Abstract
We report the case of a patient who had benefited from bilateral subthalamic nucleus deep brain stimulation for Parkinson's disease and who presented acute and reproducible manic behaviour when stimulated mainly in the substantia nigra. A positron emission tomography scan showed an activation of the right dorsolateral prefrontal and inferior temporal cortex, the left anterior cingulate cortex and a deactivation of the left insula. This suggests that changes in cortical activation related to mania are subcortically driven, involving notably the substantia nigra.
Chronic high‐frequency stimulation of the subthalamic nucleus is now considered to be the “gold standard” for the treatment of patients with Parkinson's disease with severe motor complications. Among the psychiatric complications of this treatment, transitory mania has been described in some cases.1 We report the case of a patient who has benefited from subthalamic nucleus deep‐brain stimulation (STN‐DBS) for Parkinson's disease and who presented with acute manic behaviour as a function of the stimulation conditions. This observation highlights the physiopathology of manic behaviour in patients with Parkinson's disease and also in patients with manic disorders. This allowed us to obtain a rapid thymic change, which is the main difficulty in functional imaging studies in patients with bipolar disorders.2
Case report
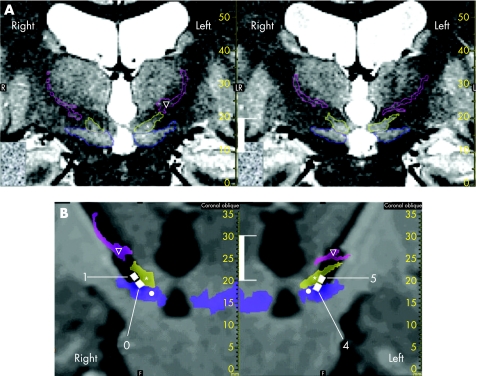
A 55‐year‐old woman, without any psychiatric medical history and having Parkinson's disease for 9 years with intractable motor fluctuations and dyskinesias, despite treatment with 200 mg levodopa and 12 mg ropinirole (GlaxoSmithKline), was administered STN‐DBS (quadripolar electrodes Medtronic 3389, Medtronic, Minneapolis, USA). DBS contacts were positioned under local anaesthesia and optimised by anatomical direct targeting (identification of subthalamic structures on T2‐weighted magnetic resonance imaging (MRI; fig 1), electrophysiological recordings, and stimulation tests conducted on the planned trajectories heading from the thalamus to the substantia nigra (10 mm) using microelectrodes (Alpha Omega, Nazareth Illit, Israel), with rigidity assessment and detection of adverse effects (gaze and vegetative troubles). The centres of contacts 1 (right hemisphere) and 5 (left hemisphere) were placed on the optimal site—that is, where we found both the best clinical improvement with the lowest stimulation current and adverse effects with the highest stimulation current. Consequently, the contacts located below (0, right; 4; left) were in the substantia nigra. We did not note behavioural changes during surgery. The initial stimulation parameters were as follows: contact 1 (left) and contact 5 (right), monopolar, 2.5 V, 130 Hz, 60 μs bilaterally. After surgery, levodopa was stopped and the patient received only ropinirole, 8 mg/day. Acute motor improvement induced by STN‐DBS at 3 months postoperatively was 46% as assessed by the Unified Parkinson's Disease Rating Scale motor part (off medication/off stimulation, 43; off medication/on stimulation, 23). After the 3‐month assessment, contacts of stimulation were changed to the most ventral ones (contacts 0 and 4) because the patient complained of dysarthria. Acute motor improvement was only 12% (off medication/off stimulation, 41; off medication/on stimulation, 36). From 2.2 V bilaterally, a complex change in behaviour was noted in 45 min. These modifications in behaviour consisted of mood exaltation, increase in self‐belief coupled with an overestimation of self capacities leading to frequent falls, logorrhoea associated with flight of ideas, distractibility, and psychomotor agitation. According to the Diagnostic and statistical manual—4th edn (DSM IV) criteria, only three of these six symptoms present to a considerable degree can evoke the diagnosis of manic episode. The symptomatology, following the criteria of DSM IV, was associated with a marked impairment in usual social activities and relationship with others (family and care givers). After these changes, the patient was exhausted and showed emotional lability. This manic state quickly disappeared when using the immediately dorsally located contacts (contacts 1 and 5). This symptomatology could be reproduced several times after the same modifications of the stimulation parameters.
Figure 1 Effective contact locations (white vertical bar, 10 mm). (A) Determination of anatomical structures on stereotactic (stereotactic frame and location box in place) preoperative T2‐weighted slices (voxel size 0.6×0.5×2 mm3). Structures were identified according to their anatomical locations afforded by the image contrast, and surrounded manually on the coronal plane. Example of two joined coronal slices (right slice located 2 mm in front of the left slice); zona incerta (∇, light pink), subthalamic nucleus (*, yellow) and substantia nigra (•, blue). (B) Effective contact locations: white squares, contact numbers 0 and 1 for the right electrode and numbers 4 and 5 for the left electrode. Slices were reconstructed in pseudocoronal planes going through the electrode tracts. Anatomical structures identified on preoperative T2‐weighted slices were merged (voxel‐to‐voxel matching; semitransparent coloured surfaces) on postoperative T1‐weighted MRI (without stereotactic frame and location box; isotropic voxel size 1.3 mm3; electrodes can be seen in a black artefact form). Contacts are shown taking into account both the location of the centre, based on the postoperative radiograph stereotactic controls, and the zoom effect of the artefact (black signal; according to deep‐brain stimulation electrode geometry).
Methods
Clinical evaluation
The regional medical school ethics committee approved the study protocol (AU 631).
A psychiatrist (AS) assessed the mood swings 12 months after surgery in a double‐blind fashion according to the change in the stimulation parameters, using the Bech and Rafaelsen3 Manic Scale. This 11‐item scale explores the different symptoms that are required for diagnosing a manic episode according to the DSM IV criteria (motor activity, verbal activity, flight of ideas, mood, self‐esteem, sleep, sexual activity, work). The evaluation takes into account the consequences and intensity of symptoms. It was carried out after a 12‐h withdrawal period of all drugs for Parkinson's disease. The delay between the changes in stimulation parameters and the clinical evaluation was 45 min, giving enough time to observe a change in the patient's mood. Five conditions were tested as follows:
Stimulation off bilateral
Euthymic stimulation: bilateral stimulation contact 1 (2.5 V) and contact 5 (2.5 V)
Manic stimulation: bilateral stimulation contact 0 (2.2 V) and contact 4 (2.2 V)
Unilateral manic stimulation: bilateral stimulation contact 1 (2.5 V) and contact 4 (2.2 V)
Unilateral manic stimulation: bilateral stimulation contact 0 (2.2 V) and contact 5 (2.5 V).
For conditions 2–5, the frequency and pulse width of the stimulation were identical (130 Hz; 60 μs). The voltage of 2.5 V (contacts 1 and 5) corresponded to that leading to the best motor control without side effects. In the same way, 2.2 V (contacts 0 and 4) corresponded to the voltage that was systematically associated with maniac behaviour. The order of assessment was randomised. The Manic Scale values were as follows: condition 1, 0; condition 2, 1; condition 3, 12; condition 4, 1; and condition 5, 1. Clinically, the beginning of the mood swing was observed within the first minutes of the modification of the stimulation parameters. Complete association of symptoms was obvious after 45 min. The main symptoms in this patient during the manic phase were psychomotor agitation, flight of ideas and inflated self‐esteem. This confirmed that manic behaviour was specifically induced by a bilateral stimulation of the deepest contacts (contacts 0 and 4).
Location of contacts
The location of the contacts was determined by two methods using stereotactic surgical software (Iplan, BrainLAB, Feldkirchen, Germany) The first method used stereotactic matching based on the fiducials of stereotactic boxes (Leksell G frame, Elekta, Sweden) between the intraoperative x ray controls and the preoperative coronal stereotactic MRI: the contacts were projected on the preoperative stereotactic MRI based on their stereotactic coordinates. The second method consisted of automated matching (voxel‐to‐voxel optimisation algorithm, the whole brain was included for the calculation) of the postoperative MRI (before placement of the neurostimulator, without stereotactic frame and location box) with the preoperative coronal stereotactic MRI. The electrodes with their four contacts were visible in a black artefact form. The contact locations were determined by taking into account the size of the artefact (zoom effect) and merged with the preoperative stereotactic MRI. As expected after the surgery, the deepest contacts were located bilaterally, mainly in the substantia nigra (fig 1).
Analysis of positron emission tomography data
A positron emission tomography (PET) scan using water with oxygen‐15 was carried out on the patient while at rest, without any motor or cognitive activation tasks, 14 months after the surgery. Three conditions were studied: (1) rest in off stimulation condition (off); (2) rest with bilateral stimulation on contacts 1 and 5 without mood changes (euthymic stimulation); and (3) rest with bilateral stimulation on contacts 0 and 4 (manic stimulation). The parameters of stimulation were the same as those used for the clinical evaluation. The first condition was no STN stimulation; the stimulator was then switched on without any stimulation‐induced dysthymia and later the stimulation parameters were changed, inducing manic behaviour, as assessed by the patient's matter during the PET scan. During the PET scan, mania could not be scaled except for the patient's elicited anxiety, emotional lability and excitation. The conditions unilateral manic stimulation (conditions 4 and 5 of the clinical evaluation) could not be tested because of the limit in the number of scans and the duration of the procedure. Each condition was repeated four times, and the conditions were pooled according to the stimulation condition in three blocks, for a total of 12 scans. The integrated counts were collected for 90 s, starting 20 s after the injection. An 8‐min interval was necessary between each condition for adequate radioactivity decay. The latency between the changes in stimulation parameters and the next block of conditions was 45 min. Covariances in global differences in cerebral blood flow (CBF) were determined for all voxels, and comparisons across conditions were made using t statistics with appropriate linear contrasts. Only voxels that exceeded a threshold of corrected p<0.05 were considered to be significant in a region comprising at least 10 voxels.
Compared with STN stimulation without mood disorders, mania induced by changes of contacts was associated with an increase of regional cerebral blood flow (rCBF) in the right superior frontal gyrus (Brodmann area (BA)10), dorsolateral prefrontal cortex; BA9 and BA46), inferior temporal gyrus (BA20) and lateral premotor cortex (BA6), as well as in the left dorsal anterior cingulate cortex (BA24; fig 2A). Simultaneously, a decrease in rCBF was seen in the left insula, inferior parietal lobe (BA40) and superior temporal gyrus (fig 2B).
Figure 2 (A) Activated areas during manic stimulation versus euthymic stimulation. During mania, an increase in regional cerebral blood flow (rCBF) was observed in the right superior frontal gyrus (Brodmann area (BA)10; p = 0.011), dorsolateral prefrontal cortex (DLPFC; BA9 and BA46; p = 0.011), inferior temporal gyrus (BA20; p<0.001); lateral premotor cortex (BA6; p = 0.011) and left anterior cingulate cortex (BA24; p = 0.017). (B) Activated areas during euthymic stimulation versus manic stimulation. During mania, a decrease in rCBF was observed in the left insula (p = 0.001), inferior parietal lobe (BA40; p = 0.008) and superior temporal gyrus (p = 0.03). The regions were overlaid on the single magnetic resonance image of the patient brain with statistical parametric mapping 99. The p values are corrected for multiple comparisons.
Discussion
The changes in behaviour were consistent with a manic state according to DSM IV criteria, with a predominance of tachypsychia, psychomotor agitation and inflated self‐esteem. The stimulation‐dependent character of this state was shown by both the reproducibility of the symptomatology and the double‐blind clinical evaluation, which confirmed that only a bilateral stimulation on the most ventral contacts was responsible for manic behaviour. Kulisevsky et al1 have also reported three patients with manic state after DBS‐STN, in whom electrodes were probably located in the substantia nigra. In the present case, we have made an effort to accurately locate contacts in the STN area, and the PET scan provided new information on the pathophysiological mechanism of mania.
The first remarkable point is the strong asymmetry of brain activation, clearly right sided during the manic phase, as reported previously.4 In addition, our study underlines the major role in mania of the dorsal anterior cingulate cortex and prefrontal cortex, which belong to the limbic and associative corticosubcortical loops.5 Interestingly, Mayberg et al6 have shown that DBS of the ventral anterior cingulate cortex dramatically improves severe depression and is associated with an increase of the activation in the dorsal anterior cingulate cortex (BA24) and dorsolateral prefrontal cortex, which fits well with our results.This could explain the dysregulated attentional and emotional process, but could also reflect a control or a compensation of the abnormal behaviour. It has been shown that activation in the right temporal cortex is correlated to the degree of mania.7 In parallel, we observed a reduction of rCBF in the left insula, a structure that is strongly implicated in regulating emotional functions.8
Two factors support the location of contacts inducing mania in the substantia nigra. Firstly, the voxel‐to‐voxel matching of the preoperative stereotactic and the postoperative MRI showed the location of the ventral contacts (0 and 4) bilaterally in the substantia nigra. This method does not use the statistics atlas and takes into account only the patient's own anatomy. Secondly, parkinsonian symptoms were not improved by stimulation of the most ventral contacts, whereas stimulation of the more dorsal contacts (1 and 5) led to an improvement of almost 50%, as is usually observed with STN stimulation. However, we cannot exclude that high‐frequency stimulation (HFS) could also have influenced the lower, associative and limbic parts of the STN, as the upper part of ventral contacts was close to the limit between the STN and the substantia nigra.
Interestingly, when stimulating the substantia nigra, Bejjani et al9 reported an acute melancholic state in a patient with Parkinson's disease. This was associated with an increased rCBF in the left orbitofrontal cortex, amygdala, globus pallidus, anterior thalamus and right inferior parietal lobe. This shows that the opposite consequences in terms of mood changes have been associated with the stimulation of the substantia nigra. To reconcile these two observations, it could be hypothesised that other parameters, such as the patient's medical and psychiatric history, could have a role in the type of mood changes inducesd.
Although the mechanism of STN‐DBS action is still debated, HFS is supposed to override the pathological activity of STN neurones by a new HFS‐driven pattern that can influence the target neurones of the STN—in other words, the output structures of the basal ganglia.10
In conclusion, our observation suggests that (1) modifications of mood state could be induced by modifications of neural activity of the substantia nigra, especially its limbic part and (2) HFS at any point of the subcorticocortical limbic network, such as the substantia nigra and the STN, could modify the functioning of this circuit and lead to changes in mood state.
Abbreviations
DBS - deep‐brain stimulation
DSM IV - Diagnostic and statistical manual—4th edn
HFS - high‐frequency stimulation
MRI - magnetic resonance imaging
PET - positron emission tomography
rCBF - regional cerebral blood flow
STN - subthalamic nucleus
Footnotes
Competing interests: None declared.
Consent was obtained for publication of the patient's details described in this report.
References
- 1.Kulisevsky J, Berthier M L, Gironell A.et al Mania following deep brain stimulation for Parkinson's disease. Neurology 2002591421–1424. [DOI] [PubMed] [Google Scholar]
- 2.Bearden C E, Hoffman K M, Cannon T D. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3: 106–50, discussion in Bipolar Disord20013151–153. [DOI] [PubMed] [Google Scholar]
- 3.Bech P. The Bech‐Rafaelsen Mania Scale in clinical trials of therapies for bipolar disorder: a 20‐year review of its use as an outcome measure. CNS Drugs 20021647–63. [DOI] [PubMed] [Google Scholar]
- 4.Caligiuri M P, Brown G G, Meloy M J.et al A functional magnetic resonance imaging study of cortical asymmetry in bipolar disorder. Bipolar Disord 20046183–196. [DOI] [PubMed] [Google Scholar]
- 5.Drevets W C, Price J L, Simpson J R.et al Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997386824–827. [DOI] [PubMed] [Google Scholar]
- 6.Mayberg H S, Lozano A M, Voon V.et al Deep brain stimulation for treatment‐resistant depression. Neuron 200545651–660. [DOI] [PubMed] [Google Scholar]
- 7.Gyulai L, Alavi A, Broich K.et al I‐123 iofetamine single‐photon computed emission tomography in rapid cycling bipolar disorder: a clinical study. Biol Psychiatry 199741152–161. [DOI] [PubMed] [Google Scholar]
- 8.Augustine J R. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 199622229–244. [DOI] [PubMed] [Google Scholar]
- 9.Bejjani B P, Damier P, Arnulf I.et al Transient acute depression induced by high‐frequency deep‐brain stimulation. N Engl J Med 19993401476–1480. [DOI] [PubMed] [Google Scholar]
- 10.Garcia L, D'Alessandro G, Bioulac B.et al High‐frequency stimulation in Parkinson's disease: more or less? Trends Neurosci 200528209–216. [DOI] [PubMed] [Google Scholar]