The persistent vegetative state (PVS) is a condition in which awareness of the self and the environment is absent. However, neuroimaging studies suggest that normal brain activity in response to speech and faces can remain in the PVS, although it is not clear if this activity reflects higher levels of perception and cognitive processes, as only meaningless stimuli were used as control conditions.1 One way to investigate such higher cognitive processes is to examine whether differential activity can be evoked by different contents of language.
We examined brain activity in a patient in the PVS during hearing his own first name compared with another name. Previous studies found activation of the medial prefrontal cortex (MPFC) in healthy subjects during hearing their own name and self referential processing.2
We investigated the case of a 50‐year‐old man, who had a myocardial infarction with cardiac arrest in March 2003. The diagnosis of PVS was confirmed by two neurologists. The patient showed normal hypothalamic and brain stem autonomic functions and a normal sleep–wake cycle, but no signs of awareness. Magnetic resonance imaging of the brain showed diffuse symmetric leucoencephalopathy and brain atrophy. Positron emission tomography showed an overall reduction in resting brain metabolism (especially marked in the bilateral frontal, parietal and cingulate cortex, as well as in the precuneus). The functional magnetic resonance imaging (fMRI) study was carried out in January 2004; the patient's legal guardian provided informed consent. The patient was still in a PVS when he died in 2005.
Event‐related fMRI was used to explore brain activity during hearing a phrase (eg, “Martin, hello Martin”) containing one's own or another first name. Stimuli (normalised to equal sound level) were recorded from 5 men and 5 women, none of which were familiar to the patient or knew his real first name. Two fMRI runs were carried out, both containing 30 own and 30 other name stimuli, and 30 silent null events (duration = 2200 ms; ISI = 1800 ms). During each run, 180 functional images (matrix 64×64, TR 2200 ms, TE 40 ms, FA 86°, 21 six‐mm thick slices) were acquired with a Philips 1.5‐T MR‐Scanner (Philips Medical Systems, Best, The Netherlands). The patient was awake with open eyes during the study.
Statistical parametric mapping software (SPM2; http://www.fil.ion.ucl.ac.uk/spm) was used for data preprocessing (movement and slice‐timing correction, linear normalisation to MNI space and 8 mm full‐width at half‐maximum smoothing) and analysis.
A t test was used to identify regions with higher activity for one's own name than for another name. The same contrast was examined in three male control subjects (aged 32, 42 and 45 years) with a conjunction analysis. Although the control group was not perfectly matched with respect to age, this should not constitute a major problem, as it was mainly included to see whether MPFC activation in response to their own name could be detected with this paradigm. A small volume correction (svc) was used for the MPFC (26 mm diameter sphere, based on previous studies, centred at x = 2, y = 52, z = 14) to correct for multiple comparisons.
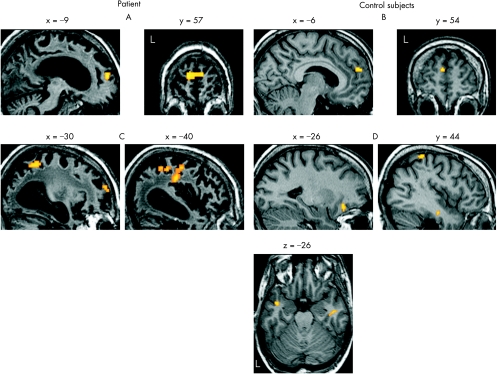
The patient showed higher brain activity during hearing his own name than another name in the bilateral MPFC (x = −6, y = 57, z = 12, p = 0.024, svc; fig 1A). The same effect was also observed in the left temporoparietal and superior frontal cortex, although only at an uncorrected significance level of p<0.005 (fig 1C). We were concerned about normalising the patient's brain owing to the widespread atrophy. However, we used only linear normalisation and manually checked that the results of the normalisation were correct. Additionally, MPFC activation was also found in an analysis using un‐normalised data.
Figure 1 Brain regions showing higher activity during hearing one's own name than another name in the medial prefrontal cortex (MPFC) (p<0.05, small volume correction (svc)) for the patient with persistent vegetative state (A) and for the three controls (B). Other brain regions showing the same effect (p<0.005, uncorrected) for the patient with persistent vegetative state (C) and for the three controls (D). Activations for the controls are shown on the structural scan from one participant.
The controls showed reliably higher activity for their own names in a comparable MPFC region, restricted to the left hemisphere (x = −6, y = 54, z = 22, p = 0.044, svc; fig 1B). At an uncorrected p<0.005, this effect was also found in bilateral inferior temporal regions, the right superior parietal and left orbital frontal cortex (fig 1D).
In summary, the patient showed differential cortical processing of his own name compared with another name in the MPFC. A comparable region showed the same effect in three controls. Visual inspection suggests that the MPFC activation was bilateral in the patient, but restricted to the left hemisphere in the controls, although the meaning of this difference remains unclear. At an uncorrected threshold, we identified regions that were activated only in the controls or in the patient. As the small sample size precluded a direct statistical comparison, no conclusions can be drawn on differences in brain activity during this paradigm between this patient with PVS and healthy subjects.
Selective cortical processing of one's own name requires the ability to perceive and access the meaning of words. Clearly, this result shows that residual language recognition can be observed in the PVS, extending previous findings.1 We do not claim that this result indicates complete awareness, as implicit perception can occur in the absence of conscious perception.
A limitation of this study is that recognising one's own name (an extremely salient stimulus) may be one of the most basic forms of language. This specific response could therefore reflect the existence of an isolated module, which may be compatible with absence of awareness, as connections between distant brain areas, which are linked to consciousness, are disrupted in the PVS.3
Furthermore, care should be taken in generalising the results of a single case study, but other fMRI results also indicate that residual language processing can be preserved in the PVS.4 One could argue that this patient was not in a PVS, but in a minimally conscious state. A selective electroencephalogram response to one's own name in the minimally conscious state was recently reported.5 However, our patient fulfilled all diagnostic criteria for the PVS, and positron emission tomography showed a compatible metabolic pattern.3
Nevertheless, this result may imply that the boundary between different disorders of consciousness is not always clearly delineated. This study shows that fMRI can provide valuable information on residual cognitive processes in the PVS. Information on whether a patient in a PVS can perceive his own name and probably other personally relevant stimuli may also be important for relatives and for planning care and rehabilitative attempts.
Acknowledgements
We thank the members of the Department of Radiology for assistance.
Footnotes
Funding: The University of Salzburg provided support to the Center for Neurocognitive Research for establishing the collaboration between the Department of Neurology at the Christian Doppler Clinic and the Department of Psychology.
Consent was obtained for publication of the patient's details described in this report.
Competing interests: None declared.
References
- 1.Owen A M, Menon D K, Johnsrude I S.et al Detecting residual cognitive function in persistent vegetative state. Neurocase 20028394–403. [DOI] [PubMed] [Google Scholar]
- 2.Northoff G, Heinzel A, de Greck M.et al Self‐referential processing in our brain—a metaanalysis of imaging studies on the self. Neuroimage 200631440–457. [DOI] [PubMed] [Google Scholar]
- 3.Laureys S, Owen A M, Schiff N D. Brain function in coma, vegetative state, and related disorders. Lancet Neurol 20043537–546. [DOI] [PubMed] [Google Scholar]
- 4.Owen A M, Coleman M R, Menon D K.et al Residual auditory function in persistent vegetativestate: a combined PET and fMRI study. Neuropsychol Rehabil 200515290–306. [DOI] [PubMed] [Google Scholar]
- 5.Laureys S, Perrin F, Faymonville M E.et al Cerebral processing in the minimally conscious state. Neurology 200463916–918. [DOI] [PubMed] [Google Scholar]