Abstract
Background
The precise time of stroke onset during sleep is difficult to specify, but this has a considerable influence on circadian variations of stroke onset.
Aim
To investigate circadian variations in situations at stroke onset—that is, in the waking state or during sleep—and their differences among subtypes.
Methods
12 957 cases of first‐ever stroke onset diagnosed from the Iwate Stroke Registry between 1991 and 1996 by computed tomography or magnetic resonance imaging were analysed. Circadian variations were compared using onset number in 2‐h periods with relative risk for the expected number of the average of 12 2‐h intervals in the waking state or during sleep in cerebral infarction (CIF), intracerebral haemorrhage (ICH) and subarachnoid haemorrhage (SAH).
Results
ICH and SAH showed bimodal circadian variations and CIF had a single peak in all situations at onset, whereas all three subtypes showed bimodal circadian variations of stroke onset in the waking state only. These variations were different in that CIF showed a bimodal pattern with a higher peak in the morning and a lower peak in the afternoon, whereas ICH and SAH had the same bimodal pattern with lower and higher peaks in the morning and afternoon, respectively.
Conclusions
Sleep or status in sleep tends to promote ischaemic stroke and suppress haemorrhagic stroke. Some triggers or factors that promote ischaemic stroke and prevent haemorrhagic stroke in the morning cause different variations in the waking state between ischaemic and haemorrhagic stroke.
Stroke occurrence shows chronobiological variations,1 such as circannual variations, circaseptan variations and circadian variations. Various patterns have been reported but no conclusions have yet been reached on circadian variations. The circadian variations of stroke onset may differ according to subtype or reporter, and are classified as cerebral infarction (CIF) with a single peak2,3,4,5,6 or double peaks,7,8 subarachnoid haemorrhage (SAH) with a single peak9 or double peaks,6,10,11,12,13,14 and intracerebral haemorrhage (ICH) with double peaks.6,10,12 Most previous studies have not treated the three major subtypes simultaneously. Only three reports6,7,8 discussed all the three subtypes, but the number of cases of ICH, especially of SAH, was too small for investigation of circadian variation. This may have led to differences in the conceived patterns of circadian variation. Large numbers of cases in population‐based samples are required to investigate and compare the circadian variations of stroke onset among subtypes. For investigation of the triggers and risk factors of stroke onset, it is necessary to determine the circadian variations of stroke onset with precise times. The precise time of stroke onset during sleep is difficult to specify, but this has a considerable influence on circadian variations of stroke onset.
We investigated circadian variation in stroke onset by situations at onset in CIF, ICH and SAH in a Japanese population, by using stroke registry data. We also investigated the differences in circadian variations, triggers and risk factors among subtypes.
Patients and methods
Stroke registry
A stroke registration programme has been instigated in the Iwate prefecture in the northern part of Honshu Island, Japan, which has a population of 1.4 million. The government of Iwate prefecture and the Iwate Medical Association have been coordinating this programme with all medical facilities (hospitals, medical offices and nursing homes) since January 1991. Registration forms are submitted to the registration office of the Iwate Medical Association by mail when a patient with stroke leaves the medical facility. All data are checked by trained staff for duplicate registration.
The registration form consists of information such as the patient's name, address, date of birth, stroke subtype, date of onset, situation at onset, symptoms and clinical findings, family history of apoplexy, histories of hypertension, diabetes and hyperlipidaemia, and use of antihypertensive or anti‐coagulant drugs before stroke onset. The results of computed tomography or magnetic resonance imaging (MRI), surgical treatment and outcome were registered. Stroke diagnostic criteria for CIF, ICH and SAH in this registry are based principally on the criteria established for the Monitoring System for Cardiovascular Disease commissioned by the Ministry of Health and Welfare.15 These criteria correspond with those published by the World Health Organization16 and define stroke as the sudden onset of neurological symptoms. Cases of traumatic ICH and SAH are not registered. A total of 16 997 cases (9121 men and 7876 women; average age 66.5 and 70.6 years, respectively) were registered between January 1991 and December 1996: 10 093 cases of CIF, 4603 cases of ICH, 1682 cases of SAH and the remaining 619 cases of other cerebrovascular stroke (transient ischaemic attack, cerebral venous thrombosis and unclassified stroke in the registry data). Registered patients hospitalised with stroke accounted for 97.5% (16 585/16 997) of the total number of patients. The patients who were diagnosed using computed tomography or MRI accounted for 95.5% (16 240/16 997) of the total number. For our study, 619 patients with other cerebrovascular stroke were excluded. Furthermore, 649 patients diagnosed without computed tomography or MRI and 2772 patients with recurrent stroke were excluded. Our study was conducted using data for the remaining 12 957 patients (7575 with CIF, 3852 with ICH and 1530 with SAH) of first‐ever stroke diagnosed using computed tomography or MRI.
Analysis of onset time
Onset time was registered in hourly intervals in the registry. In patients perceiving the occurrence of stroke on awakening, the time of perception was used as the onset time. When the precise onset time was not clear, whether the stroke occurred in the morning or in the afternoon was registered if possible. When the time of onset could not be identified, only the date of onset was registered.
The situation at stroke onset was registered in detail during exercise, during meals, while working, bathing, defecating or urinating, sleeping, drinking, chatting, watching television or in other situations. These situations were categorised simply as “in the waking state” or “during sleep”. The cases in which onset time was not registered were categorised as “unknown situation”.
For determination of the time of stroke onset, the day was divided into 12 2‐h intervals. The cases in which onset times were registered in the morning or in the afternoon only were redistributed equally between pertinent intervals, and those in which onset time was not registered were redistributed equally into 12 intervals. Data were statistically analysed with χ2 test for goodness of fit to the null model of equal distribution of stroke to evaluate the circadian variations in stroke onset. To estimate the relative risk (RR) of stroke occurring in a specific time period, the observed number of strokes was compared with the average number of 12 2‐h intervals.
Results
Table 1 shows the characteristics of the patients with first‐ever stroke having CIF, ICH and SAH, diagnosed using computed tomography or MRI.
Table 1 Characteristics of patients with first‐ever stroke, diagnosed using computed tomography or magnetic resonance imaging.
| Variable | CIF, n = 7575 | ICH, n = 3852 | SAH, n = 1530 |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 4238 (55.9) | 2079 (54.0) | 545 (35.6) |
| Female | 3337 (44.1) | 1773 (46.0) | 985 (64.4) |
| Mean age (SD), years | |||
| Men | 68.5 (11.5) | 62.9 (12.3) | 56.3 (13.4) |
| Women | 73.1 (11.4) | 68.9 (12.4) | 62.8 (13.0) |
| All | 70.5 (11.7) | 65.6 (12.7) | 60.5 (13.5) |
| Age distribution (years), n (%) | |||
| 0–49 | 313 (4.1) | 377 (9.8) | 323 (21.1) |
| 50–59 | 756 (10.0) | 751 (19.5) | 329 (21.5) |
| 60–69 | 2007 (26.5) | 1165 (30.2) | 444 (29.0) |
| 70–79 | 2433 (32.1) | 883 (22.9) | 281 (18.4) |
| Over 80 | 1574 (20.8) | 549 (14.3) | 110 (7.2) |
| Unknown | 492 (6.5) | 127 (3.3) | 43 (2.8) |
CIF, cerebral infarction; ICH, intracerebral haemorrhage; SAH, subarachnoid haemorrhage.
In all subtypes of stroke, men were about 5 years younger than women on average (men v women: CIF, 68.5 (11.5) v 73.1 (11.4); ICH, 62.9 (12.3) v 68.9 (12.4); SAH, 56.3 (13.4) v 62.8 (13.0)). Some data on the ages at onset were missing because the date of onset was not recorded in the registry.
Table 2 shows the percentages of cases in which the onset time was registered hourly, in the morning or afternoon, and unspecified cases, and the proportions of the categorised situation at onset (in the waking state, during sleep and unknown situation).
Table 2 Cases in which onset time was specified hourly, in the morning or afternoon, or was unspecified.
| Cerebral infarction | Intracerebral haemorrhage | Subarachnoid haemorrhage | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Hourly | ||||||
| In the waking state | 3726 | 49.2 | 2668 | 69.3 | 1104 | 72.2 |
| During sleep | 1079 | 14.2 | 341 | 8.8 | 146 | 9.5 |
| Unknown situation | 255 | 3.4 | 150 | 3.9 | 58 | 3.8 |
| Morning or afternoon | 788 | 10.4 | 233 | 6.1 | 74 | 4.8 |
| Unspecified | 1727 | 22.8 | 460 | 11.9 | 148 | 9.7 |
| All | 7575 | 100.0 | 3852 | 100.0 | 1530 | 100 |
The percentage of cases of CIF registered hourly was less than those of ICH and SAH (66.8% v 82.0% and 85.5%, respectively; p<0.05). The percentages of specified cases were not markedly different between the sexes in any subtype. We found no significant differences in age between cases that were specified hourly, in the morning or afternoon, and unspecified cases in any subtype. The percentage of cases of CIF, registered hourly, in which stroke onset occurred while the patient was asleep was more than those of ICH and SAH (14.2% v 8.8% and 9.5%, respectively; p<0.05). The proportions of categorised situation at onset were similar between cases of ICH and SAH.
Time‐specific onset numbers for 12 2‐h periods
The time‐specific onset numbers by sex were pooled because the characteristics of circadian variation were not markedly different between men and women in all subtypes (table 3).
Table 3 Time‐specific onset number by sex.
| Time interval (h) | Cerebral infarction | Intracerebral haemorrhage | Subarachnoid haemorrhage | |||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| 0–2 | 112 | 89 | 48 | 51 | 22 | 30 |
| 2–4 | 84 | 74 | 54 | 20 | 10 | 22 |
| 4–6 | 176 | 108 | 81 | 52 | 39 | 43 |
| 6–8 | 529 | 455 | 251 | 175 | 55 | 107 |
| 8–10 | 342 | 256 | 172 | 157 | 45 | 84 |
| 10–12 | 344 | 253 | 180 | 138 | 52 | 77 |
| 12–14 | 217 | 141 | 119 | 64 | 26 | 57 |
| 14–16 | 257 | 194 | 156 | 122 | 39 | 64 |
| 16–18 | 244 | 188 | 212 | 227 | 55 | 127 |
| 18–20 | 267 | 213 | 231 | 251 | 53 | 121 |
| 20–22 | 195 | 140 | 130 | 135 | 25 | 76 |
| 22–24 | 109 | 73 | 72 | 61 | 32 | 47 |
| Morning* | 321 | 260 | 90 | 72 | 24 | 32 |
| Afternoon† | 108 | 99 | 45 | 26 | 11 | 7 |
| Unspecified‡ | 933 | 794 | 238 | 222 | 57 | 91 |
| All | 4238 | 3337 | 2079 | 1773 | 545 | 985 |
*Onset time registered in the morning.
†Onset time registered in the afternoon.
‡Onset time not registered.
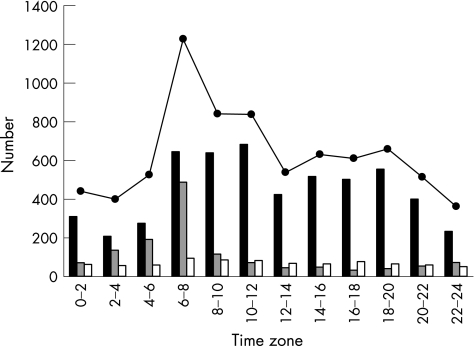
Figure 1 shows the time‐specific onset pattern of cases of CIF. In all onset situations, the circadian variation showed a sharp peak during the period from 06:00 to 07:59 (RR 194.0% (95% confidence interval (CI) 177.2% to 212.4%)), a small dip around noon, a smaller second peak from 18:00 to 19:59 (RR 104.3% (95% CI 94.0% to 115.8%)) and a nadir during the night (χ2 test, p<0.001). The cases in which onset occurred in the waking state showed two peaks: one from 10:00 to 11:59 (RR 152.2% (95% CI 136.0% to 170.4%)) and the other from 18:00 to 19:59 (RR 123.7% (95% CI 109.9% to 139.3%)), with a dip around noon and a nadir during the night (χ2 test, p<0.001). The peak in the morning was higher than that in the afternoon. The cases in which onset occurred during sleep showed a single peak during the period from 06:00 to 07:59 (RR 426.6% (95% CI 353.1% to 515.5%); χ2 test, p<0.001).
Figure 1 Time‐specific onset number for 12 2‐h intervals by situation at onset of cerebral infarction. Solid columns, in the waking state; shaded columns, during sleep; empty columns, unknown situation; solid circles, all onset situations.
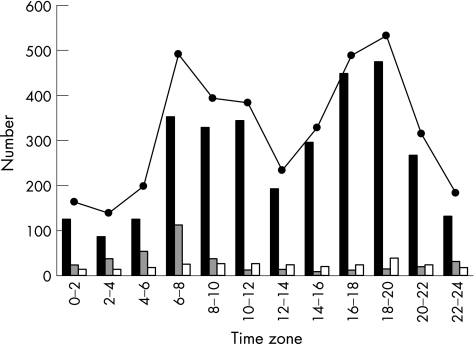
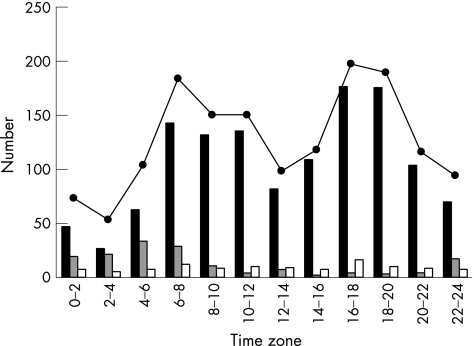
Figures 2 and 3 show the time‐specific onset patterns of ICH and SAH. For all onset situations, two peaks were observed: one from 06:00 to 07:59 (RR 153.1% (95% CI 134.0% to 174.9%) and RR 144.1% (95% CI 116.2% to 178.5%), respectively) and the other from 18:00 to 19:59 (RR 165.8% (95% CI 145.4% to 189.0%) and RR 154.8% (95% CI 125.3% to 191.2%), respectively), with a dip around noon and a nadir during the night (χ2 test, p<0.001). The cases in which onset occurred in the waking state showed variations similar to those seen in all cases. The cases with onset in the waking state showed two peaks: one from 06:00 to 07:59 (RR 133.0% (95% CI 114.3% to 154.8%) and RR 135.7% (95% CI 106.8% to 172.4%), respectively) and the other from 18:00 to 19:59 (RR 179.8% (95% CI 156.0% to 207.2%)) and from 16:00 to 17:59 (RR 168.0% (95% CI 133.7% to 211.1%)), respectively (χ2 test, p<0.001). The cases of ICH and SAH in which onset occurred during sleep showed a single peak in the period from 06:00 to 07:59 (RR 343.4% (95% CI 239.2% to 493.1%)) and from 04:00 to 05:59 (RR 252.8% (95% CI 123.2% to 457.5%)), respectively (χ2 test, p<0.001).
Figure 2 Time‐specific onset number for 12 2‐h intervals by onset situation of intracerebral haemorrhage. Solid columns, in the waking state; shaded columns, during sleep; empty columns, unknown situation; solid circles, all onset situations.
Figure 3 Time‐specific onset number for 12 2‐h intervals by onset situation of subarachnoid haemorrhage. Solid columns, in the waking state; shaded columns, during sleep; empty columns, unknown situation; solid circles, all onset situations.
Discussion
Validation of cases in the stroke registry for this study
We used the stroke registry data from the Iwate prefecture. In this registry, the annual registration rates, which were considered to be the annual incidence rates of onset of first‐ever stroke per 100 000 people from 1991 to 1996 were 88.9, 45.2 and 18.0 per year for CIF, ICH and SAH, respectively. The age‐adjusted annual incidence rates of ICH and SAH, estimated using data from the 1985 Japanese population census, were similar to those of previous reports from Japan.10,17,18 However, the rate for CIF was lower. The percentage of unregistered cases of CIF may be higher than those of ICH and SAH. The average ages of patients with CIF in our study were similar to those of patients in other studies based on the Japanese community.17,18 The percentages of cases in which onset time was unspecified were similar to those of previous reports.3,9,13,14,19,20,21 Therefore, there was probably no bias in the registry with regard to cases with a specific time zone or specific onset category.
Circadian variation of stroke onset
Previous studies showed that the circadian variation of stroke onset in patients with CIF had a single peak,2,3,4,5,6 whereas those of patients with ICH6,10,12 and SAH6,10,11,12,13,14 had double peaks. Only three previous reports have discussed circadian variation of stroke onset separated on the basis of situation at onset—that is, in the waking state or during sleep3,7,8—but the numbers of cases included were too few (n = 914, 375 and 675, respectively) for conclusions to be drawn.
In our study, ICH and SAH showed bimodal circadian variations and CIF had a single peak for all cases in all onset situations, whereas all three subtypes showed bimodal circadian variations of stroke onset in the waking state only. This difference was due to the influence of cases of CIF in which onset occurred during sleep, which accounted for about 20% of the cases in all situations and were concentrated at the time of awakening. In contrast, the cases of ICH and SAH occurring during sleep, which accounted for about 10% of the cases in all situations, had a small influence but did not affect bimodal variations. This concentration at the time of wakening corresponded not to the concentration of stroke onset but to that of its recognition. This circadian variation of stroke onset for all cases is actually a sociological variation of stroke onset, and is information that is useful when accepting patients with stroke—for example, for ambulance or hospital services. If all the cases of stroke onset during sleep and with unknown situation occurred equally between midnight and 06:00, circadian rhythm did not lose its nadir during the night in ICH and SAH, but lost it in CIF. Lower blood pressure reduces the incidence of stroke, but nocturnal low blood pressure is a risk factor for ischaemic stroke.22 Disordered breathing in sleep was reported to be a risk factor for ischaemic stroke onset at night.23 This shows that sleep or status in sleep tends to promote ischaemic stroke and suppress haemorrhagic stroke.
In the waking state, bimodal circadian variations were different in that CIF showed a bimodal pattern with a higher peak in the morning and a lower peak in the afternoon, whereas ICH and SAH had a bimodal pattern with a lower peak in the morning and a higher peak in the afternoon. Onset time in the waking state was more accurate than those during sleep or with an unknown onset situation. The bimodal circadian variation of stroke onset while awake seems useful for investigation of the trigger for stroke onset. Several previous studies have concluded that arterial blood pressure is a trigger for haemorrhagic stroke onset.9,11,13,14,20,24,25 Our results on ICH and SAH, showing very similar variations, indicated that the triggers for stroke onset were the same for ICH and SAH. Ischaemic and haemorrhagic stroke were reported previously as having the same trigger.9 In our study, the results of bimodal circadian variation in the waking state for both ischaemic and haemorrhagic stroke indicated that both types of stroke have a common trigger. However, some other factors are required to explain the difference in heights of the peaks in the morning and afternoon between ischaemic and haemorrhagic stroke. Previous studies indicated increases in the levels of haematocrit, platelet aggregability and hypercoagulability in the morning.26,27 These factors promote ischaemic events and prevent haemorrhagic events. The triggers for stroke onset seem to consist of two types of factor—that is, blood pressure, which is common to both ischaemic and hemorrhagic stroke and shows a bimodal pattern, and haemostatic functions, which promote ischaemic stroke and prevent haemorrhagic stroke in the morning.
Abbreviations
CIF - cerebral infarction
ICH - intracerebral haemorrhage
MRI - magnetic resonance imaging
SAH - subarachnoid haemorrhage
Footnotes
Competing interests: None declared.
References
- 1.Manfredini R, Gallerani M, Portaluppi F.et al Chronobiological patterns of onset of acute cerebrovascular diseases. Thromb Res 199788451–463. [DOI] [PubMed] [Google Scholar]
- 2.Elliott W J. Circadian variation in the timing of stroke onset. A meta‐analysis. Stroke 199829992–996. [DOI] [PubMed] [Google Scholar]
- 3.Lago A, Geffner D, Tembl J.et al Circadian variation in acute ischemic stroke. A hospital‐based study. Stroke 1998291873–1875. [DOI] [PubMed] [Google Scholar]
- 4.Argentino C, Toni D, Rasura M.et al Circadian variation in the frequency of ischemic stroke. Stroke 199021387–389. [DOI] [PubMed] [Google Scholar]
- 5.Marler J R, Price T R, Clark G L.et al Morning increase in onset of ischemic stroke. Stroke 198920473–476. [DOI] [PubMed] [Google Scholar]
- 6.Tsementzis S A, Gill J S, Hitchcock E R.et al Diurnal variation of and activity during the onset of stroke. Neurosurgery 198517901–904. [DOI] [PubMed] [Google Scholar]
- 7.Ricci S, Celani M G, Vitali R.et al Diurnal and seasonal variations in the occurrence of stroke: a community‐based study. Neuroepidemiology 19921159–64. [DOI] [PubMed] [Google Scholar]
- 8.Wroe S J, Sandercock P, Bamford J.et al Diurnal variation in incidence of stroke: Oxfordshire Community Stroke Project. BMJ 199218155–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feigin V L, Anderson C S, Anderson N E.et al Is there a temporal pattern in the occurrence of subarachnoid hemorrhage in the southern hemisphere? Pooled data from 3 large, population‐based incidence studies in Australasia, 1981 to 1997. Stroke 200132613–619. [DOI] [PubMed] [Google Scholar]
- 10.Inagawa T, Takechi A, Yahara K.et al Primary intracerebral and aneurysmal subarachnoid hemorrhage in Izumo city, Japan. Part I: incidence and seasonal and diurnal variations. J Neurosurg 200093958–966. [DOI] [PubMed] [Google Scholar]
- 11.Kleinpeter G, Schatzer R, Böck F. Is blood pressure really a trigger for the circadian rhythm of subarachnoid hemorrhage? Stroke 1995261805–1810. [DOI] [PubMed] [Google Scholar]
- 12.Sloan M A, Price T R, Foulkes M A.et al Circadian rhythmicity of stroke onset. Intracerebral and subarachnoid hemorrhage. Stroke 1992231420–1426. [DOI] [PubMed] [Google Scholar]
- 13.Vermeer S E, Rinkel G J E, Algra A. Circadian fluctuations in onset of subarachnoid hemorrhage. New data on aneurysmal and perimesencephalic hemorrhage and a systematic review. Stroke 199728805–808. [DOI] [PubMed] [Google Scholar]
- 14.Gallerani M, Portaluppi F, Maida G.et al Circadian and circannual rhythmicity in the occurrence of subarachnoid hemorrhage. Stroke 1996271793–1797. [DOI] [PubMed] [Google Scholar]
- 15.Study Project of Monitoring System for Cardiovascular Disease commissioned by the Ministry of Health and Welfare Manual for the registry and follow‐up of stroke [in Japanese]. Osaka, Japan: National Cardiovascular Center, 1988
- 16.World Health Organization MONICA Project Event registration data component, MONICA manual version 1.1. Document for meeting of MONICA Principal Investigators. Geneva: World Health Organization, 1986
- 17.Suzuki K, Kutsuzawa T, Takita K.et al Clinico‐epidemiologic study of stroke in Akita, Japan. Stroke 198718402–406. [DOI] [PubMed] [Google Scholar]
- 18.Kita Y, Okayama A, Ueshima H.et al Stroke incidence and case fatality in Shiga, Japan 1989–1993. Int J Epidemiol 1999281059–1065. [DOI] [PubMed] [Google Scholar]
- 19.Kelly‐Hayes M, Wolf P A, Kase C S.et al Temporal patterns of stroke onset. The Framingham Study. Stroke 1995261343–1347. [DOI] [PubMed] [Google Scholar]
- 20.Nyquist P A, Brown R D, Wiebers D O.et al Circadian and seasonal occurrence of subarachnoid and intracerebral hemorrhage. Neurology 200156190–193. [DOI] [PubMed] [Google Scholar]
- 21.Passero S, Reale F, Ciacci G.et al Differing temporal patterns of onset in subgroups of patients with intracerebral hemorrhage. Stroke 2000311538–1544. [DOI] [PubMed] [Google Scholar]
- 22.Stergiou G S, Vemmos K N, Pliarchopoulou K M.et al Parallel morning and evening surge in stroke onset, blood pressure, and physical activity. Stroke 2002331480–1486. [DOI] [PubMed] [Google Scholar]
- 23.Iranzo A, Santamaria J, Berenguer J.et al Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology 200258911–916. [DOI] [PubMed] [Google Scholar]
- 24.Fogelholm R R, Turjanmaa V M H, Nuutila M T.et al Diurnal blood pressure variations and onset of subarachnoid hemorrhage: a population‐based study. J Hypertens 199513495–498. [DOI] [PubMed] [Google Scholar]
- 25.Gallerani M, Trappella G, Manfredini R.et al Acute intracerebral haemorrhage: circadian and circannual patterns of onset. Acta Neurol Scand 199489280–286. [DOI] [PubMed] [Google Scholar]
- 26.Andrews N P, Gralnick H R, Merryman P.et al Mechanisms underlying the morning increase in platelet aggregation: a flow cytometry study. J Am Coll Cardiol 1996281789–1795. [DOI] [PubMed] [Google Scholar]
- 27.Jafri S M, VanRollins M, Ozawa T.et al Circadian variation in platelet function in healthy volunteers. Am J Cardiol 199269951–954. [DOI] [PubMed] [Google Scholar]