Abstract
Background
The clinical and immunological profiles of patients with paraneoplastic cerebellar degeneration (PCD) and non‐small‐cell lung cancer (NSCLC) are not well known.
Objective
To review the clinical and immunological features of patients with PCD, NSCLC and without well‐characterised onconeural antibodies.
Methods
The clinical features of nine patients with the diagnosis of classical PCD and NSCLC, included in our archives, were retrospectively reviewed. The presence of antibodies to cerebellar components was determined by immunohistochemistry and immunoblot of rat cerebellum. A cDNA library of human cerebellum was screened with the positive sera to identify the antigen.
Results
Nine patients with PCD and NSCLC were identified. Six patients were men, and the median age at diagnosis of PCD was 63 (range 47–73) years. PCD was completely reversed in two patients, and partially in one, after treatment of the tumour. The serum of one of the patients with PCD showed a unique reactivity with Purkinje cells. The screening of a cerebellar‐expression library resulted in the isolation of protein kinase Cγ (PKCγ). PKCγ immunoreactivity was not observed in the serum of 170 patients with non‐paraneoplastic neurological syndromes, 27 patients with PCD, no onconeural antibodies and small‐cell lung cancer, and 52 patients with NSCLC without paraneoplastic neurological syndromes. The NSCLC from 11 patients without PCD did not express PKCγ at either the RNA or protein level. However, many cells of the NSCLC of the patient with PKCγ antibodies expressed PKCγ.
Conclusion
PCD occurs in patients with NSCLC without typical onconeural antibodies and is associated with immune reactions against key proteins of the Purkinje cells.
Paraneoplastic cerebellar degeneration (PCD) is characterised by selective damage to the Purkinje cells of the cerebellum, which usually causes a severe pancerebellar syndrome.1 In patients with lung cancer, PCD is almost always associated with small‐cell lung cancer (SCLC).2 Patients with non‐small‐cell lung cancer (NSCLC) and PCD usually harbour onconeural antibodies typically associated with SCLC in the serum and cerebrospinal fluid, which probably indicate a common immune‐mediated mechanism of neuronal damage.3,4 Although a few studies have indicated the occurrence of PCD in patients with NSCLC and no onconeural antibodies, these patients were usually included in larger series of patients with PCD with different tumours2,5,6,7 or were reported as single observations.8
In this study, we describe the clinical and immunological findings of a series of patients without previously characterised onconeural antibodies who presented with PCD associated with NSCLC.
Patients and methods
We retrieved from our archives the data on patients with the final diagnosis of classical PCD, according to published criteria,9 and NSCLC. We specifically excluded patients who were positive for onconeural antibodies (Hu, Yo, Ri, CV2, Tr, Ma2, amphiphysin). Patients with PCD with this profile represented only 4% of the whole series of 121 patients with PCD registered in Barcelona's database. Serum and cerebrospinal fluid, when available, were evaluated by immunohistochemistry, on frozen sections of paraformaldehyde‐fixed rat tissues.10 Rat cerebella were homogenised in the presence of protease inhibitors and centrifuged at 3000 g for 10 min. The supernatant was ultracentrifuged at 130 000 g for 30 min and the supernatant was retained. Samples were separated by electrophoresis on a 4–12% polyacrylamide gel, transferred to nitrocellulose paper and subjected to standard immunoblot procedures using an avidin–biotin method as described previously.10
Screening of a cerebellar cDNA expression library
A Uni‐ZAP XR Library (Stratagene, La Jolla, California, USA) from human cerebellum was immunoscreened as reported previously.11 Phage‐positive clones were subcloned in pBluescript using the in vivo excision phage rescue protocol (Stratagene) and sequenced. The NCBI BLASTn program (National Center for Biotechnology Information, National Institutes of Health, Bethesda, Maryland, USA) was used to search for homologies.
Affinity purification of antibodies
Filters with purified phage plaques expressing protein kinase Cγ (PKCγ) or irrelevant Escherichia coli proteins were incubated with patient's serum or an anti‐Hu‐positive serum (dilution 1:200) for 12 h at 4°C. After extensive washing, bound antibodies were eluted with sodium citrate, pH 2.5, and neutralised with TRIS, pH 8.8. Purified antibodies were concentrated with a Centricon Plus‐20 centrifugal filter (Millipore, Billerica, Massachusetts, USA), and immunoglobulin (Ig)G was measured by nephelometry.
Analysis of PKCγ in NSCLC
RNA was isolated from five NSCLCs of patients without PCD using the RNeasy Mini Kit (Qiagen, Santa Clarita, California, USA), and treated with DNA‐free DNAse (Ambion, Austin, Texas, USA). After retrotranscription with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, California, USA), the cDNA samples were normalised by glyceraldehyde‐3‐phosphate dehydrogenase expression and then amplified with PKCγ‐specific primers.
Frozen (n = 5) or paraffin‐wax‐embedded (n = 6) NSCLC sections from patients without PCD, and paraffin‐wax‐embedded sections from the NSCLC of the patient with PCD with antibodies to PKCγ were probed with a specific polyclonal antibody to PKCγ (Santa Cruz Biotechnology, Santa Cruz, California, USA) using a standard avidin–biotin immunoperoxidase technique.
Results
We identified nine patients with classical PCD and NSCLC. Table 1 summarises the main clinical features. Six patients were men and the median age at diagnosis of PCD was 63 (range 47–73) years. Two patients had completely recovered from PCD at the time of tumour remission and the syndrome reappeared in one of them when the tumour relapsed; another patient had marked neurological improvement after tumour resection.
Table 1 Clinical features of patients with paraneoplastic cerebellar degeneration and non‐small‐cell lung cancer.
| Patient | Age (years)/ sex | Time to cancer diagnosis (months) | Rankin score | CSF pleocytosis | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1 | 47/M | 2.5 | 4 | Yes | Chemotherapy | Death from cancer, progression of PCD |
| 2 | 54/M | 1 | 3 | Yes | Chemotherapy, radiotherapy | Death from myocardial infarction 6 months later, PCD: complete recovery |
| 3 | 73/M | 30 | 3 | No | None | Death from cancer, PCD: progression |
| 4 | 65/M | 2 | 3 | No | Chemotherapy, radiotherapy | Lost to follow‐up |
| 5 | 52/M | 7 | 3 | No | Surgery, chemotherapy | Tumour in remission, PCD: stable |
| 6 | 63/M | 2 | 3 | No | Surgery, chemotherapy | Death from cancer, PCD: remission, then relapse at the time of tumour progression |
| 7 | 62/F | 3 | 4 | Yes | Chemotherapy | Death from cancer, progression of PCD |
| 8 | 56/F | 6 | 4 | Yes | Chemotherapy, radiotherapy | Tumour in remission, PCD: stable |
| 9 | 66/M | 3 | 4 | No | Surgery | Tumour in remission, PCD: partial improvement (Rankin score 2), stable for 18 months |
CSF, cerebrospinal fluid; F, female; M, male; PCD, paraneoplastic cerebellar degeneration.
Sera of eight of the nine patients did not disclose any specific immunoreactivity when tested on rat cerebellum; however, the serum of patient 1 (table 1) immunoreacted with the cytoplasm, dendrites and axons of the Purkinje cells of rat (fig 1) and human cerebellum. No reactivity was observed in systemic rat tissues. The serum of the patient recognised a single band of about 80 kDa in immunoblots of rat cerebellum (fig 2). The specific antibody to PKCγ produced the same results than the patient's serum either by rat cerebellum immunohistochemistry or immunoblot (fig 2). PKCγ immunoreactivity was not observed in the serum of 170 patients with non‐paraneoplastic neurological syndromes, 27 patients with PCD, no onconeural antibodies and SCLC, 52 patients with NSCLC without paraneoplastic neurological syndromes, and 105 patients with anti‐Hu (n = 50), anti‐Yo (n = 30) or anti‐CV2 (n = 25) antibodies. All sera were tested by both immunohistochemistry and immunoblot.
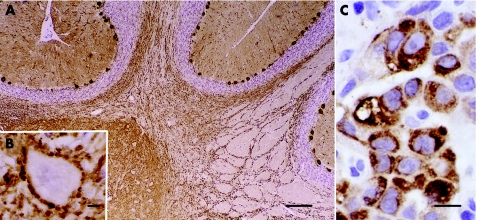
Figure 1 Frozen section of paraformaldehyde‐fixed rat cerebellum immunoreacted with the patient's serum. (A) Intense labelling of the cytoplasm, dendrites and axons of Purkinje cells. Bar = 120 μm. (B) Higher magnification showing a neurone of the deep cerebellar nucleus outlined by densely apposed axon terminals of Purkinje cells. Bar = 5 μm. (C) Paraffin‐wax‐embedded section of patient's non‐small‐cell lung cancer showing robust immunoreactivity when probed with a specific anti‐protein kinase Cγ antibody. Bar = 10 μm. Sections counterstained with haematoxylin.
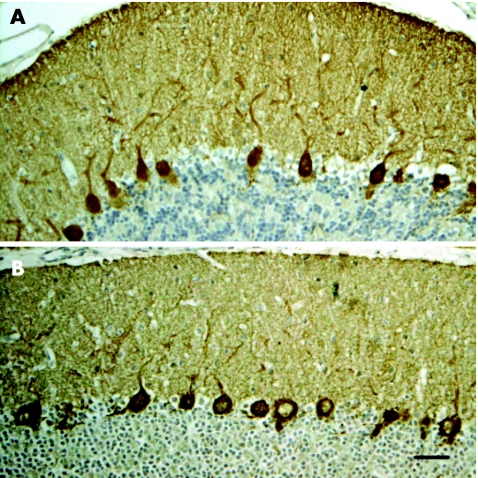
Figure 2 Rat cerebellum immunoreacted with the patient's immunoglobulin (IgG) eluted from the (A) protein kinase Cγ (PKCγ) clone and (B) with an anti‐PKCγ‐specific antibody. Bar = 30 μm. Sections counterstained with haematoxylin.
Identification of cDNA clones
Screening of the cerebellar expression library resulted in the isolation of two positive clones identical with the human PKCγ gene (GenBank accession number NM_002739). Sequencing on both strands of one of the PKCγ clones was carried out, yielding a consensus sequence spanning 2978 nucleotides that included the complete predicted open reading frame (283–2376 bp).
Analysis of affinity‐purified antibodies
To ensure the specificity of antibody recognition, IgG eluted from purified phage plaques expressing the PKCγ protein produced similar cytoplasmic staining on rat cerebellum immunohistochemistry (fig 2) and identified the same band in immunoblots of rat cerebellum (fig 3) as in the patient's serum or the polyclonal PKCγ antibody.
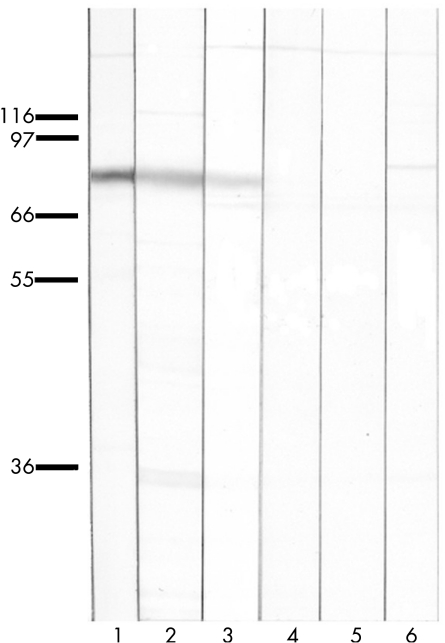
Figure 3 Immunoblots of rat cerebellum probed with an anti‐protein kinase Cγ (PKCγ)‐specific antibody (lane 1), patient's serum (lane 2), patient's immunoglobulin (Ig)G eluted from the PKCγ clone (lane 3) or an irrelevant clone (lane 4), IgG from an anti‐Hu‐positive serum eluted from the PKCγ clone (lane 5) and normal human serum (lane 6). Patient's serum and IgG eluted from the PKCγ clone recognise a band at 80 kDa also labelled with the anti‐PKCγ‐specific antibody. All lanes contain 10 µg of protein. All eluted IgGs normalised at 0.05 µg/ml.
Analysis of PKCγ in NSCLC
The NSCLC from patients without PCD did not express PKCγ at either the RNA or protein level. However, many tumour cells of the NSCLC of the patient with PKCγ antibodies stained strongly with the polyclonal PKCγ antibody (fig 1).
Discussion
Lung cancer is one of the most common tumours associated with PCD.1 Patients with SCLC and PCD, without predominant involvement of other areas of the nervous system (paraneoplastic encephalomyelitis), often do not have onconeural antibodies, although as many as 40% of them harbour voltage‐gated calcium channel (VGCC) antibodies.2 PCD in patients with NSCLC may associate with onconeural antibodies typically described in paraneoplastic syndromes linked to SCLC, mostly anti‐Hu and anti‐CV2 antibodies.3,4 However, the clinical and immunological features of a series of patients with PCD and NSCLC without onconeural antibodies had not been previously described. We identified nine patients with this profile. The cerebellar syndrome did not differ from that seen in PCD associated with other tumours.1,2 However, two patients showed complete remission of the PCD after successful treatment of the tumour, and one made a significant recovery, a rare event in PCD associated with SCLC.2,12,13
This study identified PKCγ as the antigen recognised by the anti‐Purkinje cell antibody detected in the serum of one of the nine patients of the series. A previous case report identified an anti‐Purkinje cell antibody in the serum of a patient with PCD and NSCLC, but the antibody was not further characterised.8 PKC comprises a large family of proteins, with differences in enzymological properties and tissue distribution. PKC isoenzymes are classified into three groups: the conventional PKCs that include PKCγ and are activated by Ca2+; the novel PKCs that lack the Ca2+‐binding domain; and the atypical PKCs that have been linked to cellular Ras signalling.14
At least nine PKC isoenzymes are present in the brain, where PKCγ is selectively expressed.15 In the brain, PKCγ is particularly highly expressed in the Purkinje cells of the cerebellum, where it is thought to have an important role in signal transduction and synaptic transmission. In fact, missense mutations in the regulatory domain of PKCγ have been identified as a new cause of autosomal dominant cerebellar ataxia (spinocerebellar ataxia 14).16 In transgenic mice with spinocerebellar ataxia 1, PKCγ degradation occurs before the onset of ataxia, suggesting that the loss of this protein is important for Purkinje cell dysfunction.17 High‐resolution immunogold cytochemistry to investigate the subcellular distribution of PKCγ in Purkinje cells of rat cerebellum identified robust immunoreactivity in the plasma membrane of the dendrites, cell body and, particularly, the initial segment of the Purkinje cell axons, which is assumed to be the primary locus of action potential generation in this neurone.18 PKCγ is attached to the inner aspect of the membrane, so it is unlikely that the patient's antibodies could reach the antigen in vivo to cause cerebellar dysfunction unless the antibodies also recognised other cell surface proteins closely related to PKCγ. This possibility has been observed in Lambert–Eaton myasthenic syndrome where some patients, in addition to VGCC antibodies, have antibodies to synaptotagmin, a functionally VGCC‐associated synaptic protein.19
Although the PKC family has a key regulatory role in various cancer‐associated signal transduction pathways, in line with previous work, we did not observe the expression of PKCγ in the NSCLC of patients without PCD.20 However, the NSCLC of the patient with PKCγ antibodies strongly immunoreacted with a specific antibody to PKCγ, suggesting that the expression of PKCγ by the tumour cells may have triggered an immune response that finally caused the PCD. Absence of PKCγ expression in NSCLC or SCLC21 probably explains why we did not observe other patients with PCD with antibodies to PKCγ. Despite being a unique observation, our patient proves that PCD occurs with NSCLC through immune mechanisms induced by antigens different from those typically associated with SCLC. The absence of antineuronal antibodies in the other patients of this series does not rule out the possibility that the antibodies were directed against membrane antigens and are not usually detected by the techniques used in our study.
Acknowledgements
We thank Mercè Bonastre and Eva Caballero for their excellent technical assistance.
Abbreviations
NSCLC - non‐small‐cell lung cancer
PCD - paraneoplastic cerebellar degeneration
PKCγ - protein kinase Cγ
SCLC - small‐cell lung cancer
VGCC - voltage‐gated calcium channel
Footnotes
Funding: This study was supported in part by grants 2001SGR 00387 from Generalitat de Catalunya, PI030028 from Fondo de Investigaciones Sanitarias, Madrid, Spain (to FG), and RO1CA107192 (to JD).
Competing interests: None.
References
- 1.Posner J B. Paraneoplastic syndromes. In: Neurologic complications of cancer, Philadelphia FA Davis Company, 1995353–385.
- 2.Graus F, Lang B, Pozo‐Rosich P.et al P/Q type calcium‐channel antibodies in paraneoplastic cerebellar degeneration with lung cancer. Neurology 200259764–766. [DOI] [PubMed] [Google Scholar]
- 3.Graus F, Keime‐Guibert F, Reñé R.et al Anti‐Hu‐associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 20011241138–1148. [DOI] [PubMed] [Google Scholar]
- 4.Yu Z, Kryzer T J, Griesmann G E.et al CRMP‐5 neuronal autoantibody: marker of lung cancer and thymoma‐related autoimmunity. Ann Neurol 200149146–154. [PubMed] [Google Scholar]
- 5.Wessel K, Budde‐Steffen C, Wiethölter H.et al Cerebellar dysfunction in patients with bronchogenic carcinoma: immunological investigations. J Neurol 1988235297–299. [DOI] [PubMed] [Google Scholar]
- 6.Hammack J E, Kimmel D W, O'Neill B P.et al Paraneoplastic cerebellar degeneration: a clinical comparison of patients with and without Purkinje cell cytoplasmic antibodies. Mayo Clin Proc 1990651423–1431. [DOI] [PubMed] [Google Scholar]
- 7.Anderson N E, Rosemblum M K, Posner J B. Paraneoplastic cerebellar degeneration: clinical‐immunological correlations. Ann Neurol 198824559–567. [DOI] [PubMed] [Google Scholar]
- 8.Anderson N E, Budde‐Steffen C, Wiley R G.et al A variant of the anti‐Purkinje cell antibody in a patient with paraneoplastic cerebellar degeneration. Neurology 1988381018–1026. [DOI] [PubMed] [Google Scholar]
- 9.Graus F, Delattre J Y, Antoine J C.et al Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004751135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saiz A, Arpa J, Sagasta A.et al Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late‐onset insulin dependent, and polyendocrine autoimmunity. Neurology 1997491026–1030. [DOI] [PubMed] [Google Scholar]
- 11.Bataller L, Rosenfeld M R, Graus F.et al Autoantigen diversity in the opsoclonus‐myoclonus syndrome. Ann Neurol 200353347–353. [DOI] [PubMed] [Google Scholar]
- 12.Paone J F, Jeyasingham K. Remission of cerebellar dysfunction after pneumonectomy for bronchogenic carcinoma. N Engl J Med 1980302156. [DOI] [PubMed] [Google Scholar]
- 13.Counsell C E, McLeod M, Grant R. Reversal of subacute paraneoplastic cerebellar syndrome with intravenous immunoglobulin. Neurology 1994441184–1185. [DOI] [PubMed] [Google Scholar]
- 14.Newton A C. Protein kinase C: structure, function, and regulation. J Biol Chem 199527028495–28498. [DOI] [PubMed] [Google Scholar]
- 15.Wetsel W C, Khan W A, Merchenthaler I.et al Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol 1992117121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D H, Brkanac Z, Verlinde C L M J.et al Missense mutations in the regulatory domain of PKCγ: a new mechanism for dominant nonepisodic cerebellar ataxia. Am J Hum Genet 200372839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner P J, Vierra‐Green C A, Clark H B.et al Altered trafficking of membrane proteins in Purkinje cells of SCA1 transgenic mice. Am J Pathol 2001159905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardell M, Landsend A S, Eidet J.et al high resolution immunogold analysis reveals distinct subcellular comparmentation of protein kinase Cγ and δ in rat Purkinje cells. Neuroscience 199882709–725. [DOI] [PubMed] [Google Scholar]
- 19.Leveque C, Hoshino T, David P.et al The synaptic vesicle protein synaptotagmin associates with calcium channels and is a putative Lambert‐Eaton myasthenic syndrome antigen. Proc Natl Acad Sci USA 1992893625–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark A S, West K A, Blumberg P M.et al Altered protein kinase C (PKC) isoforms in non‐small cell lung cancer cells: PKCδ promotes cellular survival and chemotherapeutic resistence. Cancer Res 200363780–786. [PubMed] [Google Scholar]
- 21.Kanashiro C A, Schally A V, Zarandi M.et al Suppression of growth of H‐69 small cell lung carcinoma by antagonists of growth hormone releasing hormone and bombesin is associated with an inhibition of protein kinase C signaling. Int J Cancer 2004112570–576. [DOI] [PubMed] [Google Scholar]