensitivity, specificity and post‐test probability were calculated for an abnormal tandem gait to identify patients with atypical parkinsonism. In addition, these were calculated for the cohort of 50–70‐year‐old patients. Owing to an age difference between both groups, we adjusted for age and calculated the age‐dependent sensitivity and specificity using regression analysis (generalised linear model with binomial distribution and additive link function).
Results
The proportion of patients with a completely normal tandem gait (score 0) was significantly greater in the Parkinson's disease group than in the atypical parkinsonism group (table 1 and fig 1). In all three patients with Parkinson's disease with an abnormal tandem gait, MRI of the brain showed mild white matter lesions, but these patients did not fulfil the formal criteria for vascular parkinsonism.7 Overall, an abnormal tandem gait test differentiated Parkinson's disease from atypical parkinsonism with a sensitivity of 82% and a specificity of 92%. In the age‐matched cohort of 53 patients aged 50–70 years, the sensitivity was 75% with a specificity of 92% (table 1). In all, 25 patients with Parkinson's disease (mean age 60.9 (standard deviation (SD) 5.9) years) and 28 patients with atypical parkinsonism (mean age 61.5 (SD 6.7) years; no statistical difference in mean age between both groups) were included in this cohort. Furthermore, age‐adjusted accuracy numbers derived from regression analysis showed that although the sensitivity increased with age, the specificity slightly decreased. For a 50‐year‐old patient, the sensitivity was 59% and the specificity 93%. For a 70‐year‐old patient, the sensitivity was 86% and the specificity 88%. This increase in diagnostic accuracy with age was explained by an age‐dependent decrease in tandem gait performance that was seen only for patients with atypical parkinsonism but not for patients with Parkinson's disease.
Figure 1 Tandem gait performance, which was scored as follows: score 0, no side steps; score 1, single side step; score 2, multiple side steps; score 3, unable to take >4 consecutive steps in a straight line, with eyes open.
Differentiating patients with Parkinson's disease is especially difficult early in the disease course. Therefore, we also calculated the accuracy of the tandem gait performance for the subgroup with a disease duration of <3 years. This subgroup consisted of 21 patients with Parkinson's disease and 20 patients with atypical parkinsonism. Only 1 (4.8%) patient with Parkinson's disease had abnormal tandem gait compared with 17 of the 20 (85%) patients with atypical parkinsonism.
History of falls correlated weakly with tandem gait performance in the atypical parkinsonism group (r2 = 0.13, p = 0.01), but not in patients with Parkinson's disease. Furthermore, the Postural Instability and Gait Disability (PIGD) scores of the Unified Parkinson's Disease Rating Scale part III correlated (r2 = 0.33; p<0.001) with tandem gait in patients with atypical parkinsonism, but not in those with Parkinson's disease.
Because of selective referral to the dedicated Parkinson Centre Nijmegen, the percentage of patients with atypical parkinsonism was relatively high. This could have led to an overestimation of percentages of tandem gait accuracy percentages. Hence, accuracy was recalculated using community‐based prevalences. Assuming that the proportion of atypical parkinsonism among all parkinsonian disorders in patients aged 50–70 years is 40% (pre‐test probability),8 the post‐test probability was calculated for an abnormal tandem gait performance for patients aged 50–70 years (table 1, n = 53). In this age cohort, abnormal tandem gait performance increased the post‐test probability of having atypical parkinsonism from 40% (pre‐test odds 0.67) to 86% (post‐test odds 6.25).
Discussion
These results suggest that a standardised execution and scoring system of tandem gait performance has a good diagnostic ability to differentiate Parkinson's disease from atypical parkinsonian disorders. The tandem gait test is easy to conduct and simple to interpret with the proposed straightforward scoring system. Tandem gait performance according to our simple scoring system could thus provide complementary diagnostic information, next to the existing clinical tests and diagnostic criteria. An abnormal tandem gait performance should alert the doctor to possible atypical parkinsonism, as is the case with other so‐called “red flags”.2 These findings confirm the clinical impression that patients with Parkinson's disease typically have a normal base of support during walking,9 whereas those with atypical parkinsonism often have mediolateral instability.6 This mediolateral instability may be explained by neuropathological findings of involvement of the superior cerebellar peduncle in PSP or cerebellar involvement in the parkinsonian variant of multiple system atrophy.6,10 Gait ataxia may also result from more extensive frontal lobe involvement in atypical parkinsonism, as occurs in PSP and often also in vascular parkinsonism.
Interestingly, neuroimaging showed concurrent mild white‐matter lesions in all three patients with Parkinson's disease with an abnormal tandem gait. Our ongoing follow‐up of this cohort will have to show whether these patients will develop full‐blown vascular parkinsonism or some other form of parkinsonism with coexisting white‐matter lesions.
The diagnostic value of the tandem gait test increased with age. This was explained by an age‐related increase in sensitivity, which was only partially offset by a slightly lower specificity. Tandem gait performance gained accuracy with age because an age‐dependent decrease in tandem gait performance existed only in the atypical parkinsonism group.
Tandem gait performance partially correlated with a history of falls in patients with atypical parkinsonism. This suggests that increased mediolateral instability may contribute to some of the falls in these patients. Tandem gait performance was also related to the global severity of balance and gait, as indexed by the PIGD items of the Unified Parkinson's Disease Rating Scale. This suggests that tandem gait performance is not an independent measure, but rather a marker or reflection of more general problems with axial motor control, particularly in patients with atypical parkinsonism. Compared with currently available tests to score the PIGD items, an advantage of assessing tandem gait is the straightforward test execution and unambiguous scoring system. As such, tandem gait performance provides a clinically feasible marker to screen for the presence of axial motor problems, and to assist other tests (such as the pull test and single‐limb stance) in estimating the risk of falling in patients with an atypical parkinsonian syndrome.11
Tandem gait is a routine clinical test incorporated in the standard neurological examination and is currently used to assess (mild) gait ataxia in patients suspected of having a cerebellar or vestibular syndrome. In patients with parkinsonian disorders, taking just a single side step may easily be overlooked and classified as normal, whereas in fact it can have additional diagnostic potential, as observed in our study. A subanalysis of patients with a short disease duration showed that tandem gait performance had a good diagnostic ability to identify atypical parkinsonism. However, additional studies will be required to further establish the diagnostic value of this test in the differential diagnosis of patients in early stages of the disease. On the basis of the present data, we recommend the inclusion of tandem gait performance in the routine clinical assessment of patients with parkinsonism, using the proposed standardised and simple scoring system. A tandem gait performance with only one side step out of 10 consecutive steps should be scored as an additional atypical parkinsonian sign (“red flag”).
Acknowledgements
We thank Dr BPC van de Warrenburg for his critical review and suggestions.
Abbreviations
MRI - magnetic resonance imaging
PIGD - postural instability and gait disability
PSP - progressive supranuclear palsy
SPECT - single photon emission computed tomography
Footnotes
Funding: WFA was supported by a research grant from the Stichting Internationaal Parkinson Fonds. This non‐corporate funding organisation had no role in study design or conduct of the study, data collection, data analysis and interpretation, and manuscript preparation.
Competing interests: None.
References
- 1.Litvan I, Bhatia K P, Burn D J.et al Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for parkinsonian disorders. Mov Disord 200318467–486. [DOI] [PubMed] [Google Scholar]
- 2.Quinn N. Multiple system atrophy—the nature of the beast. J Neurol Neurosurg Psychiatry 1989(Suppl)78–89. [DOI] [PMC free article] [PubMed]
- 3.Klein C, Brown R, Wenning G.et al The “cold hands sign” in multiple system atrophy. Mov Disord 199712514–518. [DOI] [PubMed] [Google Scholar]
- 4.Dubois B, Slachevsky A, Pillon B.et al “Applause sign” helps to discriminate PSP from FTD and PD. Neurology 2005642132–2133. [DOI] [PubMed] [Google Scholar]
- 5.Scherfler C, Seppi K, Donnemiller E.et al Voxel‐wise analysis of [123I]beta‐CIT SPECT differentiates the Parkinson variant of multiple system atrophy from idiopathic Parkinson's disease. Brain 20051281605–1612. [DOI] [PubMed] [Google Scholar]
- 6.Bloem B R, Bhatia K P. Gait and balance in basal ganglia disorders. In: Bronstein AM, Brandt T, Nutt JG, Woollacot MH, eds. Clinical disorders of balance, posture and gait. London: Arnold, 2004173–206.
- 7.Zijlmans J C, Daniel S E, Hughes A J.et al Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord 200419630–640. [DOI] [PubMed] [Google Scholar]
- 8.Schrag A, Ben‐Shlomo Y, Quinn N P. Cross sectional prevalence survey of idiopathic Parkinson's disease and Parkinsonism in London. BMJ 200032121–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlett A, Weller C, Purkiss A G.et al Breadth of base whilst walking: effect of ageing and parkinsonism. Age Ageing 19982749–54. [DOI] [PubMed] [Google Scholar]
- 10.Paviour D C, Price S L, Stevens J M.et al Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 200564675–679. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs J V, Horak F B, Tran V K.et al Multiple balance tests improve the assessment of postural stability in subjects with Parkinson's disease. J Neurol Neurosurg Psychiatry 200677322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]