Abstract
The authors describe two patients suffering from demyelinating central nervous system disease who developed intense vertigo and downbeat nystagmus upon tilting their heads relative to gravity. Brain MRI revealed in both cases a single, small active lesion in the right brachium conjunctivum. The disruption of otolithic signals carried in brachium conjunctivum fibres connecting the fastigial nucleus with the vestibular nuclei is thought to be causatively involved, in agreement with a recently formulated model simulating central positional nystagmus. Insufficient otolithic information results in erroneous adjustment of the Listing's plane in off‐vertical head positions, thus producing nystagmic eye movements.
Keywords: brachium conjunctivum, positional nystagmus, Listing's law
Multiple sclerosis is frequently associated with neuro‐ophthalmological symptoms, mainly caused by plaques in brainstem and cerebellar structures. Prominent features are internuclear ophthalmoplegia, impaired suppression of the vestibuloocular reflex (VOR), reduced saccadic velocities, and nystagmus.1 Vestibular imbalance and vertigo often accompany these signs and in some cases may present as the initial symptoms.
Vestibular reflexes originate in hair cells located either in the cristae of semicircular canals, that encode angular head acceleration, or in the maculae of otolith organs that sense gravity and linear head acceleration. Tilting the head relative to gravity (that is, to the right or left shoulder) results in torsion of the eyes around the line of sight in the opposite direction, while linear acceleration along the horizontal interaural axis evokes compensatory horizontal eye movements.2,3 Thorough knowledge of the neural circuitry involved in human otolithic functions is still lacking.3 The vestibular nuclei, along with the velocity‐to‐position integrators in the brainstem, the deep and hemispheric cerebellar structures, as well as their connections passing mainly through the medial longitudinal fasciculus (MLF) and the cerebellar pedunculi, are thought to convey otolith signals.4 Interruption of these pathways is expected to modify the influence of the gravity vector and manifest itself in oculomotor abnormalities. However, the very few well documented clinical reports allow only vague speculations on the mechanisms of human otolith physiology and pathology. We are now able to report on two patients with a single active demyelinating lesion in the brachium conjunctivum, who presented with acute onset vertigo with downbeat nystagmus. The symptoms could be triggered exclusively by tonic head tilts with respect to gravity.
Patients
Case 1
A 60 year old female had suffered a demyelinating episode with left hemiparesis at the age of 50 for the first time. Her clinical presentation, CSF findings, and positive MRI follow up met the diagnostic criteria for multiple sclerosis. Ten days before her last admission she noted acute onset of intense vertigo and vomiting elicited by certain off‐vertical head positions. The symptoms could be abruptly stopped by bringing her head back to the upright position. On examination she showed mild unsteadiness of gait. There was no limb dysmetria or incoordination. When upright, visual fixation in primary position was steady without skew deviation, square wave jerks, or nystagmus. Horizontal VOR, evaluated by means of the head thrust test, was normal. Pursuit was smooth in all directions. Horizontal and vertical saccades were normometric. Slight gaze evoked nystagmus was present both on left and right gaze. Upon changing her head position relative to gravity, she would develop intense non‐fatigable vertigo, occasional vomiting, and downbeat nystagmus with very short latency (<2 seconds), persisting as long as the off‐vertical head position was maintained. The symptoms immediately disappeared after bringing the head to the upright position. Deep tendon reflexes were hyperactive and plantar stimulation was extensor on the left limb. Precipitating positions were mainly the left ear down and, to a lesser extent, the right ear down position. In the latter position, the downbeating eye movements were accompanied by an apogeotropic component. By bringing the head (whole body movement) between a left ear down and head hanging position, the patient developed the most intense vertigo and nystagmus.
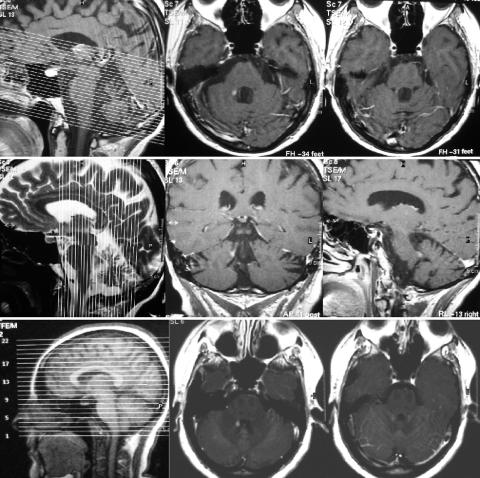
Brain MRI one day after admission showed a single contrast enhancing lesion located in the right superior cerebellar peduncle (brachium conjunctivum) dorsally to the fourth ventricle (fig 1, left). Several, non‐enhancing T2 signal abnormalities were visible in the hemispheric periventricular white matter. Intravenous administration of methylprednisolone (1000 mg/d for five days) combined with 2 mg/d clonazepam brought about gradual improvement of the symptoms.
Figure 1 T1 weighted MRI scans after gadolinium administration showing the solitary enhancing lesion in the right brachium conjuctivum in both case 1 (upper and middle panel) and case 2 (lower panel). Note the difference in scanning thickness and slice orientation: in case 1 slices were obtained in 3 mm steps, with an anterior‐posterior orientation tilted with respect to the horizontal plane (upper panel, left), while in case 2 slice thickness was 5 mm and slice orientation is more or less horizontal (lower panel, left). Sagittal and coronal slices (case 1, middle panel) demonstrate the location of the enhancing lesion in the rostro‐caudal axis.
Case 2
A 30 year old female became dizzy, nauseated, and felt severe gait unsteadiness one day before admission. Physical examination demonstrated persistent downbeat nystagmus, accompanied by vertigo and vomiting, upon bringing her head to off‐vertical positions. The nystagmic movements, as well as the associated nausea, immediately subsided in the upright standing or sitting positions. Notably, no skew deviation was observed. The precipitating characteristics of the symptoms resembled closely those of the patient presented as case 1. Left ear down head position elicited the most intense symptoms. An MRI demonstrated a single enhancing lesion located in the right brachium conjunctivum (fig 1, right), while the rest of the brain showed several non‐enhancing hemispheric white matter abnormalities. CSF findings were consistent with the diagnosis of multiple sclerosis. The symptoms improved over a period of several weeks.
Discussion
Our knowledge of otolithic pathways in animals is developing rapidly, but its relevance for human physiology and nosology is still questionable. There are about 6000 utricular and 4000 saccular afferents in humans, entering the brainstem at the level of the lateral vestibular nucleus. Considerable processing takes place within the brainstem circuitry, accomplished by the numerous interconnections between the different central and surrounding parts of the vestibular complex. The main output travels through the brachium conjunctivum, the MLF, the ascending Deiters', and the lateral vestibulospinal tracts. It is likely that the brachium conjunctivum plays a substantial role in otolithic signal processing, since it contains fibres originating from the fastigial nucleus in the cerebellum.4 It has recently been demonstrated, that a subgroup of monkey fastigial neurons is selectively responding to otolithic stimulation.5
Demyelination, the pathological hallmark of multiple sclerosis, affects fibre connections in the central nervous system. Thus, the symptoms described in our patients can be entirely attributed to disruption of neuronal routes. This adds to the localising accuracy of the clinicopathological correlation, in contrast to studies based on ischaemic and haemorrhagic lesions or tumours, which can hardly differentiate between nuclear and fibre involvement. In both subjects, MRI revealed a solitary enhancing lesion located in the right brachium conjunctivum, conceivably responsible for the reported symptoms. The location of the older, non‐enhancing lesions could by no means explain the clinical syndrome. So far, circumscribed lesions in the following areas have been associated with central positional nystagmus: dorsolateral to the fourth ventricle presumably including the caudal cerebellar peduncles (haemorrhages and tumours detected by CT scans),6 dorsal vermis,7,8,9 and diffuse cerebellar pathology.10 In contrast, ischaemic lesions in the distribution of the superior cerebellar artery including the brachium conjunctivum did not display any positional nystagmus.11
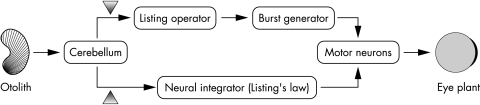
A novel approach to the pathophysiology of central positional nystagmus focuses on the connections between vestibular brainstem and cerebellar structures (for example, fastigial nucleus) carrying otolithic signals.12 The mathematical model described in this study is based on the properties of 3D ocular kinematics. A fundamental constraint of 3D eye movement control is known as Listing's law, which states that the eye positions are such that the axes of the corresponding rotations from the primary position to each of them all line up along a plane, the so called Listing's plane, given that the head remains stabilised in space. In theory, the eye could assume an infinite number of orientations for a given gaze direction, because the latter requires fixed yaw and pitch coordinates, but not roll coordinates. Listing's law helps to avoid such ambiguities and provides the basis for a neural control mechanism that constrains eye movements to a predefined pattern, thus reducing the rotational degrees of freedom.13 Three dimensional measurements of eye movements in humans under various physiological and pathological conditions have confirmed that they obey Listing's law and that this fact cannot be attributed to mechanical constraints posed by the eye plant.14 The signals, which control the eye movements according to this law, have to be implemented in various brainstem and cerebellar circuits. Glasauer's model predicts that disruption of the connections between the vestibulocerebellum and the vestibular nuclei, just like in our patients, would result in nystagmus triggered exclusively in off‐vertical head positions. The burst command for 3D eye movements (eye velocity signal), as well as the neural structures which calculate and maintain the eye position from the velocity signal (neural integrator), have to contain information necessary for shaping Listing's plane. The model incorporates the dynamic consequences of altered gravitational input on eye position, by including a saccade generator and a neural velocity to position integrator operating in Listing's coordinates. Under normal conditions, both operators are continuously receiving otolithic input, thereby controlling for changes in the orientation of Listing's plane, which necessarily occur when assuming off‐vertical head positions. Otolithic input to the integrator consists of two parts: one via the MLF, providing a steady eye position offset, and a second one via cerebello‐vestibular connections. The latter readjusts the motoneuron signals that hold the eye position by accounting for the tilt in Listing's plane. Disruption of the gravitational information inflow from the archicerebellum results in positional nystagmus in the model, which ceases in the head upright position (fig 2). The drive for the nystagmic movements is assumed to be the erroneous adjustment of Listing's plane.
Figure 2 Simplified schematic representation of the model of Glasauer et al.12 Crucial for the occurrence of positional nystagmus is the interruption of the information inflow from cerebellum to the neural integrator operating in Listing's coordinates or an assumed Listing operator placed prior to the burst generator. Simulated lesions (arrowheads) applied to either of the two connections generate central positional nystagmus when otolithic input indicates deviations from the gravity vector.
The dynamic syndrome described here is induced by changing head position in space relative to the gravity vector, either by a whole body, or a head‐to‐trunk movement. It should not be confused with the static otolithic disorder of the ocular tilt reaction triad. The latter consists of skew deviation, ocular counterroll, and head tilt.15 The orientation of these signs depends on the anatomical height of the lesion from the labyrinth to the thalamus.
Our cases also suggest that there might be anatomical and functional segregation within the brachium conjunctivum, since none of them displayed intentional tremor and/or limb ataxia, indicating that the dentatofugal fibres to the midbrain and thalamus remained unaffected.
Laboratory testing, including 3D eye and head position recordings, is needed to further validate in detail the predictions posed by simulations. Our cases add to the growing knowledge of human otolithic pathophysiology, by demonstrating a single circumscribed brachium conjunctivum lesion as the cause of central positional nystagmus and vertigo.
An extra figure and two videos are available at http://jnnp.bmjjournals.com/supplemental
Copyright © 2006 BMJ Publishing Group
Supplementary Material
Abbreviations
MLF - medial longitudinal fasciculus
VOR - vestibuloocular reflex
Footnotes
Competing interests: None.
An extra figure and two videos are available at http://jnnp.bmjjournals.com/supplemental
References
- 1.Sharpe J A, Goldberg H J, Lo A W.et al Visual‐vestibular interaction in multiple sclerosis. Neurology 198131427–433. [DOI] [PubMed] [Google Scholar]
- 2.Anastasopoulos D, Gianna C C, Bronstein A M.et al Interaction of linear and angular vestibulo‐ocular reflexes of human subjects in response to transient motion. Exp Brain Res 1996110465–472. [DOI] [PubMed] [Google Scholar]
- 3.Gresty M A, Bronstein A M, Brandt T.et al Neurology of otolith function. Peripheral and central disorders. Brain 1992115647–673. [DOI] [PubMed] [Google Scholar]
- 4.Büttner‐Ennever J A. A review of otolith pathways to brainstem and cerebellum. Ann NY Acad Sci 199987151–64. [DOI] [PubMed] [Google Scholar]
- 5.Siebold C, Anagnostou E, Glasauer S.et al Canal‐otolith interaction in the fastigial nucleus of the alert monkey. Exp Brain Res 2001136169–178. [DOI] [PubMed] [Google Scholar]
- 6.Brandt T. Positional and positioning vertigo and nystagmus. J Neurol Sci 1990953–28. [DOI] [PubMed] [Google Scholar]
- 7.Gregorius F K, Grandall P H, Baloh R W. Positional vertigo with cerebellar astrocytoma. Surg Neurol 19766283–286. [PubMed] [Google Scholar]
- 8.Watson C P, Barber H O, Peck J.et al Positional vertigo and nystagmus of central origin. Can J Neurol Sci 19818133–137. [DOI] [PubMed] [Google Scholar]
- 9.Barber H O. Positional nystagmus. Otolaryngol Head Neck Surg 198492649–655. [DOI] [PubMed] [Google Scholar]
- 10.Arbusow V, Strupp M, Brandt T. Amiodarone induced severe prolonged head positional vertigo and vomiting. Neurology 199851917. [DOI] [PubMed] [Google Scholar]
- 11.Ranalli P J, Sharpe J A. Contrapulsion of saccades and ipsilateral ataxia: a unilateral disorder of the rostral cerebellum. Ann Neurol 198620311–316. [DOI] [PubMed] [Google Scholar]
- 12.Glasauer S, Dieterich M, Brandt T. Central positional nystagmus simulated by a mathematical model of otolith‐dependent modification of Listing's plane. J Neurophysiol 2001861546–1554. [DOI] [PubMed] [Google Scholar]
- 13.Tweed D, Vilis T. Implications of rotational kinematics for the oculomotor system in three dimensions. J Neurophysiol 198758832–849. [DOI] [PubMed] [Google Scholar]
- 14.Helmchen C, Glasauer S, Büttner U. Pathological torsional eye deviation during voluntary saccades: a violation of Listing's law. J Neurol Neurosurg Psychiatry 199762253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halmagyi G M, Gresty M A, Gibson W P. Ocular tilt reaction with peripheral vestibular lesion. Ann Neurol 1979680–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.