Abstract
Background
Depersonalisation is a subjective experience of unreality and detachment from the self often accompanied by derealisation; the experience of the external world appearing to be strange or unreal. Feelings of unreality can be evoked by disorienting vestibular stimulation.
Objective
To identify the prevalence of depersonalisation/derealisation symptoms in patients with peripheral vestibular disease and experimentally to induce these symptoms by vestibular stimulation.
Methods
121 healthy subjects and 50 patients with peripheral vestibular disease participated in the study. For comparison with the patients a subgroup of 50 age matched healthy subjects was delineated. All completed (1) an in‐house health screening questionnaire; (2) the General Health Questionnaire (GHQ‐12); (3) the 28‐item depersonalisation/derealisation inventory of Cox and Swinson (2002). Experimental verification of “vestibular induced” depersonalisation/derealisation was assessed in 20 patients and 20 controls during caloric irrigation of the labyrinths.
Results
The frequency and severity of symptoms in vestibular patients was significantly higher than in controls. In controls the most common experiences were of “déjà vu” and “difficulty in concentrating/attending”. In contrast, apart from dizziness, patients most frequently reported derealisation symptoms of “feel as if walking on shifting ground”, “body feels strange/not being in control of self”, and “feel ‘spacey' or ‘spaced out'”. Items permitted discrimination between healthy subjects and vestibular patients in 92% of the cases. Apart from dizziness, caloric stimulation induced depersonalisation/derealisation symptoms which healthy subjects denied ever experiencing before, while patients reported that the symptoms were similar to those encountered during their disease.
Conclusions
Depersonalisation/derealisation symptoms are both different in quality and more frequent under conditions of non‐physiological vestibular stimulation. In vestibular disease, frequent experiences of derealisation may occur because distorted vestibular signals mismatch with the other sensory input to create an incoherent frame of spatial reference which makes the patient feel he or she is detached or separated from the world.
Keywords: depersonalisation, derealisation, dissociation, vestibular, dizziness
Altered perceptions of the self and the environment are termed “dissociation phenomena”1 and include depersonalisation which is a subjective experience of unreality and detachment from the self.2 Depersonalisation is often accompanied by derealisation; the experience of the external world appearing strange or unreal; viewed by some as a distinct disorder or as a subset of depersonalisation.3,4 A community questionnaire survey study in the USA has reported prevalence rates of 19.1% for depersonalisation, 14.4% for derealisation, and 23.4% for either dissociative experience.5 In USA and UK, student samples, using a variety of data collection methods and diagnostic criteria, prevalence of depersonalisation/derealisation symptoms has been reported as varying from 8.5% to 73.9%.6 On the other hand, in consecutive psychiatric inpatient admissions with a mixture of diagnoses, prevalence of depersonalisation/derealisation symptoms in USA, UK, and Canada has varied from 1% to 86%.6
Depersonalisation/derealisation symptoms are also commonly described accompanying a wide variety of psychiatric and neurological disorders,3,7 including epilepsy.8,9 Of pertinence, Blanke et al propose that autoscopic phenomena (seeing one's body in extra personal space) in epilepsy tend to occur when there is coexisting vestibular dysfunction.10 Recent studies suggest that unambiguous self location and egocentric visuospatial perspective are related to neural activity at the temporoparietal junction.11 A functional imaging study of patients with depersonalisation disorder has suggested abnormalities in the sensory cortex and areas responsible for an integrated body schema, specifically area 7B, consistent with the proposal that the inferior parietal cortex is concerned with spatial orientation, visuomotor, and vestibular function.12,13
The relationship between vestibular function and feelings of unreality was recognised decades ago, when Schilder emphasised its connection with depersonalisation.14 This tendency for abnormal vestibular stimulation to provoke feelings of unreality has also been found in normal healthy subjects undergoing calorics;15,16,17 furthermore, false perceptions of orientation may be a consequence of vestibular disorders.18 Even after head trauma, depersonalisation syndrome has been associated with the feature of vertigo.19 However, reports of depersonalisation/derealisation symptoms in patients with vestibular disease are scarce.20 The present study attempts to identify the prevalence of depersonalisation/derealisation experiences in vestibular patients in comparison with a control population of healthy subjects. We also sought experimental confirmation of the association between depersonalisation/derealisation and vestibular dysfunction by probing for depersonalisation/derealisation experiences during caloric stimulation of the labyrinths.
Methods
Subjects
The 171 subjects, comprising 121 healthy adults and 50 patients with peripheral vestibular disease, gave their informed consent to participate in the study which was approved by the Riverside Hospitals Ethics Committee. All were volunteers and no pay was offered for participation. Their relevant characteristics are presented in table 1. Initially, 184 healthy subjects attending a health and fitness centre in London were invited to participate; one did not accept the invitation; data from other 62 subjects were excluded from the study because of histories of dizziness or vestibular disease (32 subjects), self‐reports of anxiety or depression (18 subjects), visual field or hearing disorders (4 subjects), and incomplete information (3 subjects). The 121 healthy subjects who participated were 66 females and 55 males (mean age 37 (SD 13) years, range 21–79 years). Subjects had no history of vertigo, unsteadiness, hearing loss, or neurological disorder nor were they under psychiatric care or on psychotropic medication. For comparison with the patients, an age matched subgroup of 50 healthy subjects comprising 23 females and 27 males (mean age 48 (SD 11) years, range 29–79 years) was selected from the main group. The subjects with vestibular disease were recruited by invitation from consecutive patients, who presented sequentially to outpatients clinics at tertiary referral centres (University Hospitals) in central London and who received the diagnosis of peripheral vestibular disorder. All invited patients accepted to participate, resulting in 26 females and 24 males (mean age 52 (SD 13) years, range 26–78 years).
Table 1 General characteristics of the 121 healthy subjects and 50 vestibular patients who participated in the survey.
| Variables | Healthy subjects | Vestibular patients |
|---|---|---|
| Highest education level | ||
| University | 60% | 44% |
| Secondary school | 25% | 44% |
| Primary school | 15% | 12% |
| Employment status | ||
| Employed | 74% | 50% |
| Student | 11% | 10% |
| Retired | 6% | 22% |
| Unemployed | 9% | 16% |
| Marital status | ||
| Single | 52% | 20% |
| Married | 43% | 68% |
| Divorced or widowed | 5% | 12% |
| Health habits | ||
| Smokers | 15% | 18% |
| No alcohol | 22% | 34% |
| 1–5 units/week (beer, spirits, wine) | 36% | 28% |
| 6–10 units/week | 23% | 14% |
| >10 units/week | 19% | 24% |
Vestibular disorder was diagnosed by neuro‐otological evaluation including: eye movement examination, positional manoeuvres, and caloric testing (30° and 44°C). Clinical diagnoses are shown in table 2. Patients were classified as having recent symptoms of dizziness or imbalance, if these were the main presenting symptoms at the time of evaluation (table 2). All patients denied having a history of neurological or psychiatric disorders (under psychiatric care or on psychotropic medication). None had strabismus or ophthalmologic disorders other than corrected refractive errors. Hearing was normal in 33 patients; nine patients had mild to moderate, high frequency, bilateral hearing loss; five had mild or moderate, low frequency, unilateral hearing loss, and three patients had moderate to severe, unilateral hearing loss (all frequencies). In all cases, hearing loss was concomitant with vestibular disease or due to presbyacusis.
Table 2 Clinical diagnosis of the patients with peripheral vestibular disease who participated in the study.
| Diagnosis | Total | Recent balance symptoms | No recent balance symptoms |
|---|---|---|---|
| Unilateral canal paresis | 17 | ||
| Vestibular neuritis | 6 | 5 | 1 |
| Unknown origin | 7 | 5 | 2 |
| Vestibular schwannoma | 4 | 1 | 3 |
| Bilateral hypofunction | 13 | 9 | 4 |
| BPPV | 20 | ||
| Normal horizontal VOR | 12 | 10 | 2 |
| Unilateral canal paresis | 8 | 7 | 1 |
| Total | 50 | 37 | 13 |
In the first part of the study all subjects completed three written questionnaires:
An in‐house 27‐item health screening questionnaire about general medical history, particularly headache, dizziness, hearing loss, visual problems, neurological symptoms, and smoking and alcohol consumption (available on request from the authors).
The 12‐item General Health Questionnaire (GHQ‐12) to identify symptoms of common mental disorders (for example, anxiety and depression).21,22,23
The 28‐item depersonalisation/derealisation inventory by Cox and Swinson (table 3).24
Table 3 Frequency for each of the symptoms of the Cox and Swinson (2002) depersonalisation/derealisation inventory (2002) and median score for items scored >0. *p<0.05.
| Depersonalisation/derealisation symptoms | Healthy subjects | Vestibular patients | ||||
|---|---|---|---|---|---|---|
| (n = 121) | (n = 50) | (n = 50) | ||||
| Frequency | If positive, score range (median) | Frequency | If positive, score range (median) | Frequency | If positive, score range (median) | |
| 1. Surroundings seem strange and unreal | 17% | 1–2 (1) | 8% | 1–2 (1) | 40%* | 1–4 (2) |
| 2. Time seems to pass very slowly | 36% | 1–2 (1) | 18% | 1–2 (1) | 24% | 1–4 (2) |
| 3. Body feels strange or different in some way | 15% | 1–2 (1) | 10% | 1–1 (1) | 50%* | 1–4 (2) |
| 4. Feel like you've been here before (déjà vu) | 65% | 1–4 (1) | 56% | 1–3 (1) | 32%* | 1–2 (2) |
| 5. Feel as though in a dream | 22% | 1–3 (1) | 10% | 1–2 (1) | 46%* | 1–4 (1) |
| 6. Body feels numb | 9% | 1–2 (1) | 6% | 1–1 (1) | 26%* | 1–3 (1) |
| 7. Feeling of detachment or separation from surroundings | 18% | 1–2 (1) | 14% | 1–2 (1) | 40%* | 1–4 (2) |
| 8. Numbing of emotions | 20% | 1–3 (1) | 10% | 1–1 (1) | 24% | 1–4 (2) |
| 9. People and objects seem far away | 10% | 1–3 (1) | 2% | 1–1 (1) | 24%* | 1–4 (2) |
| 10. Feeling detached or separated from body | 7% | 1–2 (1) | 6% | 1–2 (1) | 30%* | 1–3 (2) |
| 11. Thoughts seem blurred | 27% | 1–2 (1) | 20% | 1–2 (1) | 46%* | 1–3 (2) |
| 12. Events seem to happen in slow motion | 13% | 1–2 (1) | 4% | 1–1 (1) | 26%* | 1–3 (1) |
| 13. Your emotions seem disconnected from yourself | 14% | 1–2 (1) | 6% | 1–1 (1) | 30%* | 1–3 (2) |
| 14. Feeling of not being in control of self | 15% | 1–3 (1) | 10% | 1–1 (1) | 50%* | 1–3 (2) |
| 15. People appear strange or unreal | 12% | 1–2 (1) | 4% | 1–1 (1) | 22%* | 1–3 (2) |
| 16. Dizziness | 21% | 1–2 (1) | 8% | 1–1 (1) | 88%* | 1–4 (3) |
| 17. Surroundings appear covered with a haze | 9% | 1–1 (1) | 10% | 1–1 (1) | 30%* | 1–4 (2) |
| 18. Vision is dulled | 7% | 1–1 (1) | 6% | 1–1 (1) | 40%* | 1–3 (2) |
| 19. Feel as if walking on shifting ground | 6% | 1–2 (1) | 4% | 1–1 (1) | 64%* | 1–4 (2) |
| 20. Difficulty understanding what others say to you | 21% | 1–3 (1) | 12% | 1–2 (1) | 40%* | 1–3 (2) |
| 21. Difficulty focusing attention | 36% | 1–3 (1) | 18% | 1–2 (1) | 60%* | 1–4 (2) |
| 22. Feel as though in a trance | 13% | 1–2 (1) | 6% | 1–1 (1) | 32%* | 1–4 (2) |
| 23. The distinction between close and distant is blurred | 3% | 1–2 (1) | 2% | 2–2 (2) | 24%* | 1–3 (2) |
| 24. Difficulty concentrating | 49% | 1–3 (1) | 30% | 1–2 (1) | 60%* | 1–4 (2) |
| 25. Feel as though your personality is different | 15% | 1–2 (1) | 8% | 1–1 (1) | 22% | 1–4 (2) |
| 26. Feel confused or bewildered | 19% | 1–3 (1) | 8% | 1–1 (1) | 36%* | 1–4 (2) |
| 27. Feel isolated from the world | 11% | 1–2 (1) | 8% | 1–2 (2) | 26%* | 1–3 (2) |
| 28. Feel “spacey” or “spaced out” | 22% | 1–2 (1) | 4% | 1–1 (1) | 48%* | 1–4 (2) |
The instrument was designed to assess this phenomenology in clinically anxiety states, rather than in the context of dissociative disorders, with the purpose of enabling the correlation of symptoms with concurrent neurophysiological variables.25 The severity of each inventory item is coded on a five‐point scale where 0 = does not occur, 1 = mild, 2 = moderate, 3 = severe, and 4 = very severe. The inventory was administered stating the relevant time frame: for healthy subjects “Have you ever had these types of experiences?”; for vestibular patients “Since you had vertigo for the first time, have you ever had these types of experiences?”. Repeatability of responses was evaluated in 21 healthy subjects, 21–81 years old (12 female and 9 male), all of whom had a GHQ‐12 score ⩽2. After completing the inventory twice, within the same day, the total score was repeatable in 91% of the cases (repeatability coefficient of 2). Responses to each item were repeatable in 91% to 100% of the cases.
After completing the questionnaires, a subset of 20 vestibular patients (mean age 48 (SD 13) years old) and 20 healthy volunteers (mean age 38 (SD 12) years old) were studied during caloric stimulation (30° and 44°C). In patients the caloric test was a routine part of their clinical diagnosis. Although patients with bilateral vestibular loss were not included in this subgroup of 20 patients, to control for the conditions in which the caloric test was performed, four additional patients with bilateral vestibular loss who received calorics as part of their clinical evaluation were also questioned about depersonalisation/derealisation experiences.26
The caloric test was administered with the head orientated at 30° with respect to horizontal, using a water caloric stimulator (NCI‐480, ICS Medical, Taastrup, Denmark), and eye movements were recorded using video‐nystagmography (ChartrVNG, ICS Medical, Taastrup, Denmark). Immediately after testing, the subjects once more reported their depersonalisation/derealisation symptoms on the Cox and Swinson inventory.24 The examiner issued the specific instruction “During the caloric test, did you have these types of experiences?”
Data processing and analysis
The score for the GHQ‐12 was obtained using the “GHQ method” of 0‐0‐1‐1.21 The score for the depersonalisation/derealisation inventory was calculated as the sum of the individual scores of each of the 28 items. Statistical analysis was performed using ANCOVA, t for proportions, Mann‐Whitney U test, Wald Wolfovitz runs test, t test, Spearman's correlation coefficient, and Discriminant Function Analysis. Significance was set at the ⩽0.05 level.
Results
Healthy subjects survey
The number of symptoms reported by all the healthy subjects in the depersonalisation/derealisation inventory ranged from 0 to 20 (average 5) and the range of the total score was from 0 to 28 (average 6). Individual scores on each item were low (table 3). After “déjà vu” (65%) the most frequent symptoms described by healthy subjects were: “difficulty concentrating” (49%), “difficulty focusing attention” (36%), “time seems to pass very slowly” (36%), and “thoughts seem blurred” (27%).
A significant correlation between the depersonalisation/derealisation symptoms and the general characteristics of the subjects was found only for the déjà vu item; greater age was related to both lower symptom frequency (Spearman's r = −0.21, p<0.05) and lower symptom severity (r = −0.33, p<0.02). No significant correlation was found with the other general characteristics of the subjects.
In healthy subjects, the range of the GHQ‐12 total score was from 0 to 2, median of 0. There was a significant positive correlation between the depersonalisation/derealisation total score and the GHQ‐12 total score (Spearman's r = 0.28, p<0.01) and the age of the subjects (r = 0.32, p<0.01). However, when these two variables were considered together with the general characteristics, only the age of subjects (adjusted r2 = 0.08, p<0.01) had a significant influence on the depersonalisation/derealisation score. However, it is doubtful that the responses do reflect lifetime experiences as the inventory purports, since one would expect experiences to accumulate through time. Failure to accumulate symptoms may be due to failure to recall inconsequential experiences, suppression of unpleasant memories, and negative connotations of psychological symptoms, which may bias reporting.
Symptoms in vestibular patients versus healthy subjects
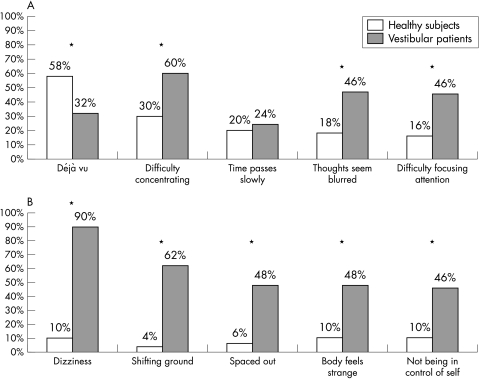
Compared to the matched subgroup of healthy subjects, the frequency and severity of the depersonalisation/derealisation symptoms reported by the vestibular patients were significantly higher on 25 of the 28 items (table 3). Frequency distribution of the five most frequent symptoms of each group (fig 1) were also significantly different (p<0.05).
Figure 1 The five most frequent symptoms reported by (A) healthy subjects and by (B) vestibular patients (apart from “difficulty concentrating). *p<0.05.
In the vestibular patients the number of depersonalisation/derealisation symptoms reported ranged from 1 to 28 (median 7), while in the age matched controls the number of symptoms reported ranged from 0 to 16 (median 2) (p<0.01). In the patients the range of the depersonalisation/derealisation total score was from 1 to 97 (average 21) and in the age matched controls the range of the total score was from 0 to 16 (average 4) (p<0.01). In particular, the symptoms “feel ‘spacey' or ‘spaced out'”, “feeling of detachment or separation from surroundings”, and “feel as though in a dream” were reported by about 50% of the patients, while they were rare in the age matched controls (p<0.01).
The GHQ‐12 total score also differed between the two groups. The GHQ‐12 total score in the vestibular patients ranged from 0 to 10 (median 2) and in the age matched controls it ranged from 0 to 2 (median 0) (p<0.01). The simple correlation between the GHQ‐12 total score and the depersonalisation/derealisation score was significant (Spearman's r = 0.57, p<0.001). No interaction was observed between the GHQ‐12 score and age or any other general characteristic of the subjects.
Patients with unilateral canal paresis (>20% caloric asymmetry) reported 2–24 depersonalisation/derealisation symptoms (median 10) with total scores of 2–67 (average 17). Patients with benign paroxysmal positional vertigo (BPPV) reported 1–28 symptoms (median 6) with total scores of 2–52 (average 16). BPPV patients with or without canal paresis reported a similar number of symptoms and total scores (average score 18 (SD 13) v 15 (SD 9)). Patients with bilateral vestibular dysfunction reported the highest number of symptoms, 2–28 symptoms (median 17), and had the highest total scores (4–97, average 36). In contrast, the four patients with vestibular schwannoma reported just 1–5 symptoms with low total scores (1–12).
Vestibular patients with recent balance symptoms had a higher depersonalisation/derealisation score (range 2–97, average 25) than those without recent symptoms (1–41, average 9) and the age matched controls (0–16, average 4) (p<0.01). However, patients with recent symptoms also showed a trend for higher GHQ‐12 scores; their median GHQ‐12 score was 2 (range 0–10), while patients with no recent symptoms had a median score of 1 (0–8) (p = 0.09). This finding is consistent with the significant correlation found between the GHQ‐12 score and the individual score of several items (items 5, 7, 14, 20, and 24 described in table 3), with Spearman's r values between 0.3 and 0.5 (p<0.05). However, analysis of depersonalisation/derealisation scores of healthy subjects and vestibular patients (with/without recent balance symptoms), considering covariance of the GHQ‐12 score, showed significant influence only of the group (p<0.001), either for the total depersonalisation/derealisation score or for the partial score given by the sum of the items described as a result of discriminant function analysis.
Discriminant function analysis of both frequency and severity rating on each item was used to identify the items of the inventory, which could discriminate between vestibular patients and healthy subjects. Since half of the patients had some hearing loss, item 20 (“difficulty understanding what others say to you”) was not included in this analysis. Given that “dizziness” and “feel like walking on shifting ground” are symptoms that arise because sensory integration is compromised by the altered vestibular signal of spatial reference,27 a preliminary analysis was performed considering only these two symptoms, which were enough to classify 100% of the healthy subjects and 78% of the patients (Wilk's lambda of 0.36, p<0.0001; squared Mahalanobis distance of 6.88, p<0.0001). Next, we focused on other symptoms of depersonalisation/derealisation, performing the analysis excluding these two items. A combination of eight items best discriminated 100% of healthy subjects and 62% of the patients (Wilk's lambda of 0.64, p<0.0001; squared Mahalanobis distance of 2.21, p<0.0001). These items were (1) “surroundings seem strange and unreal”; (3) “body feels strange or different in some way”; (5) “feel as though in a dream”; (7) “feeling of detachment or separation from surroundings”; (10) “feeling detached or separated from your body”; (14) “feeling of not being in control of self”; (25) “feel as though your personality is different”; and (28) “feel ‘spacey' or ‘spaced out'”. In this analysis, to feel “spacey” was the most important factor discriminating 96% of healthy subjects and 48% of the patients (Wilk's lambda of 0.77, p<0.0001; squared Mahalanobis distance of 1.18, p<0.0001). In a third analysis, the combination of all 10 items discriminated 100% of healthy subjects and 84% of patients (Wilk's lambda 0.31, p<0.0001; squared Mahalanobis distance of 8.88, p<0.0001).
When vestibular patients were divided into those with and those without recent balance symptoms, the 10 items discriminated 100% of healthy subjects, but only 53% of patients without symptoms and 67% of the patients with symptoms (Wilk's lambda 0.23, p<0.0001, squared Mahalanobis distances from 3.07 to 11.9, p<0.01). However, when only patients with recent symptoms were considered in the analysis, discrimination was appropriate in 100% of healthy subjects and 86% of the patients (Wilk's lambda 0.21, p<0.0001, squared Mahalanobis distance 14.99, p<0.0001).
Caloric stimulation
On the first inventory, given before calorics, the depersonalisation/derealisation symptoms reported by the subset of healthy subjects and the vestibular patients were representative of the groups as wholes.
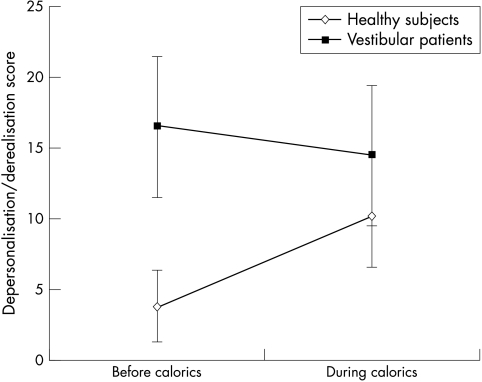
The depersonalisation/derealisation total scores obtained before and during the caloric stimulation differed in healthy subjects (p<0.01) but not in vestibular patients (fig 2). Healthy subjects reported that calorics provoked symptoms they had not previously experienced and many of these were similar to the ones reported by vestibular patients (table 4). Apart from dizziness, the symptoms that had a significantly higher frequency during caloric stimulation were mostly the derealisation symptoms reported by patients. Most healthy subjects denied having had these types of symptoms previously and were experiencing them for the first time during calorics.
Figure 2 Mean and 95% confidence interval of the mean of depersonalisation/derealisation scores before and during caloric stimulation of healthy subjects and of patients with vestibular disease.
Table 4 Frequency of symptoms included in the Cox and Swinson depersonalisation/derealisation inventory (2002).
| Depersonalisation/derealisation symptoms | Healthy subjects | Vestibular patients | ||
|---|---|---|---|---|
| Before calorics | During calorics | Before calorics | During Calorics | |
| 1. Surroundings seem strange and unreal** | 10% | 40%* | 35% | 45% |
| 2. Time seems to pass very slowly** | 30% | 70%* | 40% | 45% |
| 3. Body feels strange/different in some way** | 10% | 50%* | 55% | 60% |
| 4. Feel like you've been here before (déjà vu)** | 40% | 10%* | 30% | 20% |
| 5. Feel as though in a dream | 10% | 10% | 30% | 20% |
| 6. Body feels numb** | 0% | 20% | 15% | 10% |
| 7. Feeling of detachment or separation from surroundings | 10% | 50%* | 30% | 55% |
| 8. Numbing of emotions | 10% | 10% | 20% | 10% |
| 9. People and objects seem far away | 10% | 30% | 20% | 15% |
| 10. Feeling detached or separated from body | 10% | 30% | 15% | 25% |
| 11. Thoughts seem blurred | 30% | 20% | 40% | 45% |
| 12. Events seem to happen in slow motion | 20% | 20% | 15% | 20% |
| 13. Your emotions seem disconnected from yourself | 10% | 10% | 15% | 20% |
| 14. Feeling of not being in control of self** | 20% | 40% | 35% | 40% |
| 15. People appear strange or unreal | 10% | 10% | 15% | 15% |
| 16. Dizziness** | 20% | 90%* | 85% | 90% |
| 17. Surroundings appear covered with a haze | 10% | 10% | 35% | 30% |
| 18. Vision is dulled | 10% | 20% | 45% | 35% |
| 19. Feel as if walking on shifting ground | 0% | 20% | 60% | 50% |
| 20. Difficulty understanding what others say to you | 20% | 10% | 35% | 20% |
| 21. Difficulty focusing attention | 30% | 40% | 45% | 35% |
| 22. Feel as though in a trance | 10% | 20% | 25% | 30% |
| 23. The distinction between close and distant is blurred | 10% | 10% | 10% | 20% |
| 24. Difficulty concentrating** | 30% | 30% | 50% | 30% |
| 25. Feel as though your personality is different | 20% | 10% | 10% | 10% |
| 26. Feel confused or bewildered | 10% | 20% | 30% | 25% |
| 27. Feel isolated from the world** | 10% | 30% | 20% | 20% |
| 28. Feel “spacey” or “spaced out” | 10% | 40%* | 30% | 50% |
Reported by 20 healthy subjects and 20 vestibular patients, before and during caloric stimulation, within each group (*) and between group changes (**) p<0.05.
Vestibular patients reported that the symptoms induced by caloric stimulation were similar to the symptoms that they had experienced since their vestibular disorder was diagnosed (table 4). However, there was a trend to increased occurrence of “feeling of detachment or separation from surroundings” (p = 0.1) (table 4).
In the four patients with bilateral vestibular loss, caloric stimulation reassuringly induced almost no symptoms. Before the caloric test, the depersonalisation/derealisation scores of these patients ranged from 17–97 but during the caloric test, the scores were 1–24. The most frequent symptom was “time seems to pass very slowly”.
Discussion
Prevalence of depersonalisation/derealisation symptoms
In agreement with findings from dissociation surveys,28 our younger subjects reported depersonalisation/derealisation symptoms more frequently than the older subjects. There was no influence of gender, marital status, employment status, or formal education. Failure to find any relationship with tobacco or alcohol consumption may be due to the low numbers of heavy smokers and high alcohol consumers in our sample.
The most frequent symptom reported by almost two thirds of the healthy subjects was “déjà vu”, a finding consistent with numerous other studies.29,30 The second most common symptom reported was “difficulty concentrating”, endorsed by almost half of the subjects. Although interference between mental activity and orientation is known,31 difficulty concentrating is a common symptom whose significance is difficult to assess when taken out of context.
Patient ratings of depersonalisation/derealisation were different both in quality and quantity from those reported by normal subjects who showed a strong loading specifically on derealisation items. Vestibular disease causes primary symptoms of vertigo and feelings that the ground is unstable—“mal de debarquement”—which are more marked in distinct, acute episodes. These immediate symptoms are, by definition, unreal experiences since the body is not spinning and the ground is not heaving, but they are readily understandable as perceptions derived directly from abnormal sensory signals. Vestibular dysfunction could also compromise more general precepts of stable relationships between the self and the environment because the process of sensory integration which makes a coherent whole of sensory‐motor transactions relies on a veridical vestibular signal of spatial reference.27 Apart from acute episodes of vertigo, the vestibular patient is also likely to suffer a continual disorder of sensory integration since all head movement is accompanied by a deranged vestibular signal which is mismatched to both corroborative sensory input and expectations. Hence the vestibular patient is continually exposed to mismatches or incoherencies in his sensory experiences. The evidence for derealisation due to this failure of sensory integration are the symptoms “feel ‘spacey' or ‘spaced out'”, “feeling of detachment or separation from surroundings”, and “feel as though in a dream”, which were reported by 50% of the patients while being rare in the matched controls (p<0.01).
Analysis of depersonalisation/derealisation symptoms allowed discrimination of all healthy subjects and 84% of the patients from healthy subjects. Conversely, discrimination between patients with and without recent symptoms was poor. The latter finding could be explained by the fact that, although their scores were significantly different, the symptoms reported were similar. However, the different scores suggest that patients with poor or incomplete recovery, who are still disoriented, are more prone to manifest derealisation symptoms than those patients who do not have recent balance symptoms. Interestingly, patients who had vestibular schwannoma were indistinguishable from healthy subjects on the derealisation items. The slowly progressive loss of function with a schwannoma does not provoke marked symptoms of vertigo or imbalance and allows time for adaptation to take place.
The depersonalisation/derealisation score in normal subjects was related to their GHQ‐12 score, a self‐report of symptoms of common mental disorders. This finding is consistent with the high frequency of depersonalisation/derealisation reported in patients with panic disorder,7,24 and the common finding of anxiety and depression as comorbid diagnoses in depersonalisation disorder.7,32,33 However, according to Simeon,34 mood, anxiety, and personality disorders are often comorbid with depersonalisation disorder but none predicts symptom severity; the most common immediate precipitants of the disorder are severe stress, depression, and panic, and marijuana and hallucinogen ingestion. Since anxiety is common among vestibular patients, it may have increased patients' scores,35,36 as shown by the correlation found between their GHQ‐12 score and both item scores and total depersonalisation/derealisation scores. However, when GHQ‐12 score and group classification (healthy/vestibular) were considered together, group classification was shown to have a major influence on the results.
Caloric stimulation
In healthy subjects caloric stimulation induced depersonalisation/derealisation symptoms. During the test, all subjects experienced a degree of dizziness, as expected, accompanied by symptoms that they previously denied. In particular, about half of them experienced feelings of unreality of both the body and the environment. Curiously, some healthy subjects and patients reported that caloric stimuli induced “feeling as if walking on shifting ground” although, during the test, subjects were supine! This suggests that subjects reported their sensations without prejudice, and that they may have identified the sensation induced by caloric stimuli with previous experiences.
In vestibular patients, caloric stimulation was able to reproduce symptoms that they had experienced during the course of their disease. Both vestibular patients and healthy subjects also experienced “feeling of detachment or separation from surroundings”. This symptom may have been influenced by the dim lighting and quiet environment of the caloric room, which imply some sensory deprivation. However, in three of the four patients with bilateral vestibular loss the only symptom reported during caloric stimulation was “time seems to pass very slowly”. This finding suggests that vestibular stimulation, and not the unusual circumstances of the test, was the main provocateur of depersonalisation/derealisation symptoms.
The high frequency of derealisation reported by patients is in agreement with evidence from functional imaging showing that dissociation and depersonalisation scores in subjects with depersonalisation disorder are significantly related to metabolic activity in the parietal cortex12 and vestibular caloric stimuli also induces regional cerebral blood flow changes in this region.37 This concurrence of cerebral localisation together with the presence of a vestibular component to derealisation in epilepsy10,11 support that notion that derealisation experiences are a specific feature in primary vestibular disease.
The findings suggest that disorientation due to deficient sensory integration could contribute to derealisation. However, this study was designed to identify a possible association between vestibular disease and depersonalisation/derealisation symptoms. Further studies are needed to evaluate the meaning of vestibular function and self‐orientation on depersonalisation/derealisation experiences.
Conclusions
Depersonalisation/derealisation symptoms scored by questionnaire are both different in quality and frequency in vestibular patients, who report more experiences of derealisation than healthy subjects. Caloric labyrinthine stimulation provokes similar derealisation symptoms to those experienced in vestibular disease. We propose that derealisation occurs in vestibular patients because their distorted vestibular signals create a misleading frame of spatial reference which mismatches with the other senses, giving rise to illusory, “unreal” perceptions of the patient's transactions with the physical world. Since experiences of unreality were powerful items in discriminating between healthy subjects and symptomatic vestibular patients, further studies are needed to evaluate their influence on the patient's attitude to his or her disease.
Acknowledgements
KJR was supported by Instituto Mexicano del Seguro Social.
Footnotes
Competing interests: None.
Procedures in the study were approved by Riverside Research Ethics Committee of Hammersmith
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). Washington DC: American Psychiatric Press, 1994
- 2.Simeon D, Knutelska M, Nelson D.et al Feeling unreal: a depersonalization disorder update of 117 cases. J Clin Psychiatry 200364990–997. [DOI] [PubMed] [Google Scholar]
- 3.Coons P M. The dissociative disorders. Rarely considered and underdiagnosed. Psychiatr Clin North Am 199821637–648. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs J R, Bovasso G B. Toward the clarification of the construct of depersonalization and its association with affective and cognitive dysfunctions. J Pers Assess 199259352–365. [DOI] [PubMed] [Google Scholar]
- 5.Aderibigbe Y A, Bloch R M, Walker W R. Prevalence of depersonalization and derealization experiences in a rural population. Soc Psychiatry Psychiatr Epidemiol 20013663–69. [DOI] [PubMed] [Google Scholar]
- 6.Hunter E C, Sierra M, David A S. The epidemiology of depersonalisation and derealization. A systematic review. Soc Psychiatry Psychiatr Epidemiol 2004399–18. [DOI] [PubMed] [Google Scholar]
- 7.Cassano G B, Petracca A, Perugi G.et al Derealization and panic attacks: a clinical evaluation on 150 patients with panic disorder/agoraphobia. Compr Psychiatry 1989305–12. [DOI] [PubMed] [Google Scholar]
- 8.Lambert M V, Sierra M, Phillips M L.et al The spectrum of organic depersonalization: a review plus four new cases. J Neuropsychiatry Clin Neurosci 200214141–154. [DOI] [PubMed] [Google Scholar]
- 9.Bancaud J, Brunet‐Bourgin F, Chauvel P.et al Anatomical origin of déjà vu and vivid ‘memories' in human temporal lobe epilepsy. Brain 199411771–90. [DOI] [PubMed] [Google Scholar]
- 10.Blanke O, Landis T, Spinelli L.et al Out‐of‐body experience and autoscopy of neurological origin. Brain 2004127243–258. [DOI] [PubMed] [Google Scholar]
- 11.Blanke O, Mohr C, Michel C M.et al Linking out‐of‐body experience and self processing to mental own‐body imagery at the temporoparietal junction. J Neuroscience 200525550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simeon D, Guralnik O, Hazlett E A.et al Feeling unreal: a PET study of depersonalization disorder. Am J Psychiatry 20001571782–1788. [DOI] [PubMed] [Google Scholar]
- 13.Brandt T, Dieterich M. The vestibular cortex. Its locations, functions, and disorders. Ann NY Acad Sci 1999871293–312. [DOI] [PubMed] [Google Scholar]
- 14.Schilder P.The image and appearance of the human body. Studies in the constructive energies of the psyche. New York: John Wiley & Sons Inc, 1964,
- 15.Cappon D, Banks R. Orientational perception. A review and preliminary study of distortion in orientational perception. Arch Gen Psychiatry 19615380–392. [DOI] [PubMed] [Google Scholar]
- 16.Cappon D, Banks R. Orientational perception. II. Body perception in depersonalization. Arch Gen Psychiatry 196513375–379. [DOI] [PubMed] [Google Scholar]
- 17.Cappon D. Orientational perception: 3. Orientational percept distortions in depersonalization. Am J Psychiatry 19691251048–1056. [DOI] [PubMed] [Google Scholar]
- 18.Brandt T.Vertigo: its multisensory syndromes. 2nd ed. London: Springer‐Verlag, 1999
- 19.Grisby J, Kaye K. Incidence and correlates of depersonalization following head trauma. Brain Injury 19937507–513. [DOI] [PubMed] [Google Scholar]
- 20.Grigsby J P, Johnston C L. Depersonalization, vertigo and Meniere's disease. Psychol Rep 198964527–534. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg D, Williams P.A users guide to the General Health Questionnaire. London: nferNelson, 1988
- 22.Furukawa T, Goldberg D P. Cultural invariance of likelihood ratios for the General Health Questionnaire. Lancet 1999353561–562. [DOI] [PubMed] [Google Scholar]
- 23.Weich S, Holt G R, Twigg L.et al Geographic variation in the prevalence of common mental disorders in Britain: a multilevel investigation. Am J Epidemiol 200319109–116. [DOI] [PubMed] [Google Scholar]
- 24.Cox B J, Swinson R P. Instrument to assess depersonalization‐derealization in panic disorder. Depress Anxiety 200215172–175. [DOI] [PubMed] [Google Scholar]
- 25.Kruger C, Mace C J. Psychometric validation of the State Scale of Dissociation (SSD). Psychol Psychother 20027533–51. [DOI] [PubMed] [Google Scholar]
- 26.Rinne T, Bronstein A M, Rudge P.et al Bilateral loss of vestibular function: clinical findings in 53 patients. J Neurol 1998245314–321. [DOI] [PubMed] [Google Scholar]
- 27.Mergner T, Maurer C, Peterka R J. A multisensory posture control model of human upright stance. Prog Brain Res 2003142189–201. [DOI] [PubMed] [Google Scholar]
- 28.Seedat S, Stein M B, Forde D R. Prevalence of dissociative experiences in a community sample: relationship to gender, ethnicity, and substance use. J Nerv Ment Dis 2003191115–120. [DOI] [PubMed] [Google Scholar]
- 29.Baker D, Hunter E, Lawrence E.et al Depersonalisation disorder: clinical features of 204 cases. Br J Psychiatry 2003182428–433. [PubMed] [Google Scholar]
- 30.Segui J, Marquez M, Garcia L.et al Depersonalization in panic disorder: a clinical study. Compr Psychiatry 200041172–178. [DOI] [PubMed] [Google Scholar]
- 31.Yardley L, Papo D, Bronstein A.et al Attentional demands of continuously monitoring orientation using vestibular information. Neuropsychologia 200240373–383. [DOI] [PubMed] [Google Scholar]
- 32.Adachi N, Adachi T, Kimura M.et al Demographic and psychological features of deja vu experiences in a nonclinical Japanese population. J Nerv Ment Dis 2003191242–247. [DOI] [PubMed] [Google Scholar]
- 33.Sno H N, Draaisma D. An early Dutch study of deja vu experiences. Psychol Med 19932317–26. [DOI] [PubMed] [Google Scholar]
- 34.Simeon D. Depersonalisation disorder: a contemporary overview. CNS Drugs 200418343–354. [DOI] [PubMed] [Google Scholar]
- 35.Grunfeld E A, Gresty M A, Bronstein A M.et al Screening for depression among neuro‐otology patients with and without identifiable vestibular lesions. Int J Audiol 200342161–165. [DOI] [PubMed] [Google Scholar]
- 36.Yardley L, Luxon L M, Haacke N P. A longitudinal study of symptoms, anxiety and subjective well‐being in patients with vertigo. Clin Otolaryngol Allied Sci 199419109–116. [DOI] [PubMed] [Google Scholar]
- 37.Emri M, Kisely M, Lengyel A.et al Cortical projection of peripheral vestibular signaling. J Neurophysiol 2003892639–2646. [DOI] [PubMed] [Google Scholar]