Abstract
To explore the aetiology of pathological laughing, a 65‐year‐old woman with pathological laughing was examined by 3‐T functional magnetic resonance imaging (fMRI) before and after treatment with drugs. Here, we report that the patient consistently showed exaggerated pontine activation during the performance of three tasks before treatment, whereas abnormal pontine activation was no longer found after successful treatment with the selective serotonin reuptake inhibitor, paroxetine. Our findings in this first fMRI study of pathological laughing suggest that serotonergic replacement decreases the aberrant activity in a circuit that involves the pons.
Pathological laughing and crying (PLC) is defined as a sudden loss of emotional control, occurs in response to non‐specific stimuli, and lacks an associative and matching mood state.1 Previous studies have shown that PLC appears in association with various brain disorders such as pseudo‐bulbar palsy, subcortical and brain stem infarctions and injury, tumours in the cerebellopontine region, multiple sclerosis, and amyotrophic lateral sclerosis.1,2,3,4,5,6,7,8 However, the pathogenesis of PLC remains unclear and to date there have not been any functional neuroimaging studies of PLC. We conducted functional magnetic resonance imaging (fMRI) using a 3‐T magnetic resonance scanner to investigate the brain function of a patient with pathological laughing before and after treatment with drugs, and compared these findings with fMRI data from normal volunteers.
Case report
A 65‐year‐old right‐handed woman with pathological laughing developed episodes of inappropriate and uncontrollable laughing at the age of 62 years. The laughing episodes were not accompanied by a sense of joy or any other pleasurable feeling. These spells occurred even in stressful situations such as when she was reproved by her husband. As her episodes of uncontrolled laughing began to occur almost every day, she was admitted to our hospital.
She had no history of neurological or psychiatric disease, and there was no sign of drug or alcohol abuse. Motor strength and stretch reflexes were normal. On testing of all sensory modalities, no apparent abnormalities were observed. The status of all cranial nerve nuclei (including the facial nucleus) was also normal. Scores on Hamilton rating scale for both anxiety (5 points) and depression (3 points) were low, and there were no apparent psychiatric diseases such as anxiety disorders or depression. Brain magnetic resonance imaging, including fast fluid‐attenuated inversion‐recovery and diffusion‐weighted imaging, showed no apparent abnormal findings such as tumour, haemorrhage, infarction or severe cortical atrophy. 99mTc‐ECD SPECT and electroencephalogram were also unremarkable. Mini Mental State Examination showed a full score of 30 points. We could not detect any apparent organic brain disorders such as epilepsy, infarction or dementia.
On the basis of reports of the effectiveness of the selective serotonin reuptake inhibitor (SSRI) on PLC,5,7,9,10 treatment with paroxetine was started at 10 mg daily 2 weeks after hospitalisation. Paroxetine 10 mg/day led to a gradual reduction in the number of laughing episodes at 2 weeks, which eventually disappeared within 6 weeks after an increase to 20 mg/day.
Methods
We conducted fMRI using T2*‐weighted, gradient echo, echo planar imaging (EPI) sequences with a 3‐T magnetic resonance scanner (Signa Horizon; General Electric Medical Systems, Milwaukee, Wisconsin, USA). Three types of experimental tasks were performed to establish responses to non‐specific stimuli. The protocol was approved by the Ethical Committee of Fukui Medical University (Fukui, Japan), and the patient gave written informed consent for the study.
During scanning, stimuli were projected onto a semitransparent screen using an liquid crystal display projector connected to a personal computer that generated the stimuli. The subject saw the stimuli through a tilted mirror attached to the head coil of the scanner. The subject's head was immobilised with comfortable foam and taped to the head folder to prevent motion.
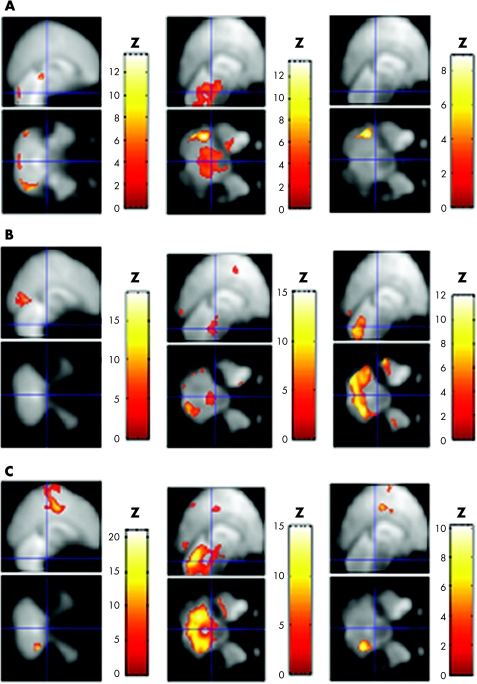
fMRI analysis was performed based on a block design, alternating control and task, as previously reported.11 (A) During the sex discrimination task, the patient was instructed to judge the sex of face pairs, and to discriminate the size of two rectangles during the control condition (control/task block = 32/96 s, repetition time or echo time(TR/TE) = 4000/30 ms, flip angle = 90°, 64×64 matrix and 44 slices, 3 mm slice thickness, 136 images in total). (B) During the semantic decision task, she was told to push the button if the presented word was an animal name, and to push the button if the presented cross‐character disappeared during the control condition (control/task block = 27/27 s, TR/TE = 3000/30 ms, flip angle = 90°, 64×64 matrix and 36 slices, 4 mm slice thickness, 126 images in total). (C) During the finger‐tapping task, she was instructed to alternately press the buttons with her right index and ring fingers at about 1 Hz, and she remained at rest during the control condition (control/task block = 28/28 s, TR/TE = 4000/30 ms, flip angle = 90°, 64×64 matrix and 44 slices, 3 mm slice thickness, 112 images in total). The patient experienced laughing episodes only during the scanning of the sex discrimination task before treatment. Each task performance was over 90% correct. Imaging processing and statistical analyses were performed using Statistical Parametric Mapping 99 software (Wellcome Department of Cognitive Neurology, University College London, London, UK). To correct for dislocations caused by head motion, all EPI images were realigned. These images were then normalised to the Montreal Neurological Institute atlas12 using the parameter obtained from the normalisation process of the anatomical image that was coregistered to the first EPI image beforehand. The images were smoothed by using an 8‐mm gaussian kernel. The statistical threshold was set at p<0.05 (corrected) for each voxel (fig 1). Then, we compared with data of control subjects obtained from our previous studies.13
Figure 1 Functional magnetic resonance imaging findings of normal volunteers and the patient with pathological laughing before and after treatment. (A) During the sex discrimination task (12 normal volunteers and the patient). (B) During the semantic decision task (11 normal volunteers and the patient). (C) During the finger‐tapping task (six normal volunteers and the patient). The figures depict brain areas showing significant activation during each task compared with each control condition in normal volunteers (left column) and the patient with pathological laughing before (middle column) and after (right column) treatment with paroxetine. The task‐related increase in magnetic resonance signal was superimposed on the mean T2‐weighted image (control subjects, left column) and the patient's own normalised T2‐weighted image (middle and right column; p<0.05, corrected). Abnormal pontine activation during the experimental tasks was shown only in the patient before treatment. The blue lines indicate the pons, at Talairach coordinates [0, −26, −30].
Results
During the sex discrimination task, as compared with the control condition (fig 1A, table 1), the patient showed broad and significant activation in the pons and cerebellum before treatment with paroxetine, which was not observed in any of the 12 normal volunteers. During the semantic decision task (fig 1B, table 1), significant pontine activation was also observed only in the patient, although the bilateral prefrontal, parietal and occipital cortices and cerebellum were activated in the patient and the 11 normal volunteers. During the finger‐tapping task (fig 1C, table 1), the pons was considerablly activated only in the patient, although significant activation was found in the motor and temporal cortices, supplementary motor area and cerebellum in the patient and the six normal volunteers. None of the normal control subjects showed significant pontine response to any experimental task in intra‐subject analysis. Although the patient experienced laughing episodes during scanning, the magnitude of head movement during all tasks was <0.6 mm.
Table 1 Brain areas of significant activation during the sex discrimination, semantic decision and finger‐tapping tasks in normal volunteers and the patient with pathological laughing before and after treatment.
| Normal group | The patient with pathological laughing | |
|---|---|---|
| Without paroxetine | With paroxetine | |
| (A) The sex discrimination task (12 normal volunteers and the patient) | ||
| Rt. Fusiform gyrus | Pons | Lt. fusiform gyrus |
| (40, −78, −16) | (6, −36, −22) | (−38, −42, −28) |
| (Z = 5.55) | (Z = 5.27) | (Z = 7.46) |
| Lt. Fusiform gyrus | Lt. fusiform gyrus | Lt. inferior temporal gyrus |
| (−38, −76, −16) | (−38, −42, −28) | (−40, −66, −2) |
| (Z = 4.85) | (Z = 8.00) | (Z = 4.38) |
| Rt. amygdala | Lt. amygdala | |
| (16, −6, −18) | (−22, −10, −14) | |
| (Z = 4.33) | (Z = 5.78) | |
| Lt. amygdala | Lt. inferior temporal gyrus | |
| (−16, −6, −18) | (−40, −68, 2) | |
| (Z = 4.47) | (Z = 5.15) | |
| Rt. inferiot frontal gyrus | Rt. cerebellum (MCP) | |
| (46, 14, 26) | (12, −40, −30) | |
| (Z = 5.57) | (Z = 4.63) | |
| Lt. middle frontal gyrus | Lt. cerebellum (MCP) | |
| (−36, 8, 34) | (−8, −42, −38) | |
| (Z = 4.20) | (Z = 4.40) | |
| Rt. hippocampus | ||
| (34, −16, −16) | ||
| (Z = 4.05) | ||
| Rt. lingual gyrus | ||
| (22, −70, 2) | ||
| (Z = 4.17) | ||
| (B) The semantic decision task (11 normal volunteers and the patient) | ||
| Rt. lingual gyrus | Pons | Rt. lingual gyrus |
| (32, −80, −20) | (2, −34, −32) | (22, −72, −18) |
| (Z = 5.76) | (Z = 4.35) | (Z = 7.37) |
| Lt. lingual gyrus | Rt. lingual gyrus | Lt. lingual gyrus |
| (−10, −90, −14) | (18, −90, −10) | (−22, −82, −22) |
| (Z = 7.62) | (Z = 7.35) | (Z = 8.00) |
| Lt. Fusiform gyrus | Lt. lingual gyrus | Rt. superior parietal lobe |
| (−38, −74, −16) | (−16, −86, −14) | (28, −74, 22) |
| (Z = 7.72) | (Z = 8.00) | (Z = 7.04) |
| Lt. inferior frontal gyrus | Rt. superior parietal lobe | Lt. superior parietal lobe |
| (−52, 28, 32) | (28, −74, 18) | (−20, −68, 38) |
| (Z = 5.72) | (Z = 4.95) | (Z = 7.82) |
| Rt. postcentral gyrus | Lt. superior parietal lobe | Rt. inferior temporal gyrus |
| (52, −14, 40) | (−22, −66, 26) | (54, −40, −14) |
| (Z = 4.75) | (Z = 6.05) | (Z = 4.54) |
| Lt. postcentral gyrus | Lt. inferior temporal gyrus | Lt. inferior temporal gyrus |
| (−44, −28, 58) | (−56, −42, −14) | (−54, −44, −22) |
| (Z = 5.36) | (Z = 6.09) | (Z = 7.38) |
| Rt. middle frontal gyrus | Lt. middle temporal gyrus | Rt. middle temporal gyrus |
| (42, 10, 52) | (−44, −36, −4) | (44, −28, −10) |
| (Z = 4.56) | (Z = 7.01) | (Z = 4.45) |
| Rt. Superior parietal lobe | Rt. inferior frontal gyrus | Lt. middle temporal gyrus |
| (14, −54, 54) | (26, 32, −18) | (−44, −36, −8) |
| (Z = 4.53) | (Z = 3.72) | (Z = 7.15) |
| Rt. cerebellum | Lt. middle frontal gyrus | Rt. inferior frontal gyrus |
| (20, −72, −18) | (−44, 6, 50) | (38, −12, 30) |
| (Z = 3.90) | (Z = 7.09) | (Z = 4.30) |
| Lt. inferior frontal gyrus | Lt. middle frontal gyrus | |
| (−26, 30, −14) | (−26, 0, 28) | |
| (Z = 5.52) | (Z = 8.00) | |
| Lt. supplementary motor area | Rt. cerebellum | |
| (−8, 10, 60) | (24, −56, −32) | |
| (Z = 5.88) | (Z = 8.00) | |
| Rt. precentral gyrus | Lt. cerebellum | |
| (60, −4, 32) | (−24, −52, −34) | |
| (Z = 5.79) | (Z = 6.36) | |
| Rt. cerebellum | ||
| (22, −72, −40) | ||
| (Z = 5.90) | ||
| (C) The finger‐tapping task (six normal volunteers and the patient) | ||
| Lt. precentral gyrus | Pons | Rt. precentral gyrus |
| (−24, −30, 66) | (−6, −36, −38) | (62, 2, 24) |
| (Z = 4.59) | (Z = 5.22) | (Z = 5.70) |
| Lt. Superior temporal gyrus | Rt. precentral gyrus | Lt. precentral gyrus |
| (−52, 8, 10) | (60, −4, 28) | (−30, −10, 64) |
| (Z = 3.62) | (Z = 5.25) | (Z = 6.88) |
| Lt. supplementary motor area | Lt. precentral gyrus | Lt. supplementary motor area |
| (−6, −6, 68) | (−30, −24, 42) | (−8, 2 84) |
| (Z = 4.22) | (Z = 6.94) | (Z = 5.66) |
| Rt. cerebellum | Lt. inferior temporal gyrus | Lt. anterior cingulate |
| (22, −50, −20) | (−40, −14, −26) | (−6, −12 50) |
| (Z = 3.98) | (Z = 5.92) | (Z = 5.22) |
| Lt. superior temporal gyrus | Lt. inferior frontal gyrus | |
| (−58, −26, 22) | (−48, 10, 28) | |
| (Z = 5.36) | (Z = 5.01) | |
| Rt. lingual gyrus | Rt. cerebellum | |
| (18, −88, −6) | (28, −50, −28) | |
| (Z = 5.93) | (Z = 6.40) | |
| Rt. superior frontal gyrus | ||
| (20, 66 26) | ||
| (Z = 5.04) | ||
| Rt. superior occipital gyrus | ||
| (36, −74, 10) | ||
| (Z = 4.95) | ||
| Rt. cerebellum | ||
| (20, −56, −30) | ||
| (Z = 7.44) | ||
| Lt. cerebellum | ||
| (−20, −52, −32) | ||
| (Z = 8.00) | ||
MCP, middle cerebellar peduncle; Rt., right; Lt, left.
The fMRI was also examined after the complete disappearance of the patient's symptoms (ie, after 8 weeks of treatment with drugs). This analysis showed that abnormal pontine activation during the performance of each task was no longer found after successful treatment with paroxetine. Although significant cerebellum activation seen before treatment with paroxetine also disappeared during the sex discrimination task, it persisted during the other two tasks after treatment (fig 1, table 1).
Discussion
This is the first fMRI study of pathological laughing in which a patient consistently showed pontine hyperactivity during different types of tasks.
There are two major hypotheses regarding PLC. One is that lesions of the voluntary paths from the motor cortex to the brain stem laughing and crying centre, which controls facial and respiratory muscles, can cause involuntarily initiated laughing and crying.2 The other is that dysfunction of the cerebro‐ponto‐cerebellar pathways causes inappropriate laughing or crying behaviour because the cerebellum fails to adjust the execution of laughing or crying to the cognitive and situational context of a potential stimulus.10 In the present study, three types of fMRI experiment tasks, facial recognition, semantic decision and motor function, were used to establish the response to non‐specific stimuli. The patient showed consistent abnormal pontine activation not seen in any control subject during the performance of all tasks before treatment, and when the laughing episodes disappeared after treatment with paroxetine, the exaggerated pontine activation was normalised. Thus, it was suggested that the pons might be a part of the aetiological focus for pathological laughing. In addition to the pons, significant cerebellum activation was also found before treatment in our patient. Although it was seen to some extent even in the control subjects and activation change in the cerebellum after treatment was not as dramatic as that in the pons, abnormal cerebellar function might also be related to the emergence of PLC in accord with the second hypothesis.
There is also increasing evidence that SSRIs are effective against PLC.5,7,9,10 Our fMRI results, in which the abnormal pontine activity disappeared with clinical improvement after treatment with paroxetine, also suggested some SSRI efficacy in PLC. Although the response to SSRI in our patient was relatively slow compared with other reports in which rapid improvement was seen within 1 week,14,15,16 a favourable response was obtained in 2 weeks in our patient.
Whereas pathological laughing was present in only one task (sex discrimination), abnormal brain stem activation was present for all task comparisons. One of the possible explanations for this discrepancy was that the pons, or a putative pathway for PLC associated with the pons, might always remain functionally abnormal during the disease episodes, and then clinical episodes of PLC would occur episodically in response to non‐specific triggering until successful treatment; however, it remains unclear how the threshold for triggering these attacks is set or modulated.
As there are also some fMRI studies that reported brain stem activation associated with the pons during auditory stimulation or sensory stimulation in response to faces,17,18,19 it is considered that pontine hyperactivity in the present findings was not likely to be caused by mere motion artefacts. Further, the patient experienced only one laughing episode during the task, and this episode was transient and lasted for less than a minute without vocalisation. The subject's head movement was very slight (ie, <0.6 mm), but even if pontine hyperactivity was an artefact due to gross head motion, breathing motion or heart beat, it is hard to consider that these elements happened only during the task, and not during the control periods. Although further studies with a large number of patients and age‐matched control subjects are needed, the present findings contribute to our understanding of the neuronal mechanism responsible for PLC and suggest treatment strategies for this condition.
Acknowledgements
This study was supported by the 21st Century COE program “Biomedical Imaging Technology Integration Program” from the Japan Society of the Promotion of Science (JSPS).
Abbreviations
EPI - echo planar imaging
fMRI - functional magnetic resonance imaging
PLC - pathological laughing and crying
SSRI - selective serotonin reuptake inhibitor
TR/TE - repetition time or echo time
Footnotes
Consent was obtained for publication of the patient's details described in this report.
Competing interests: None declared.
The protocol was approved by the Ethical Committee of University of Fukui and the patient gave written informed consent for the study.
References
- 1.Poeck K. Pathological laughter and crying. In: Fredericks JAM, ed. Handbook of clinical neurology. Vol. 1. Amsterdam: Elsevier Science Publishers B, V. 1985219–225.
- 2.Wilson S A K. Some problems in neurology. II. Pathological laughing and crying. J Neurol Psychopathol 19244299–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson R G, Parikh R M, Lipsey J R.et al Pathological laughing and crying following stroke: validation of a measurement scale and a double‐blind treatment study. Am J Psychiatry 1993150286–293. [DOI] [PubMed] [Google Scholar]
- 4.Andersen G, Ingeman‐Nielsen M, Vestergaard K.et al Pathoanatomic correlation between poststroke pathological crying and damage to brain areas involved in serotonergic neurotransmission. Stroke 1994251050–1052. [DOI] [PubMed] [Google Scholar]
- 5.Müller U, Murai T, Bauer‐Wittmund T.et al Paroxetine versus citalopram treatment of pathological crying after brain injury. Brain Inj 199913805–811. [DOI] [PubMed] [Google Scholar]
- 6.Bhatjiwale M G, Nadkarni T D, Desai K I.et al Pathological laughter as a presenting symptom of massive trigeminal neuromas: report of four cases. Neurosurgery 200047469–471. [DOI] [PubMed] [Google Scholar]
- 7.McCullagh S, Feinstein A. Treatment of pathological affect: variability of response for laughter and crying. J Neuropsychiatry Clin Neurosci 200012100–102. [DOI] [PubMed] [Google Scholar]
- 8.Feinstein A, Feinstein K, Gray T.et al Prevalence and neurobehavioral correlates of pathological laughing and crying in multiple sclerosis. Arch Neurol 1997541116–1121. [DOI] [PubMed] [Google Scholar]
- 9.Andersen G, Vestergaard K, Riis J O. Citalopram for post‐stroke pathological crying. Lancet 1993342837–839. [DOI] [PubMed] [Google Scholar]
- 10.Parvizi J, Anderson S W, Martin C O.et al Pathological laughing and crying: a link to the cerebellum. Brain 20011241708–1719. [DOI] [PubMed] [Google Scholar]
- 11.Kosaka H, Omori M, Iidaka T.et al Neural substrates participating in acquisition of facial familiarity: an fMRI study. Neuroimage 2003201734–1742. [DOI] [PubMed] [Google Scholar]
- 12.Evans A C, Kamber M, Collins D L.et al An MRI‐based probablistic atlas of neuroanatomy. In: Shorvon S, et al eds. Magnetic resonance scanning and epilepsy, NATO ASI series A. Vol. 264. New York: Life Sciences, Plenum, 1994263–274.
- 13.Iidaka T, Okada T, Murata T.et al Age‐related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus 200212352–362. [DOI] [PubMed] [Google Scholar]
- 14.Nahas Z, Arlinghaus K A, Kotrla K J.et al Rapid response of emotional incontinence to selective serotonin reuptake inhibitors. J Neuropsychiatry Clin Neurosci 199810453–455. [DOI] [PubMed] [Google Scholar]
- 15.van Wattum P J, Chiles C. Rapid response to low dose citalopram in pathological crying. Gen Hosp Psychiatry 200123167–168. [DOI] [PubMed] [Google Scholar]
- 16.Andersen G, Stylsvig M, Sunde N. Citalopram treatment of traumatic brain damage in a 6‐year‐old boy. J Neurotrauma 199916341–344. [DOI] [PubMed] [Google Scholar]
- 17.Hesselmann V, Wedekind C, Kugel H.et al Functional magnetic resonance imaging of human pontine auditory pathway. Heart Res 2001158160–164. [DOI] [PubMed] [Google Scholar]
- 18.DaSilva A F, Becerra L, Makris N.et al Somatotopic activation in the human trigeminal pain pathway. J Neurosci 2002228183–8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komisaruk B R, Mosier K M, Liu W C.et al Functional localization of brainstem and cervical spinal cord nuclei in humans with fMRI. Am J Neuroradiol 200223609–617. [PMC free article] [PubMed] [Google Scholar]