Abstract
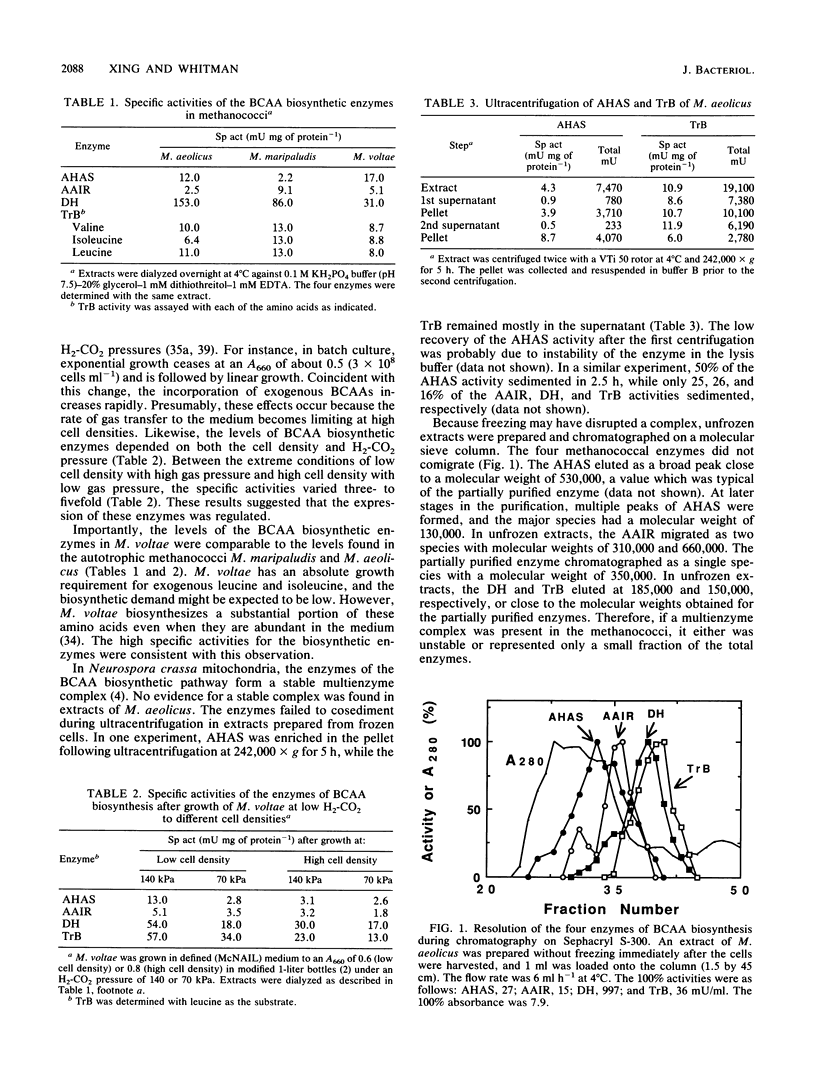
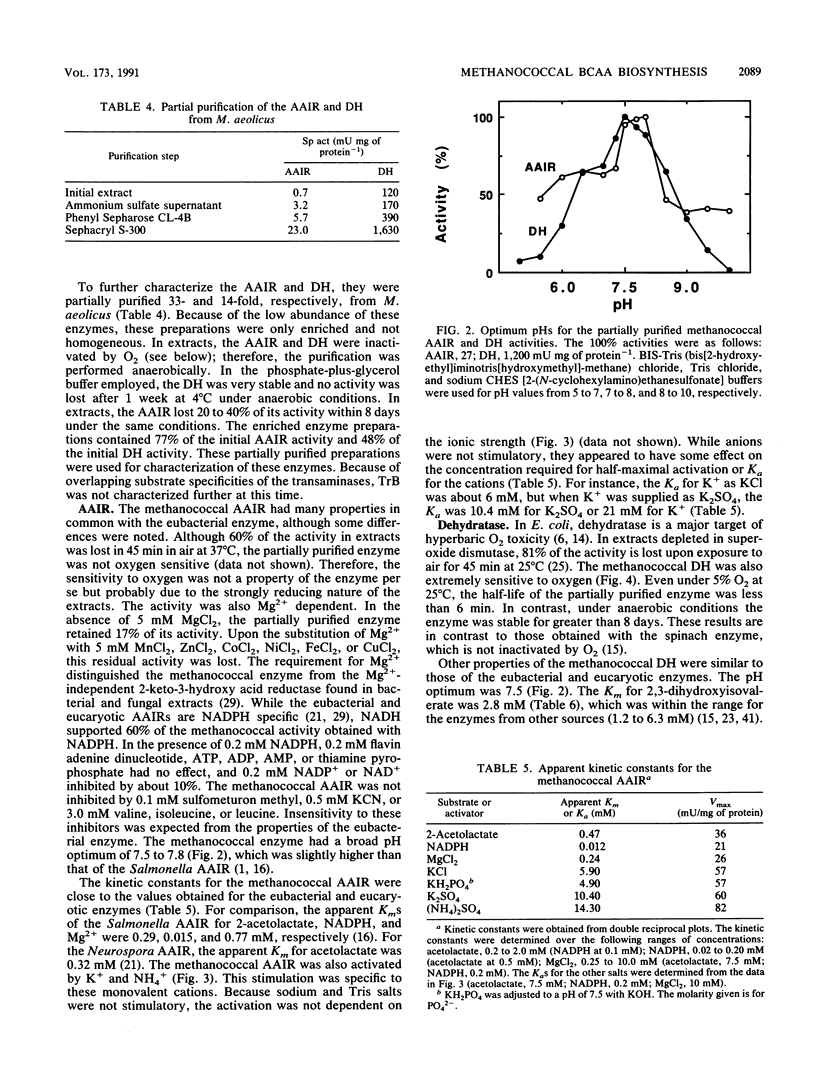
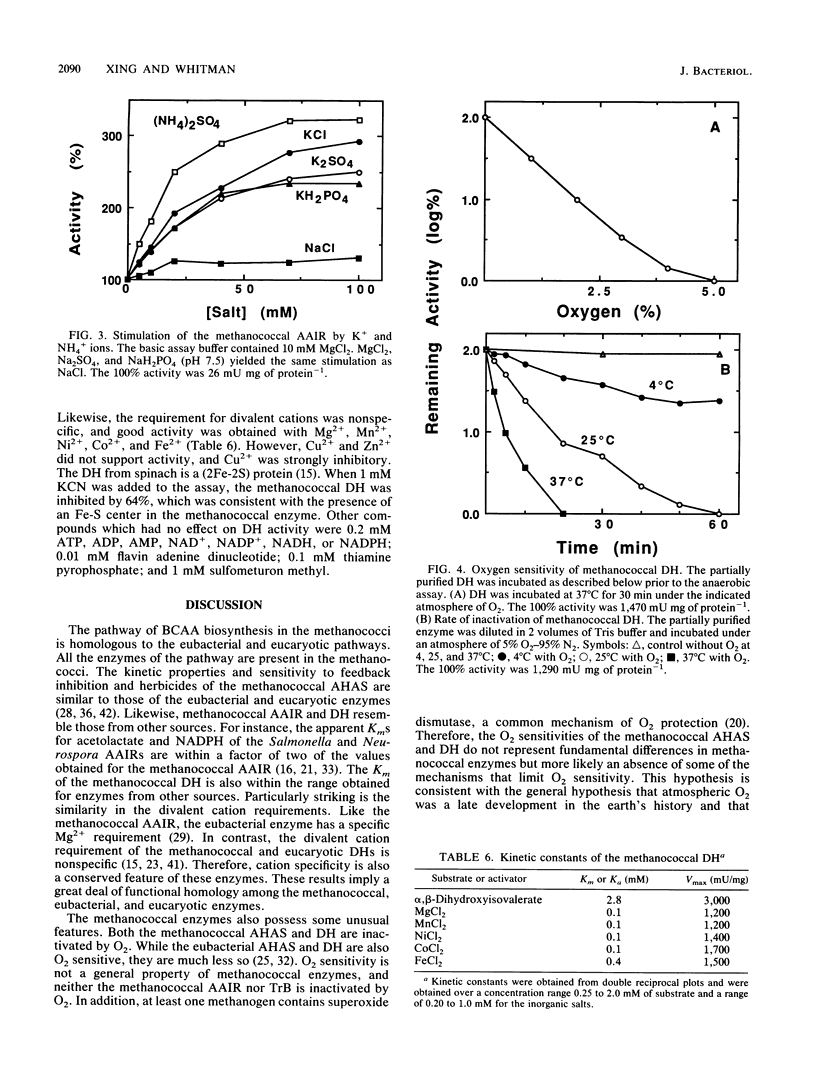
Methanococcus aeolicus, Methanococcus maripaludis, and Methanococcus voltae contain similar levels of four enzymes of branched-chain amino acid biosynthesis: acetohydroxy acid synthase, acetohydroxy acid isomeroreductase, dihydroxy acid dehydratase, and transaminase B. Following growth at low partial pressures of H2-CO2, the levels of these enzymes in extracts of M. voltae are reduced three- to fivefold, which suggests that their synthesis is regulated. The enzymes from M. aeolicus were found to be similar to the eubacterial and eucaryotic enzymes with respect to molecular weights, pH optima, kinetic properties, and sensitivities to O2. The acetohydroxy acid isomeroreductase has a specific requirement for Mg2+, and other divalent cations were inhibitory. It was stimulated threefold by K+ and NH4+ ions and was able to utilize NADH as well as NADPH. The partially purified enzyme was not sensitive to O2. The dihydroxy acid dehydratase is extremely sensitive to O2, and it has a half-life under 5% O2 of 6 min at 25 degrees C. Divalent cations were required for activity, and Mg2+, Mn2+, Ni2+, Co2+, and Fe2+ were nearly equally effective. In conclusion, the archaebacterial enzymes are functionally homologous to the eubacterial and eucaryotic enzymes, which implies that this pathway is very ancient.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfin S. M., Umbarger H. E. Purification and properties of the acetohydroxy acid isomeroreductase of Salmonella typhimurium. J Biol Chem. 1969 Mar 10;244(5):1118–1127. [PubMed] [Google Scholar]
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckler G. S., Reeve J. N. Conservation of primary structure in the hisI gene of the archaebacterium, Methanococcus vannielii, the eubacterium Escherichia coli, and the eucaryote Saccharomyces cerevisiae. Mol Gen Genet. 1986 Jul;204(1):133–140. doi: 10.1007/BF00330200. [DOI] [PubMed] [Google Scholar]
- Bergquist A., Eakin E. A., Murali D. K., Wagner R. P. A pyruvate-valine enzyme complex that is dependent upon the metabolic state of the mitochondria. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4352–4355. doi: 10.1073/pnas.71.11.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W., Daniels C. J., Reeve J. N. Gene structure, organization, and expression in archaebacteria. Crit Rev Microbiol. 1989;16(4):287–338. doi: 10.3109/10408418909105479. [DOI] [PubMed] [Google Scholar]
- Brown O. R., Yein F. Dihydroxyacid dehydratase: the site of hyperbaric oxygen poisoning in branch-chain amino acid biosynthesis. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1219–1224. doi: 10.1016/0006-291x(78)90672-1. [DOI] [PubMed] [Google Scholar]
- Cioffi E. A., Shaw K. J., Bailey W. F., Berg C. M. Improved synthesis of the sodium salt of DL-alpha, beta-dihydroxyisovaleric acid. Anal Biochem. 1980 May 15;104(2):485–488. doi: 10.1016/0003-2697(80)90104-9. [DOI] [PubMed] [Google Scholar]
- Cubellis M. V., Rozzo C., Nitti G., Arnone M. I., Marino G., Sannia G. Cloning and sequencing of the gene coding for aspartate aminotransferase from the thermoacidophilic archaebacterium Sulfolobus solfataricus. Eur J Biochem. 1989 Dec 8;186(1-2):375–381. doi: 10.1111/j.1432-1033.1989.tb15219.x. [DOI] [PubMed] [Google Scholar]
- Duggan D. E., Wechsler J. A. An assay for transaminase B enzyme activity in Escherichia coli K-12. Anal Biochem. 1973 Jan;51(1):67–79. doi: 10.1016/0003-2697(73)90453-3. [DOI] [PubMed] [Google Scholar]
- Ekiel I., Jarrell K. F., Sprott G. D. Amino acid biosynthesis and sodium-dependent transport in Methanococcus voltae, as revealed by 13C NMR. Eur J Biochem. 1985 Jun 3;149(2):437–444. doi: 10.1111/j.1432-1033.1985.tb08944.x. [DOI] [PubMed] [Google Scholar]
- Falco S. C., Dumas K. S., Livak K. J. Nucleotide sequence of the yeast ILV2 gene which encodes acetolactate synthase. Nucleic Acids Res. 1985 Jun 11;13(11):4011–4027. doi: 10.1093/nar/13.11.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint D. H., Emptage M. H. Dihydroxy acid dehydratase from spinach contains a [2Fe-2S] cluster. J Biol Chem. 1988 Mar 15;263(8):3558–3564. [PubMed] [Google Scholar]
- Hofler J. G., Decedue C. J., Luginbuhl G. H., Reynolds J. A., Burns R. O. The subunit structure of alpha-acetohydroxyacid isomeroreductase from Salmonella typhimurium. J Biol Chem. 1975 Feb 10;250(3):877–882. [PubMed] [Google Scholar]
- Jarrell K. F., Koval S. F. Ultrastructure and biochemistry of Methanococcus voltae. Crit Rev Microbiol. 1989;17(1):53–87. doi: 10.3109/10408418909105722. [DOI] [PubMed] [Google Scholar]
- Jones W. J., Nagle D. P., Jr, Whitman W. B. Methanogens and the diversity of archaebacteria. Microbiol Rev. 1987 Mar;51(1):135–177. doi: 10.1128/mr.51.1.135-177.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T. W., Lancaster J. R., Jr, Fridovich I. Isolation and characterization of the iron-containing superoxide dismutase of Methanobacterium bryantii. Arch Biochem Biophys. 1981 Aug;210(1):140–148. doi: 10.1016/0003-9861(81)90174-0. [DOI] [PubMed] [Google Scholar]
- Kuo C. F., Mashino T., Fridovich I. alpha, beta-Dihydroxyisovalerate dehydratase. A superoxide-sensitive enzyme. J Biol Chem. 1987 Apr 5;262(10):4724–4727. [PubMed] [Google Scholar]
- Morris C. J., Reeve J. N. Conservation of structure in the human gene encoding argininosuccinate synthetase and the argG genes of the archaebacteria Methanosarcina barkeri MS and Methanococcus vannielii. J Bacteriol. 1988 Jul;170(7):3125–3130. doi: 10.1128/jb.170.7.3125-3130.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B. E., Larsen P. K., Lemmich J. The stereochemistry of 1,2,3-trihydroxy-3-methylbutane and 2,3-dihydroxy-3-methylbutyric acid. Acta Chem Scand. 1969;23(3):967–970. doi: 10.3891/acta.chem.scand.23-0967. [DOI] [PubMed] [Google Scholar]
- Poulsen C., Stougaard P. Purification and properties of Saccharomyces cerevisiae acetolactate synthase from recombinant Escherichia coli. Eur J Biochem. 1989 Nov 6;185(2):433–439. doi: 10.1111/j.1432-1033.1989.tb15133.x. [DOI] [PubMed] [Google Scholar]
- RADHAKRISHANAN A. N., WAGNER R. P., SNELL E. E. Biosynthesis of valine and i43soleucine, 3. alpha-Keto-beta-hydroxy acid reductase and alpha-hydroxy-beta-Keto acid reductoisomerase. J Biol Chem. 1960 Aug;235:2322–2331. [PubMed] [Google Scholar]
- Ragland T. E., Kawasaki T., Lowenstein J. M. Comparative aspects of some bacterial dehydrogenases and transhydrogenases. J Bacteriol. 1966 Jan;91(1):236–244. doi: 10.1128/jb.91.1.236-244.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanangelantoni A. M., Barbarini D., Di Pasquale G., Cammarano P., Tiboni O. Cloning and nucleotide sequence of an archaebacterial glutamine synthetase gene: phylogenetic implications. Mol Gen Genet. 1990 Apr;221(2):187–194. doi: 10.1007/BF00261719. [DOI] [PubMed] [Google Scholar]
- Schloss J. V., Van Dyk D. E., Vasta J. F., Kutny R. M. Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry. 1985 Aug 27;24(18):4952–4959. doi: 10.1021/bi00339a034. [DOI] [PubMed] [Google Scholar]
- Shematek E. M., Diven W. F., Arfin S. M. Subunit structure of -acetohydroxy acid isomeroreductase from Salmonella typhimurium. Arch Biochem Biophys. 1973 Sep;158(1):126–131. doi: 10.1016/0003-9861(73)90604-8. [DOI] [PubMed] [Google Scholar]
- Shieh J., Mesbah M., Whitman W. B. Pseudoauxotrophy of Methanococcus voltae for acetate, leucine, and isoleucine. J Bacteriol. 1988 Sep;170(9):4091–4096. doi: 10.1128/jb.170.9.4091-4096.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh J., Whitman W. B. Autotrophic acetyl coenzyme A biosynthesis in Methanococcus maripaludis. J Bacteriol. 1988 Jul;170(7):3072–3079. doi: 10.1128/jb.170.7.3072-3079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprott G. D., Usher J. R. The electrochemical proton gradient and phenylalanine transport in Escherichia coli irradiated with near-ultraviolet light. Can J Microbiol. 1977 Dec;23(12):1683–1688. doi: 10.1139/m77-242. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Kuwana H. A basal unit of valine-sensitive acetolactate synthase of Neurospora crassa. Biochem Biophys Res Commun. 1984 Sep 17;123(2):418–423. doi: 10.1016/0006-291x(84)90246-8. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- WIXOM R. L., SHATTON J. B., STRASSMAN M. Studies on a dehydrase in valine biosynthesis in yeast. J Biol Chem. 1960 Jan;235:128–131. [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman W. B., Sohn S., Kuk S., Xing R. Role of Amino Acids and Vitamins in Nutrition of Mesophilic Methanococcus spp. Appl Environ Microbiol. 1987 Oct;53(10):2373–2378. doi: 10.1128/aem.53.10.2373-2378.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing R. Y., Whitman W. B. Sulfometuron methyl-sensitive and -resistant acetolactate synthases of the archaebacteria Methanococcus spp. J Bacteriol. 1987 Oct;169(10):4486–4492. doi: 10.1128/jb.169.10.4486-4492.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


