Abstract
Objective
To document and critically analyse the impact of the revised WHO 2000 histological classification for meningiomas on postoperative radiotherapy/radiosurgery indications and MRI follow up protocols.
Methods
The current (2000) WHO classification was used to grade 57 meningiomas treated surgically at one institution. These had been reviewed previously in 1999. All German neurosurgical departments carrying out intracranial microsurgery were asked to detail their guidelines for radiation therapy and follow up for meningiomas of different WHO grades.
Results
Use of the current criteria downgraded seven of 15 atypical meningiomas (WHO grade II, MII) to grade I (MI), and four of six anaplastic tumours (WHO grade III, MIII) to grade II. Indications for radiotherapy/radiosurgery and MRI follow up protocols varied substantially with the histological grade and between institutions—for example, after an incomplete resection, radiotherapy/radiosurgery recommendations differed between MI and MII in 30 of 58 units (52%), and between MII and MIII in 34 of 56 units (61%).
Conclusions
Correlative studies combining treatment and outcome data with a standardised histopathological analysis are warranted to define properly the indications for radiotherapy/radiosurgery and follow up protocols after surgery for meningiomas of different histological grades. The use of changing grading paradigms during recent years renders decision making based on local and published experience difficult. The relatively large number of meningiomas classified as atypical/WHO grade II in current practice would argue against an uncritically aggressive approach to these tumours.
Keywords: meningioma, WHO classification, radiotherapy, radiosurgery, follow up
Meningiomas are common tumours of the central nervous system arising from cells of the meningeal coverings of the brain and spinal cord. Most meningiomas can be resected, often providing immediate relief from neurological deficits, seizures, and other symptoms. Many studies have delineated the extent of resection as a powerful predictor of recurrence.1,2,3 The clinical significance of tumour recurrence is high and the management of these patients poses specific challenges.
Conventional external beam radiation (EBR) and more recently conformal radiotherapy and radiosurgery are valuable additions to the therapeutic armamentarium. Radiosurgery is often recommended for non‐resectable benign and non‐benign tumours—that is, in the cavernous sinus, sometimes after tumour progression has been confirmed by serial magnetic resonance imaging (MRI).4,5,6,7,8,9,10 A significant body of data supports adjuvant conventional EBR for patients with malignant meningiomas.4,11,12,13,14
Meningioma recurrence rates also strongly depend on the intrinsic biological properties of the tumours, as reflected in their histopathological appearance.1,3,14,15,16,17,18,19,20,21,22 The 1979 World Health Organisation (WHO) classification of central nervous system tumours recognised anaplastic/malignant meningiomas as a distinct tumour subtype.23 Several studies have since established the existence of an intermediate category of meningioma which carries a worse prognosis than the classic grade I tumour, but clearly displays a more favourable behaviour than frankly malignant meningiomas.18,24 The 1993 WHO classification adopted the term “atypical meningioma WHO grade II” for such tumours.24 Recurrence rates as high as 38–52% and 50–84% after five years have been reported for atypical and anaplastic/malignant meningiomas, respectively.1,3,13,14,15,17,18,19,21,22 In 2000, a substantially revised WHO classification of meningiomas was published.25
Published reports provide no specific guidelines for postoperative radiotherapy or radiosurgery or MRI follow up protocols for grade I v atypical v anaplastic/malignant meningiomas. We have been impressed by an increasing proportion of tumours labelled as atypical/WHO grade II in recent years. At our institution, up to four meningiomas (0–4.9%) were classified as atypical every year from 1994 to 1996, while from 1997–2003 numbers increased to 10 to 20 tumours (14.5–23.3% of all meningiomas). Similarly, in all recent large series the number of non‐benign meningiomas approaches or exceeds 20%, in contrast to earlier cohorts.1,3,14,15,16,17,18,20,23,24,25,26 This latter observation led us to investigate the impact of the recent revision of the WHO classification on two specific clinical management issues—postoperative radiotherapy or radiosurgery, and MRI follow up.
Methods
Patients
The impact of the revised 2000 WHO classification for the diagnosis of atypia and malignancy in meningiomas was investigated by reviewing the histological grading of 57 tumours treated surgically in our department between February 1996 and September 1997. These tumours were reviewed in 1999 for an unrelated publication, allowing for a comparison of the 1999 and 2004 diagnoses.27 All diagnoses were made at the German Reference Centre for Brain Tumour Neuropathology (Deutsches Hirntumour‐Referenzzentrum). Tumours with a papillary, rhabdoid, clear cell, or chordoid differentiation were excluded. Relevant clinical information was obtained through review of the clinical notes.
All patients gave informed consent before surgery to have their tumours included in a tumour bank for further studies. Approval of the ethics committee at the University of Bonn Medical Centre was obtained for research using the tumour bank.
The WHO 1993 criteria were adhered to in 1999 with one exception. In addition and paralleling a worldwide debate among neuropathologists, a MIB1 index of >5% was deemed sufficient to allow for the diagnosis of atypia during the 1999 review.24,27,28,29 The WHO 1993 classification details contradictory views on brain invasion, with some neuropathologists maintaining that gross brain invasion even in the absence of histological malignancy may qualify a tumour for designation as malignant.24 We considered brain invasion as a marker of malignancy in 1999. For the current review, the WHO 2000 classification was used. The WHO 2000 classification does not list brain invasion among the criteria for malignancy. The study was not blinded.
Survey of indications for postoperative radiotherapy/radiosurgery and follow up protocols
To assess the impact of meningioma grading (and of any changes in the grading) on the management policies for tumours of different histological grades, a questionnaire was sent to all German neurosurgical units undertaking surgery for intracranial meningiomas (n = 123), based on a current (September 2003) listing (www.dgnc.de), published by the German Neurosurgical Society (Deutsche Gesellschaft für Neurochirurgie). The chairmen were asked to detail the department's policies for postoperative radiotherapy and follow up after surgery for WHO grade I, atypical WHO grade II, and anaplastic/malignant WHO grade III meningiomas.
Specifically, we asked if the centres would recommend conventional radiotherapy or conformal radiotherapy/radiosurgery after a complete (Simpson grades I‐III) versus an incomplete resection of a WHO grade I, grade II, and grade III meningioma. Dates and intervals for MRI surveillance examinations could be ticked on the questionnaire as follows: postoperatively; first follow up visit after <3, 3, 6, and 12 months; follow up intervals <6, 6, 12, and 24 months in the first one to two years after surgery; follow up intervals <6, 6, 12, 24, and >24 months two to five years after surgery; follow up intervals <6, 6, 12, 24, and >24 months five to 10 years after surgery; and follow up intervals 6, 12, 24, and >24 months >10 years after surgery.
Sixty four questionnaires were returned; 61 of these (61 of 123, 49.6%) could be used for further analysis. The WHO classification was used for meningioma grading in 59 institutions. A modified system was employed in one centre. No information was available for one department.
Results
Comparison of the 1993 and 2000 WHO grading for meningiomas: review of 57 tumours
The histopathological grade of 36 WHO grade I tumours was confirmed. The results of applying two consecutive WHO grading schemes to 21 tumours from 20 patients initially classified as atypical or anaplastic, as well as treatment and outcome information, are presented in detail in table 1.
Table 1 Histopathological grading (1999 v WHO 2000), treatment, and outcome of 20 patients with atypical and anaplastic/malignant meningiomas.
| Tumour No | Age (y)/sex | Tumour grade (1999 review) | Tumour grade (WHO 2000) | Location | Degree of resection (Simpson grade at initial surgery and surgery for recurrent tumour) | Radiotherapy/radiosurgery | Follow up/outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | 49/M | II | I | L fronto‐dorsal falx | Initial surgery: Simpson 2 | 90 mo: no tumour | ||||||
| 2 | 106 | 76/M | II | I | L frontal convexity | Initial surgery: Simpson l | 1 mo: death (unrelated) | ||||||
| 3 | 234 | 48/M | II | I | R frontodorsal convexity | Initial surgery: Simpson 1 | 86 mo: no tumour | ||||||
| 4 | 287 | 74/F | II | I | L postcentral convexity | Initial surgery: Simpson 1 | 79 mo: no tumour | ||||||
| 5 | 320 | 47/F | II | I | L fronto‐dorsal parasagittal | Initial surgery: Simpson 3–4 | 60 mo: stable residual tumour | ||||||
| 6 | 417 | 38/F | II | I | L fronto‐dorsal falx | Initial surgery: Simpson 1 | 73 mo: no tumour | ||||||
| 117 | 22/F | II | I | L fronto‐median convexity | Initial surgery: Simpson 1 (WHO°I) | ||||||||
| 1st rec (34 mo; tumour 117): Simpson 3 | |||||||||||||
| 303 | 23/F | II | II | Superior sagittal sinus | 2nd rec (43 mo; tumour 303 = rec tumour 117): Simpson 1 | ||||||||
| 8 | 174 | 66/F | II | II | L med‐lat sphenoid wing | Initial surgery: Simpson 2 (WHO°I) | |||||||
| 1st rec (71 mo; tumour 174): Simpson 1 | 81 mo: no tumour | ||||||||||||
| 9 | 262 | 69/F | II | II | R fronto‐dorsal falx | Initial surgery: Simpson 2 | |||||||
| surgery for 2nd men (31 mo, WHO°I) | 50 mo: no tumour | ||||||||||||
| 10 | 268 | 54/F | II | II | R cavernous sinus | Initial surgery: Simpson 4 | 5 mo: stable residual tumour | ||||||
| 11 | 314 | 49/F | II | II | L frontal parasagittal | Initial surgery: Simpson 2 | 2 mo: no tumour | ||||||
| 12 | 378 | 60/F | II | II | L fronto‐lat convexity | Initial surgery: Simpson 1 | 74 mo: no tumour | ||||||
| 13 | 391 | 62/M | II | II | L fronto‐parietal convexity | Initial surgery: Simpson 1 | EBR (after 2nd rec) | 26 mo: death (tumour related) | |||||
| 1st rec (6 mo): Simpson 3 (WHO°III) | |||||||||||||
| 2nd rec (8 mo): Simspon 3 (WHO°III) | |||||||||||||
| 3rd rec (17 mo): Simpson 3 (WHO°III) | |||||||||||||
| 4th rec (22 mo): Simpson 3 (WHO°III) | |||||||||||||
| 14 | 409 | 24/F | II | II | L parieto‐occipital falx | Initial surgery: Simpson 1 | 55 mo: no tumour | ||||||
| 15 | 54 | 74/F | III | II | L frontal convexity | Initial surgery: Simpson 1 Simpson 1 | 1 mo: death (unrelated) | ||||||
| 16 | 83/123 | 56/M | III | II | Mult men: L parietal convexity (tumour 83), L fronto‐basal (tumour 123), L frontal parasagittal, L tentorium | Initial surgery: Simpson 2 (“benign”) | |||||||
| 1st rec (106 mo): Simpson 2 (WHO°I) | EBR (after 2nd rec) | 134 mo: death (tumour‐related) | |||||||||||
| 2nd rec (123 mo; tumours 83, 123 etc.): Simpson 3–4 | |||||||||||||
| 17 | 93 | 70/F | III | II | L med sphenoid wing | Initial surgery: Simpson 2 | 0 mo: death (perioperative) | ||||||
| 18 | 280 | 41/F | III | II | L fronto‐dorsal convexity | Initial surgery: Simpson 1 | 78 mo: no tumour | ||||||
| 19 | 212 | 53/F | III | III | R frontal parasagittal | Initial surgery: Simpson 1 (WHO°III) | |||||||
| 1st rec (48 mo; tumour 212): Simpson 1 | EBR (after 1st surgery) | 48 mo: death (perioperative) | |||||||||||
| 20 | 329 | 44/F | III | III | L fronto‐parietal convexity | Initial surgery: Simpson 1 | |||||||
| 1st rec (4 mo): Simpson 3–4 | RS (after 1st surgery) | 7 mo: death (tumour related) |
EBR, external beam radiotherapy; L, left; lat, lateral; med, medial; men, meningiomas; mo, months; mult, multiple; R, right; rec, recurrence; RS, radiosurgery; sx, surgery; y, years.
Three meningiomas (234, 320, 117) were considered atypical during the first review only because of a raised MIB1 index. Four tumours (54, 83/123, 93, 280) were assigned the WHO grade III during the 1999 review solely because of the presence of brain invasion. Tumour numbers are the same as in Simon et al, 2000.27
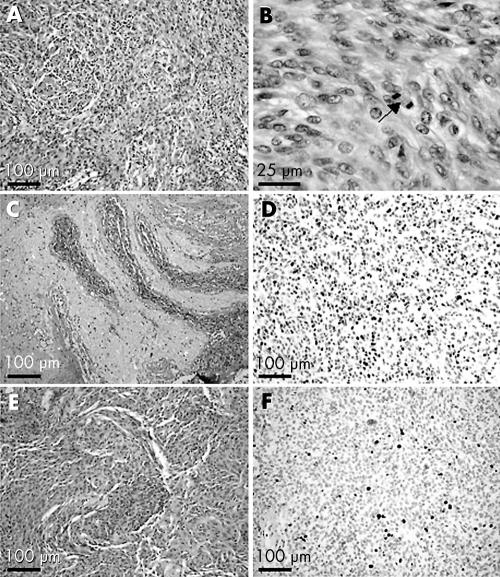
Seven of 15 (47%) tumours were reclassified as WHO grade I using the current WHO classification. Notably, in three of these seven cases the 1999 diagnosis of a WHO grade II tumour was made on the basis of a raised MIB1 index (>5%). Four of six meningiomas (67%) were no longer considered malignant (WHO grade III) in 2004. In all four cases the diagnosis of malignancy was made in 1999 on the basis of the presence of brain invasion rather than frank histopathological anaplasia. Examples of meningiomas assigned a WHO grade I or II based on MIB1 immunostaining, and tumours classified as malignant because of brain invasion, are shown in fig. 1.
Figure 1 Examples of histopathological meningioma grading. (A)–(D): This meningioma (tumour 280) shows increased cellularity (A), prominent nucleoli as well as mitotic figures (12 in 10 HPF (B)), and infiltration of central nervous tissue (C). The MIB1 labelling index exceeds 10% of the tumour cells (D). In particular, because of the presence of brain invasion this tumour was graded as an anaplastic meningioma WHO grade III during the 1999 review. This tumour was regraded in 2004 as an atypical meningioma WHO grade II. (E)–(F): An increased MIB1 labelling index of more than 5% of the tumour cells led to a diagnosis of an atypical meningioma WHO grade II during the 1999 review (tumour 234). With only two mitotic figures in 10 HPFs, the tumour was downgraded to a WHO grade I meningioma in 2004. HPF, high power field.
Postoperative radiation therapy for meningiomas
The majority of the departments recommend radiotherapy/radiosurgery for patients with malignant tumours (after complete resection, n = 40; after incomplete resection, n = 54) and for residual atypical meningioma (n = 40). A few centres (n = 10) radiate at least some patients with completely resected atypical meningiomas. Half of the centres (n = 23) consider some form of radiation therapy (mainly radiosurgery) for at least some patients with incompletely resected WHO grade I meningioma. Two units would not prescribe radiation therapy after surgery at all, even after incomplete resection of a malignant meningioma. Radiosurgery or conformal radiation is primarily used for incompletely resected tumour, while conventional EBR is used most often for malignant meningiomas (table 2). Some centres commented that they would reserve radiotherapy/radiosurgery for tumour recurrence or progression rather than using adjuvant radiation treatment. The format of the questionnaire did not specifically require the centres to report their policy for tumour recurrence.
Table 2 Recommendations for radiotherapy after complete and incomplete resections of WHO grade I, atypical WHO grade II, and anaplastic WHO grade III meningiomas.
| WHO grade I | WHO grade II | WHO grade III | ||||
|---|---|---|---|---|---|---|
| Complete (n = 58) | Incomplete (n = 57) | Complete (n = 56) | Incomplete (n = 58) | Complete (n = 56) | Incomplete (n = 56) | |
| Conventional EBR | 0 | 1 (1.7%) | 2 (3.6%) | 10 (17.2%) | 28 (50.0%) | 34 (60.7%) |
| Radiosurgery/conformal radiotherapy | 0 | 16 (28.1%) | 3 (5.4%) | 26 (44.8%) | 21 (37.5%) | 35 (62.5%) |
| Any radiotherapy | 0 | 23 (40.4%) | 10 (17.9%) | 43 (74.1%) | 40 (71.4%) | 54 (96.4%) |
Numbers exclude centres reserving radiotherapy specifically for progressive/recurrent disease or specific tumour locations (for example, cavernous sinus).
EBR, external beam radiotherapy; WHO, World Health Organisation.
The WHO grade significantly influences recommendations for adjuvant radiation therapy in many centres. Table 3 details the consequences which would result from changing the histological grade of a meningioma, for example by using a different grading system. Nine of 56 centres recommend conventional radiotherapy or conformal radiotherapy/radiosurgery after complete removal of a WHO grade II but not a WHO grade I tumour. Eighteen of 58 units radiate incompletely resected atypical but not benign meningiomas. More than half of the departments will use different radiation therapy algorithms for WHO grade III meningiomas when compared with WHO grade II meningiomas. Most commonly, EBR (after complete resection, 20; after incomplete resection, 16) or radiosurgery/conformal radiotherapy (after complete resection, 16; after incomplete resection, 9) is given for grade III but not grade II meningiomas.
Table 3 Regrading of meningiomas would change recommendations for postoperative radiotherapy/radiosurgery.
| After complete resection | After incomplete resection | |||
|---|---|---|---|---|
| WHO grade I → II (n = 56) | WHO grade II → III (n = 55) | WHO grade I → II (n = 58) | WHO grade II → III (n = 56) | |
| No change | 47 (83.9%) | 20 (36.4%) | 28 (48.3%) | 22 (39.3%) |
| No radiotherapy → EBR | 2 (3.6%) | 20 (36.4%) | 10 (17.2%) | 16 (28.6%) |
| No radiotherapy → radiosurgery/conformal radiotherapy | 3 (5.4%) | 16 (29.1%) | 12 (20.7%) | 9 (16.1%) |
| No radiotherapy → radiotherapy | 9 (16.1%) | 31 (56.4%) | 18 (31.0%) | 21 (37.5%) |
| Other changes* | 0 | 5 (9.1%) | 6 (10.3%) | 16 (28.6%) |
The table details how changing the histological grade (for example, by applying a different grading system) would result in significantly different recommendations for postoperative radiation therapy. Numbers exclude centres recommending radiotherapy only for progressive/recurrent disease or specific tumour locations (for example, cavernous sinus).
*Includes recommending EBR instead of conformal radiotherapy/radiosurgery and vice versa, and applying radiotherapy in selected cases v recommending it to all patients.
EBR, external beam radiotherapy; WHO, World Health Organisation.
Follow up after meningioma resection
In the majority of the centres meningioma patients are followed for more than 10 years after surgery. However, three to six centres (depending on tumour grade and degree of resection) routinely order follow up MRIs only for one year. Fifteen of the 59 centres used early postoperative MRI to verify the presumed degree of resection after complete resection of a benign or atypical meningioma, and 25 used early postoperative MRI for incompletely resected tumours (table 4).
Table 4 MRI follow up after complete and incomplete resections of WHO grade I, atypical WHO grade II, and anaplastic WHO grade III meningiomas.
| WHO grade I | WHO grade II | WHO grade III | ||||
|---|---|---|---|---|---|---|
| Complete | Incomplete | Complete | Incomplete | Complete | Incomplete | |
| Postoperative MRI | 15/59 (25%) | 24/59 (41%) | 15/59 (25%) | 25/59 (42%) | 21/59 (36%) | 25/59 (42%) |
| 1st f‐up MRI | n = 61 | n = 61 | n = 61 | n = 61 | n = 61 | n = 59 |
| ⩽3 mo | 34 (56%) | 42 (69%) | 42 (69%) | 50 (82%) | 50 (82%) | 54 (92%) |
| 6 mo | 17 (28%) | 18 (30%) | 17 (28%) | 11 (18%) | 11 (18%) | 5 (9%) |
| 1 y | 10 (16%) | 1 (2%) | 2 (3%) | 0 | 0 | 0 |
| F‐up intervals 0–2 y | n = 55 | n = 57 | n = 57 | n = 57 | n = 56 | n = 56 |
| ⩽6 mo | 7 (13%) | 18 (32%) | 22 (39%) | 37 (65%) | 44 (79%) | 48 (86%) |
| ⩾1 y | 48 (87%) | 39 (68%) | 35 (61%) | 20 (35%) | 12 (21%) | 8 (14%) |
| MRIs/0–2 y* | 2.9 (0.9), 1–5 | 3.6 (1.0), 2–6 | 3.6 (1.1), 1–7 | 4.4 (1.1), 2–7 | 4.6 (1.4), 1–9 | 4.9 (1.3), 1–9 |
| F‐up intervals 2–5 y | n = 55 | n = 57 | n = 55 | n = 56 | n = 56 | n = 55 |
| ⩽6 mo | 0 | 4 (7%) | 7 (13%) | 15 (27%) | 27 (48%) | 31 (55%) |
| 1 y | 24 (44%) | 42 (74%) | 41 (74%) | 37 (66%) | 27 (48%) | 21 (38%) |
| ⩾2 y | 31 (56%) | 11 (19%) | 7 (13%) | 4 (7%) | 2 (4%) | 2 (4%) |
| MRIs/0–5 y* | 4.6 (1.7), 1–8 | 6.1 (1.7), 2–11 | 6.4 (2.2), 1–13 | 7.8 (2.4), 2–13 | 8.8 (3.0), 1–15 | 9.5 (2.8),1–15 |
| F‐up intervals 5–10 y | n = 45 | n = 49 | n = 49 | n = 47 | n = 50 | n = 46 |
| ⩽1 y | 5 (11%) | 19 (39%) | 17 (35%) | 31 (66%) | 38 (76%) | 38 (83%) |
| 2 y | 30 (67%) | 23 (47%) | 29 (59%) | 14 (30%) | 10 (20%) | 6 (13%) |
| >2 y | 10 (22%) | 7 (14%) | 3 (6%) | 2 (4%) | 2 (4%) | 2 (4%) |
| MRIs/0–10 y* | 6.5 (2.6), 1–12 | 8.8 (3.0), 2–16 | 9.0 (3.7), 1–18 | 11.0 (3.9), 2–18 | 12.8 (5.4), 1–30 | 13.6 (5.2), 1–30 |
| F‐up: >10th y | n = 33 | n = 38 | n = 33 | n = 37 | n = 33 | n = 35 |
| ⩽1 y | 2 (6%) | 12 (32%) | 7 (21%) | 20 (54%) | 16 (48%) | 23 (66%) |
| 2 y | 4 (12%) | 10 (26%) | 10 (30%) | 9 (24%) | 10 (30%) | 9 (26%) |
| >2 y | 27 (82%) | 16 (42%) | 16 (48%) | 8 (22%) | 7 (21%) | 3 (9%) |
Not all questionnaires could be evaluated for all tumour grades and complete v incomplete resection. Overall numbers decline with longer follow up, reflecting differences in follow up durations between centres. Numbers include centres obtaining MRIs at the indicated intervals only in specific situations. Because of rounding errors percentages may not always add up to 100.
*Mean (SD) and range.
F‐up, follow up; mo, months; MRI, magnetic resonance imaging; WHO, World Health Organisation; y, years.
All departments scanned their patients at least once in the first year after surgery, most commonly after three months (31 of 59 centres after complete resection of a benign tumour, 51 of 59 units after incomplete surgery for malignant meningioma). Intervals between control MR scans were prolonged with time. In general, incomplete resection and increasing tumour grade correlated with an earlier first follow up MRI, shorter follow up intervals, and hence a larger overall number of MRIs carried out for follow up. The precise follow up protocols differed quite significantly between centres. On face value, the same patient may be followed in one unit for one year with one MRI and in another for >10 years with >30 MRIs. Results are presented in detail in table 4.
Twelve centres seemed to believe in a surgical cure for WHO grade I rather than for WHO grade II/III tumours. Correspondingly, follow up was longer for at least some atypical and malignant meningiomas. Other units (n = 6) apparently felt that, because of the generally greater growth rates of non‐benign meningiomas, these tumours will regrow earlier. Therefore, a shorter follow up should suffice after surgery for atypical or anaplastic meningioma.
Discussion
Histological grading for meningiomas
The WHO grading for meningiomas has undergone significant changes in recent years. This study was therefore primarily motivated by growing concerns about the validity of clinical decision making based on the histological grading of meningiomas. The data presented suggest that, in part, the WHO diagnostic labels “grade I”, “grade II”, and “grade III” meningioma have been applied to different tumours before compared with after the introduction of the new WHO classification in 2000. Specifically, among 57 meningiomas reviewed for this paper, almost half those diagnosed as atypical in 1999 (7/15 = 47%) were reclassified as WHO grade I. The majority of anaplastic/malignant tumours were regraded as atypical (4/6 = 67%) using the current WHO criteria.
Of note, no longer using two specific criteria to diagnose atypia and malignancy—that is, an increased MIB1 index and brain invasion—resulted in downgrading of as many as three atypical/WHO grade II and four anaplastic/WHO grade III meningiomas. Employing immunohistochemical proliferation markers, in particular the MIB1 index, as an adjunct to the diagnosis of atypia has been amply discussed in the neuropathological literature.28,29 However, none of these markers has been included in the WHO classification. Brain invasion was once considered the hallmark of malignancy in meningiomas.26 More recent studies suggest a different view.22 The 2000 WHO classification does not list brain invasion among the criteria for malignancy.25
The clinical outcome of our patients with non‐benign tumours (table 1) may allow for some cautious comments with respect to the classifications schemes used. Tumours assigned to WHO grade II during the 1999 review, as well as by the WHO 2000 criteria, did not generally take a particularly adverse course. No tumour progression was seen in patients diagnosed with a meningioma WHO grade I instead of grade II using the more stringent WHO 2000 criteria. The dismal outcome observed in patient 13 was neither predicted by the older nor by the WHO 2000 criteria. Patient 20 died from progressive disease, but treatment related complications and co‐morbidities contributed significantly to this clinical outcome. In conclusion, use of the WHO 2000 criteria would not have resulted in underestimating the aggressive potential of the tumours this series.
Radiation therapy after meningioma resection
In the majority of centres, indications for postoperative radiotherapy/radiosurgery or conformal radiotherapy were in good agreement with the data available in the current literature. Various retrospective studies have indicated that radiation therapy can considerably delay tumour regrowth.4,8,11,21,30 Radiosurgery and conformal radiotherapy seem to effectively control small, difficult to resect tumours, or tumour remnants—that is, in the cavernous sinus and at other skull base locations.6,7,9,10 Hence, incompletely resected tumours should be radiated at some point during the course of the disease, when the risks of repeat surgery seem to outweigh the possible complications of radiotherapy and radiosurgery. A dose–response relation for EBR after surgery for malignant meningioma has been described in two independent studies.4,11 Many consider EBR to be standard therapy after resection of these tumours.
However, a significant number of centres employed more controversial radiation therapy algorithms. Ten centres recommended radiation therapy for at least some completely resected atypical meningiomas. Forty departments felt that residual atypical tumour should be radiated. Twenty five units would prescribe radiotherapy or radiosurgery for incompletely resected atypical but not benign tumours. Such policies rely heavily on the assumption that patients with an atypical tumour will do significantly worse than patients with benign meningiomas. The data provided in table 1 would not support this notion.
Two of 56 centres would not recommend radiotherapy or radiosurgery directly after incomplete removal of a malignant meningioma. Sixteen of 56 units replied that they did not suggest radiation therapy for completely resected malignant tumours. It might well be that some of these units reserved radiation treatment for documented tumour progression, which would not be properly reflected in our survey results
MRI follow up after meningioma surgery
To our knowledge there are no systematic studies analysing the duration of follow up and follow up intervals after meningioma surgery. The present paper allows the formulation of recommendations based on averaged expert opinions: obtaining a first follow up scan during the first year after surgery, commonly after three to six months, seems advisable. Thereafter, follow up intervals can probably be safely increased. Follow up intervals of one to more than two years, depending on the time elapsed after the surgery, would appear appropriate for benign tumours. Non‐benign tumours may require shorter intervals—that is, six month intervals for the first two years (WHO grade II) or for the first five years (WHO grade III), respectively. Incompletely resected tumours may need to be followed somewhat more closely. Tumours may require follow up for more than 10 years regardless of tumour grade and the completeness of resection.
The recommendations outlined are supported by clinical experience and by published growth rates and tumour doubling times. WHO grade II/III meningiomas may regrow within months after incomplete removal, and WHO grade I tumours can recur even after many years. We operated for a first recurrence of a malignant meningioma five months after the first surgery, and 19.75 years after the first surgery for a WHO grade I meningioma. Several reports contain growth rates and tumour doubling times of incidental meningiomas and recurrent tumours after subtotal or complete resection.31,32,33,34,35,36 Assuming exponential growth, these data suggest follow up intervals of approximately four to 10 months for non‐benign tumours, and 16 months to three years for benign tumours, depending on the maximum acceptable size of the potential regrowth at the time of detection (1.5 to 2.9 cm). Longer follow up intervals would be safe after approximately 2.5 and 9 years of uneventful follow up, respectively. Absolute growth rates of up to 18 mm/year and 20 ml/year for “benign”, and 70 mm/year and 59 ml/year for atypical and malignant meningiomas have been reported, which would theoretically require somewhat shorter follow up intervals initially (less than six months for benign and less than two months for non‐benign meningiomas, respectively) (for calculations see the appendix).
Finally, it seems important to recognise that following patients by MRI has significant socioeconomic implications. Based on the figures presented, average postoperative surveillance imaging for a completely resected meningioma costs €2.300 for a WHO grade I tumour, €3.200 for a WHO grade II tumour, and €4.400 for a WHO grade III tumour (allowing €500 per MRI study) over five years.
Clinical implications
The data presented in this paper suggest that significant numbers of atypical and anaplastic/malignant meningiomas are graded differently when using the current WHO classification compared with the criteria employed previously. Therefore, published and local experience with these tumours may be seriously flawed by the use of diagnostic labels characterising different tumours over time. On the other hand, our survey clearly documents the critical role of the histological grade of a meningioma in clinical practice. Classification issues may partly explain the quite variable policies reported by the neurosurgical units participating in our survey.
The outcome data reported in this paper could be cautiously interpreted as supporting more stringent criteria for atypia and malignancy in meningiomas. We conclude that further correlative studies combining detailed treatment and outcome data with a standardised histopathological analysis (that is, using the WHO 2000 criteria) are warranted. The histological grades defined in the WHO 2000 classification, rather than individual variables, have not been analysed as prognostic indicators.3,20,22
In the meantime, a careful reading of the neuropathological report beyond the final grading diagnosis is recommended, and averaged expert opinions with respect to radiotherapy and follow up duration/intervals after surgery for meningiomas of different histological grade can be found in this paper.
Acknowledgements
We would like to thank the chairmen of the neurosurgical departments who participated in the survey. We would also like to thank PD Dr Tjoung Won Park‐Simon, Universitätsfrauenklinik Bonn, and Dr Christian Simon, Universitäts‐Hals‐Nasen‐Ohren‐Klinik Tübingen, Germany, for helpful comments. The histopathological diagnoses were reviewed at the German Brain Tumour Reference Centre (Deutsches Hirntumour‐Referenzzentrum), Universität Bonn (Prof O D Wiestler, Prof T Pietsch, PD Dr V H J Hans). R Mahlberg, Neurochirurgische Universitätsklinik, Universität Bonn, helped with the preparation of the manuscript. The authors have no personal or institutional financial interest in drugs, materials, or devices mentioned in the manuscript. No financial support was received in conjunction with the generation of this paper.
Abbreviations
EBR - external beam radiotherapy
HPF - high power field
Appendix
A complete resection and negative MRI both probably correspond to residual tumour measuring less than 0.5 to 0.6 cm in diameter = 0.06 to 0.1 ml (assuming spherical growth) = 0.6–1×108 cells.37 If detection of tumour recurrence at a diameter of 1.5 to 2.9 cm = 1.6 to 10 ml = 1.6×109−1×1010 cells is deemed desirable, follow up intervals of four to 10 tumour doubling times are appropriate after a negative scan or after complete tumour removal. This would correspond to follow up intervals of approximately four to 10 months for WHO grade II/III tumours, and 16 months to three years for WHO grade I tumours, depending on the maximum acceptable size of the potential regrowth. A 1 cm residual tumour will have grown to 2.5 cm in diameter after four tumour doubling times, requiring at least follow up intervals of 4 and 16 months for WHO grade II/III and grade I meningiomas, respectively.
A single cell clone will have to undergo 27 cell divisions (227≈108) to become unequivocally detectable on MRI. Hence, tumour doubling times of >30 and 120 days can only be assumed after >27×30 and 120 days (approximately 2.5 and 9 years) of uneventful follow up, respectively. Calculations with the absolute growth rates detailed above rather than tumour doubling times suggest somewhat shorter follow up intervals initially (that is, <6 months for WHO grade I and <2 months for grade II/III meningiomas). However, assumption of near linear growth allows one to estimate the individual growth rate of a tumour (particularly after incomplete resection) relatively early and to increase follow up intervals correspondingly. Most centres seem to follow this latter rationale to some degree (or believe in longer tumour doubling times than outlined above). None of the calculations outlined above take into account the possible emergence of a fast growing (malignant) subclone. Malignant progression of a meningioma is not altogether uncommon.16 We observed one case in our series of recurrent meningiomas.
Footnotes
Competing interests: None declared.
References
- 1.Mirimanoff R O, Dosoretz D E, Linggood et al Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 19856218–24. [DOI] [PubMed] [Google Scholar]
- 2.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 19572022–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stafford S L, Perry A, Suman V J.et al Primarily resected meningiomas: outcome and prognostic factors in 581 Mayo clinic patients, 1978 through 1988. Mayo Clin Proc 199873936–942. [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith B J, Wara W M, Wilson C B.et al Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg 199480195–201. [DOI] [PubMed] [Google Scholar]
- 5.Mesic J B, Hanks G E, Doggett R L. The value of radiation therapy as an adjuvant to surgery in intracranial meningiomas. Am J Clin Oncol 19869337–340. [DOI] [PubMed] [Google Scholar]
- 6.Hakim R, Alexander E, Loeffler J S.et al Results of linear accelerator‐based radiosurgery for intracranial meningiomas. Neurosurgery 199842446–453. [DOI] [PubMed] [Google Scholar]
- 7.Kondziolka D, Niranjan A, Lunsford L D.et al Stereotactic radiosurgery for meningiomas. Neurosurg Clin North Am 199910317–325. [PubMed] [Google Scholar]
- 8.Nutting C, Brada M, Brazil L.et al Radiotherapy in the treatment of benign meningioma of the skull base. J Neurosurg 199990823–827. [DOI] [PubMed] [Google Scholar]
- 9.Debus J, Wuendrich M, Pirzkall A.et al High efficacy of fractionated stereotactic radiotherapy of large base‐of‐skull meningiomas: long‐term results. J Clin Oncol 2001193547–3553. [DOI] [PubMed] [Google Scholar]
- 10.Stafford S L, Pollock B E, Foote R L.et al Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery 2001491029–1037. [DOI] [PubMed] [Google Scholar]
- 11.Milosevic M F, Frost P J, Laperriere N J.et al Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys 199634817–822. [DOI] [PubMed] [Google Scholar]
- 12.Dziuk T W, Woo S, Butler E B.et al Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol 199837177–188. [DOI] [PubMed] [Google Scholar]
- 13.Goyal L K, Suh J H, Mohan D S.et al Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys 20004657–61. [DOI] [PubMed] [Google Scholar]
- 14.Jääskeläinen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol 198625233–242. [DOI] [PubMed] [Google Scholar]
- 15.Jääskeläinen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol 198626461–469. [DOI] [PubMed] [Google Scholar]
- 16.Rohringer M, Sutherland G R, Louw D F.et al Incidence and clinicopathological features of meningioma. J Neurosurg 198971665–672. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood A, Caccamo D V, Tomecek F J.et al Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery 199333955–963. [DOI] [PubMed] [Google Scholar]
- 18.Maier H, Ofner D, Hittmair A.et al Classic, atypical, and anaplastic meningioma: three histopathological subtypes of clinical relevance. J Neurosurg 199777616–623. [DOI] [PubMed] [Google Scholar]
- 19.Palma L, Celli P, Franco C.et al Long‐term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg 199786793–800. [DOI] [PubMed] [Google Scholar]
- 20.Perry A, Stafford S L, Scheithauer B W.et al Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 1997211455–1465. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy B J, Davis F G, Freels S.et al Factors associated with survival in patients with meningioma. J Neurosurg 199888831–839. [DOI] [PubMed] [Google Scholar]
- 22.Perry A, Scheithauer B W, Stafford S L.et al “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer 1999852046–2056. [DOI] [PubMed] [Google Scholar]
- 23.Zülch K J.Histologic typing of tumours of the central nervous system. Geneva: World Health Organisation, 197953–58.
- 24.Kleihues P, Burger P D, Scheithauer B W. Histological typing of tumors of the central nervous system. World Health Organisation international histological classification of tumors. Berlin: Springer, 199333–37.
- 25.Louis D N, Scheithauer B W, Budka H.et al Meningiomas. In: Kleihues P, Cavenee WK, editors. Pathology and genetics of tumours of the nervous system. World Health Organisation classification of tumours. Lyon: IARC Press, 2000176–184.
- 26.Russel D S, Rubinstein L J. Tumours of the meninges and related tissues. In: Russel DS, Rubinstein LJ, editors. Pathology of tumors of the nervous system. 5th ed. London: Edward Arnold, 1989449–532.
- 27.Simon M, Park T W, Leuenroth S.et al Telomerase activity and expression of the telomerase catalytic subunit, hTERT, in meningioma progression. J Neurosurg 200092832–840. [DOI] [PubMed] [Google Scholar]
- 28.Hsu D W, Pardo F S, Efird J T.et al Prognostic significance of proliferative indices in meningiomas. J Neuropathol Exp Neurol 199453247–255. [DOI] [PubMed] [Google Scholar]
- 29.Perry A, Stafford S L, Scheithauer B W.et al The prognostic significance of MIB‐1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer 1998822262–2269. [PubMed] [Google Scholar]
- 30.Maire J P, Caudry M, Guerin J.et al Fractionated radiation therapy in the treatment of intracranial meningiomas: local control, functional efficacy, and tolerance in 91 patients. Int J Radiat Oncol Biol Phys 199533315–321. [DOI] [PubMed] [Google Scholar]
- 31.Firsching R P, Fischer A, Peters R.et al Growth rate of incidental meningiomas. J Neurosurg 199073545–547. [DOI] [PubMed] [Google Scholar]
- 32.Olivero W C, Lister J R, Elwood P W. The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg 199583222–224. [DOI] [PubMed] [Google Scholar]
- 33.Niiro M, Yatsushiro K, Nakamura K.et al Natural history of elderly patients with asymptomatic meningiomas. J Neurol Neurosurg Psychiatry 20006825–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura M, Roser F, Michel J.et al The natural history of incidental meningiomas. Neurosurgery 20035362–70. [DOI] [PubMed] [Google Scholar]
- 35.Jääskelainen J, Haltia M, Laasonen E.et al The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg Neurol 198524165–172. [DOI] [PubMed] [Google Scholar]
- 36.Jung H W, Yoo H, Paek S H.et al Long‐term outcome and growth rate of subtotally resected petroclival meningiomas: experience with 38 cases. Neurosurgery 200046567–574. [DOI] [PubMed] [Google Scholar]
- 37.Cho K G, Hoshino T, Nagashima T.et al Prediction of tumor doubling time in recurrent meningiomas. Cell kinetics studies with bromodeoxyuridine labeling. J Neurosurg 198665790–794. [DOI] [PubMed] [Google Scholar]