Abstract
Objective
To determine the earliest symptoms of anosognosia in people with Alzheimer's disease and to validate a criteria‐guided strategy to diagnose anosognosia in dementia.
Methods
A consecutive series of 750 patients with very mild or probable Alzheimer's disease attending a memory clinic, as well as their respective care givers, was assessed using a comprehensive psychiatric evaluation.
Results
The factors of anosognosia for (1) basic activities of daily living (bADL), (2) instrumental activities of daily living (iADL), (3) depression and (4) disinhibition were produced by a principal component analysis on the differential scores (ie, caregiver score minus patient score) on the anosognosia questionnaire for dementia. A discrepancy of two or more points in the anosognosia‐iADL factor was found to have a high sensitivity and specificity to identify clinically diagnosed anosognosia in people with Alzheimer's disease. By logistic regression analysis, the severity of dementia and apathy were both shown to be noticeably associated with anosognosia in people with Alzheimer's disease.
Conclusion
Anosognosia in those with Alzheimer's disease is manifested as poor awareness of deficits in iADL and bADL, depressive changes and behavioural disinhibition. The frequency of anosognosia is found to increase considerably with the severity of dementia. The validity of a specific set of criteria to diagnose anosognosia in people with Alzheimer's disease was shown, which may contribute to the early identification of this condition.
Anosognosia (from the Greek “nosos” (illness) and “gnosis” (knowledge)) is a term coined by Babinski to refer to the phenomenon of denial of hemiplegia.1 From an etymological perspective, the term anosognosia may be construed as the lack of knowledge or awareness of an illness. Anosognosia has also been reported among patients with Wernicke's aphasia, who do not attempt to correct paraphasias and who may become irritable with others when their jargon‐loaded speech is not properly understood. Anton's syndrome occurs in patients with cortical blindness, who deny being blind and confabulate responses when asked to recognise visually presented objects. In the context of people with Alzheimer's disease, anosognosia was construed as the denial or lack of awareness of impairments in activities of daily living (ADL) or about neuropsychological deficits.2,3 Different strategies have been used to assess anosognosia in Alzheimer's disease, and these are briefly described as follows (see Clare4,5 for a thorough review).
Clinician rating of patients' awareness of illness
After a routine clinical interview, the examiner classifies the patient as having full, shallow or no awareness of deficits.3,6,7,8,9,10,11 This strategy assumes that anosognosia is a symptom that can be reliably assessed during a clinical interview.4,5 The main problem with this strategy is that clinical interviews are not structured and the extent of the cognitive and functional assessment may differ greatly between studies. Moreover, the three categories of awareness are not based on standardised criteria. The reliability of this diagnostic scheme has rarely been considered, and its validity is unknown.
Prediction–performance discrepancies
This strategy is based on the patients' oral report about their performance on a given neuropsychological task (usually a test of anterograde verbal memory).12,13,14,15 Anosognosia is scored as the difference between the patients' own estimation of performance on a given test and the score they obtained on that test. Problems with this strategy are both conceptual and methodological. The conceptual problem is that anosognosia in people with Alzheimer's disease refers to loss of awareness about functional deficits, and not about putative deficits on a given neuropsychological test. Thus, some patients could deny problems with their ADL while providing an accurate estimation about their neuropsychological performance, and vice versa. The methodological problem with this strategy is that no canonical concept of normal performance exists on neuropsychological tests (at least among lay people). Thus, patients may guess their level of performance on the basis of idiosyncratic beliefs or perceived attitudes of the examiner. Secondly, tests scores are not on a par with the score of patients' judgements of performance, and this mismatch may influence the final results considerably.4,5
Patient–care giver discrepancy scores
This strategy is based on comparing the ratings given by the patients' on their own level of performance on several of the ADL with ratings provided by their respective care givers.16,17,18 Thus, anosognosia is diagnosed whenever patients rate their functioning as better than that given by their care givers'. Here, the information provided by the care giver is the standard against which the patient's report is compared. A limitation of this strategy is that the care giver's report may be influenced by several factors, such as their emotional and cognitive state and the amount of time they usually spend with the patient. Snow and coworkers19 tried to reduce potential biases from care givers' reports by also including clinicians' reports. They found a noticeable correlation between the reports of the care givers and those of the clinicians, but the clinicians' reports provided no additional information. Another limitation is that the validity of this procedure has rarely been examined, and anosognosia is diagnosed on the basis of arbitrary cut‐off scores.
The main aim of this study was to develop a valid and practical method to diagnose anosognosia in Alzheimer's disease using standardised criteria. We assessed a large series of patients with Alzheimer's disease with very mild to severe dementia by administering the Anosognosia Questionnaire for Dementia (AQ‐D), an instrument with proven reliability and validity, to rate the severity of anosognosia in people with Alzheimer's disease.18 We used principal component analysis to identify specific factors, and established the validity of our diagnostic scheme on the basis of independent clinical assessments. In addition, we aimed to determine the earliest symptoms of anosognosia in people with Alzheimer's disease and to examine the neuropsychological correlates of this condition.
Methods
Alzheimer's disease group
This group consisted of 750 consecutive patients with progressive cognitive decline who visited the dementia clinic at a large tertiary medical centre in Buenos Aires, Argentina. All patients met the National Institute of Neurological and Communicative Disorders and Stroke criteria and the Alzheimer's Disease and Related Disorders Association criteria for probable Alzheimer's disease.20 None of them had a history of stroke, evidence of focal lesions on an MRI of the brain (T1‐weighted), or CT scan or a Hachinski Ischaemic Score>4.21
Healthy comparison group
This group included 32 elderly people (61 to 89 years, mostly volunteers from the community) with normal neurological and psychiatric evaluation results, who had no history of neurological disorders or closed head injuries, and had a normal CT or MRI scan.
Psychiatric examination
After the methodology of the study had been fully explained, written informed consent was obtained from the patients and their respective care givers and from the healthy controls. A care giver or informant was defined as a first‐degree relative currently responsible for, or in regular contact (more than twice a week) with, the participant. All except four of our patients were living in their respective homes, and informants were the spouse, a sibling or a son or daughter living with the patient. Only spouses acted as informants for the healthy controls. The evaluation included administration of the following:
Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders‐IV (DSM‐IV),22 a semi‐structured diagnostic interview for assessing signs and symptoms necessary for the major axis I DSM‐IV diagnoses
Mini‐Mental State Examination (MMSE),23 a global measure of cognitive deficits
Hamilton Depression Rating Scale,24 a 17‐item interviewer‐rated scale for rating the severity of symptoms of depression
Clinical Dementia Rating,25 a global rating device for dementia stages
Disinhibition Scale,26 a 26‐item questionnaire assessing abnormal motor behaviours, stereotyped routines, hypomanic behaviour and poor self‐care
Functioning Independence Measure,18,27 an 18‐item ordinal scale assessing self‐care, sphincter control, mobility, locomotion, communication and social cognition.
Higher scores indicate less impairment in ADL. All patients were also assessed with the AQ‐D.18 This is a 30‐item questionnaire divided into two sections. The first section assesses performance of basic activities of daily living (bADL) and instrumental activities of daily living (iADL). The second section examines changes in mood and behaviour. Two forms of this questionnaire are used: form A is answered by the patient alone, whereas form B is answered by a next of kin or care giver. Forms A and B are rated blinded to each other, and the final score is obtained by subtracting the scores on form B from those on form A. Thus, positive scores indicated that the care giver rated the patient as more impaired than the patient's own self‐evaluation.
Patients with Alzheimer's disease were interviewed first. Simultaneously, care givers, who were blinded to the results of these interviews, rated the patients' behaviours with the corresponding instruments. Finally, the psychiatrist administered the Structured Clinical Interview for DSM‐IV to each patient, with both the patient and the care giver present. We previously showed the reliability and validity of the above instruments in people with Alzheimer's disease.18,26,28,29,30
Neuropsychological examination
The cognitive evaluation was carried out after the psychiatric assessment by a neuropsychologist, who was blinded to the other clinical findings. It consisted of the following:
Boston Naming Test,31 which examines the ability to name pictured objects
Controlled Oral Word Association Test,32 which examines access to semantic information with time constraint
Buschke Selective Reminding Test,33 which measures verbal learning and memory during a multiple‐trial list‐learning task (the total recall was used as the outcome measure)
Digit Span,34 which examines auditory attention, includes two parts. In the first part (Digits Forward), the patient is asked to repeat a string of numbers as exactly as it is given, whereas in the second part (Digits Backwards) the patient must repeat the digit string in reverse order
Block Design,34 which examines constructional praxis
Token Test,32 which assesses verbal comprehension.
Statistical analysis
Statistical analyses for continuous variables were carried out by two‐way analysis of variance, Tukey's honest significant difference for unequal n and stepwise regression analyses. A principal components analysis for the AQ‐D was carried out with orthogonal rotation (varimax). Eigen values >1 and the Scree plot (visual break at the elbow) were used to inspect for factor solution accuracy. The overall χ2 tests from logistic regression were used to test the clinical predictors of anosognosia. If the overall test was significant, we followed up with individual tests. A receiver–operating characteristics curve was constructed to analyse sensitivity and 1‐specificity for the possible scores on the AQ‐D for identifying clinically significant anosognosia. Associations were tested with a stepwise regression analysis. Associations appearing in frequency distributions were tested using χ2 and Fisher's exact tests. All p values are two tailed.
Results
Sample characteristics
Table 1 shows the demographic findings for patients with Alzheimer's disease and healthy controls. Healthy controls were younger than patients with severe Alzheimer's disease (F (4, 777) = 17.0, p<0.0001), and had significantly higher education (F (4, 777) = 10.4, p<0.0001) and MMSE scores (F (4, 777) = 352.3, p<0.0001) than patients with mild, moderate or severe Alzheimer's disease.
Table 1 Demographic and clinical findings of patients with Alzheimer's disease and healthy controls.
| No of participants (n) | Control group (n = 32) | Very mild Alzheimer's disease (n = 219) | Mild Alzheimer's disease (n = 313) | Moderate Alzheimer's disease (n = 169) | Severe Alzheimer's disease (n = 49) |
|---|---|---|---|---|---|
| Age in years | 68.2 (7.5) | 68.4 (8.4) | 72.6 (7.0) | 72.9 (7.2) | 75.3 (8.3) |
| Education in years | 16.3 (5.7) | 13.3 (5.0) | 11.3 (5.8) | 10.8 (5.8) | 9.1 (5.7) |
| Mini‐Mental State Examination score | 29.0 (1.1) | 26.9 (2.5) | 22.3 (4.1) | 16.7 (4.6) | 8.1 (4.1) |
| Hamilton Depression Scale score | 7.5 (4.6) | 8.3 (7.0) | 10.6 (7.8) | 11.1 (7.5) | 13.3 (6.1) |
| Sex female, n (%) | 25 (71) | 129 (59) | 198 (63) | 107 (63) | 35 (71) |
Values are mean (SD) unless otherwise stated.
Principal component analysis of the AQ‐D
A varimax‐rotated principal component analysis on the AQ‐D (caregiver scores minus patient scores) produced four factors (table 2). Factor 1 (eigen value = 9.05, variance 30%) loaded on the items of recalling the date, orienting to new places, recalling telephone calls, remembering the location of objects at home, understanding conversations, understanding the plot of a movie, keeping belongings in order, handling money, doing mental calculations, remembering shopping lists, remembering appointments and performing clerical work. This factor was construed as anosognosia for deficits in iADL. Factor 2 (eigen value = 1.54, variance 5%) loaded on the items of writing their signature, orienting inside the house, loss of bladder control and feeding oneself. This factor was construed as anosognosia for deficits in bADL. Factor 3 (eigen value = 1.65, variance 6%) loaded on the items of frequent crying episodes, decreased interests, increased stubbornness and selfishness, increased irritability and increased sadness. This factor was construed as anosognosia for depression. Factor 4 (eigen value = 1.22, variance 4%) loaded on the items of inappropriate laughing and increased interest in sex. This factor was construed as anosognosia for disinhibition.
Table 2 Anosognosia Questionnaire for Dementia: factor loadings (varimax normalised).
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
|---|---|---|---|---|
| Problems with remembering dates | 0.70 | −0.02 | 0.16 | −0.00 |
| Problems with orienting in new places | 0.60 | 0.15 | 0.27 | 0.02 |
| Problems with remembering telephone calls | 0.67 | 0.05 | 0.16 | 0.02 |
| Problems with understanding conversations | 0.50 | 0.31 | 0.23 | 0.09 |
| Problems with keeping belongings in order | 0.56 | 0.16 | 0.21 | 0.04 |
| Problems with remembering where things were left | 0.67 | 0.06 | 0.21 | 0.04 |
| Problems with handling money | 0.60 | 0.27 | 0.15 | 0.13 |
| Problems with remembering appointments | 0.70 | 0.02 | 0.22 | 0.00 |
| Problems doing mental calculations | 0.56 | 0.29 | 0.12 | 0.00 |
| Problems with remembering shopping lists | 0.67 | 0.16 | 0.09 | 0.01 |
| Problems with understanding the plot of a movie | 0.50 | 0.31 | 0.17 | 0.14 |
| Problems with doing clerical work | 0.57 | 0.24 | 0.16 | 0.13 |
| Problems with signing the name | 0.23 | 0.61 | 0.00 | 0.00 |
| Problems with bladder control | 0.02 | 0.50 | 0.29 | 0.08 |
| Problems with orienting in the house | 0.05 | 0.52 | 0.30 | −0.05 |
| Problems with feeding oneself | −0.02 | 0.64 | 0.11 | 0.19 |
| More rigid and inflexible about decisions | 0.26 | 0.13 | 0.66 | 0.17 |
| More egotistical and self‐centred | 0.15 | 0.16 | 0.62 | 0.27 |
| More irritable | 0.19 | −0.06 | 0.68 | 0.24 |
| More frequent crying episodes | 0.19 | 0.15 | 0.50 | 0.00 |
| Less interest in favourite activities | 0.38 | 0.15 | 0.53 | −0.09 |
| More depressed | 0.24 | 0.19 | 0.63 | −0.18 |
| Laughing inappropriately | 0.12 | 0.12 | 0.16 | 0.67 |
| Increased sexual interest | 0.06 | 0.07 | 0.00 | 0.78 |
Values in bold indicate items included into a given factor
Validity of information given by patients and care givers on ADL
The validity of patients' and care givers' information on ADL was assessed with a multiple regression analysis, with AQ‐D scores from patients and care givers as the dependent variables, and age, education, MMSE and Hamilton Depression Rating Scale scores as the independent variables. Care givers AQ‐D ratings showed a significant overall correlation (R2 = 0.38, F (2, 766) = 239.9, p<0.0001), and the variables that accounted for a significant part of the variance were the MMSE (R2 = 0.34, p<0.0001) and Hamilton Depression Rating Scale scores (R2 = 0.05, p<0.0001). AQ‐D ratings of the patients' showed a significant overall correlation (R2 = 0.05, F (2, 766) = 14.1, p<0.0001), and the MMSE was the only variable that accounted for a significant part of the variance (R2 = 0.05, p<0.0001). Thus, the association between AQ‐D ratings and MMSE scores was seven times higher when using ratings given by care givers than those given by the patients.
Concurrent validity—clinical diagnosis of anosognosia
After a routine clinical evaluation, and blinded to all other clinical information, a neuropsychiatrist (SES) with experience in the diagnosis of anosognosia in people with dementia, carried out a clinical interview with a random series of 104 patients with Alzheimer's disease from the original cohort. The assessment was based on a non‐structured interview of the patient and a relative or care giver, which consisted of recording a general medical and psychiatric history, performing a structured neurological examination and assessing intellectual functioning, mood and behaviour. On the basis of this evaluation, 34 (33%) patients were diagnosed with anosognosia, defined as having poor or no awareness of intellectual and functional deficits. A multivariate analysis of variance, with group (with anosognosia v without anosognosia) as the independent variable and weighted scores on the four domains of anosognosia as the dependent variables (ie, the total score for each domain divided by the number of items in the domain), showed a significant main effect (Wilks' λ = 0.40, Rao's r = 36.0, df = 4, 99, p<0.0001). On individual comparisons, patients with a clinical diagnosis of anosognosia had significantly higher scores (ie, more severe anosognosia) on all four domains as compared with patients without a clinical diagnosis of anosognosia (table 3).
Table 3 Scores of anosognosia for deficits in instrumental (iADL) and basic activities of daily living (bADL), depression and disinhibition in patients with or without anosognosia based on a clinical examination or on a care giver's report.
| Without anosognosia group (n = 70) | With anosognosia group (n = 34) | |
|---|---|---|
| Clinical examiner's diagnosis | ||
| Anosognosia for iADL | 0.00 (0.53) | 1.39 (0.67) |
| Anosognosia for bADL | −0.13 (0.40) | 0.58 (0.62) |
| Anosognosia for depression | 0.04 (0.62) | 0.96 (0.55) |
| Anosognosia for disinhibition | 0.06 (0.48) | 0.47 (0.77) |
| Care giver's diagnosis | n = 320 | n = 73 |
| Anosognosia for iADL | 0.58 (0.69) | 1.14 (0.77) |
| Anosognosia for bADL | 0.19 (0.51) | 0.38 (0.58) |
| Anosognosia for depression | 0.39 (0.66) | 0.99 (0.70) |
| Anosognosia for disinhibition | 0.12 (0.44) | 0.31 (0.58) |
Values are mean (SD).
Concurrent validity—care giver diagnosis
Care givers were asked to rate the patient's level of awareness by using the following categories included in the insight question of the Disinhibition Scale: 0, full awareness about cognitive or behavioural problems (ie, no anosognosia); 1, minimisation of cognitive or behavioural changes; 2, complete lack of awareness of cognitive or behavioural changes; and 3, complete lack of awareness of cognitive or behavioural changes; the patient becomes irritable whenever limitations are pointed out.26 The Disinhibition Scale was included into our assessment battery at a later stage of the project, and scores were obtained on a consecutive series of 393 patients of the complete cohort with Alzheimer's disease. On the basis of answers given by the care givers, patients with Alzheimer's disease were divided into those with anosognosia (ie, scores of 2 or 3 on the insight question of the Disinhibition Scale; n = 73, 19%) or those without anosognosia (ie, scores of 0 or 1 on the same question; n = 320, 81%). A multivariate analysis of variance with anosognosia as the grouping factor and the four anosognosia domains as the dependent variables was significant (Wilks' λ = 0.87, Rao's r = 14.3, df = 4, 388, p<0.0001). On individual comparisons, we found considerable care giver–patient differences on all four anosognosia domains (table 3). Moreover, we found a significant correlation between Functional Independence Measure scores and both iADL (r = −0.32, p<0.01) and bADL (r = −0.35, p<0.01) as scored by the care giver. These correlations were no longer significant when rated by patients (iADL r = 0.03; bADL r = −0.09).
Information on anosognosia from both care givers and the clinical examiner was available for 31 patients. We found a significant agreement between the examiners' and the care givers' diagnoses of anosognosia (χ2 = 14.6, df = 1, p<0.001): 7 patients were classified with anosognosia and 20 without anosognosia by both raters; 2 patients were diagnosed with anosognosia by the examiner but not by the care giver; and 2 patients were diagnosed with anosognosia by the care giver but not by the examiner.
Diagnosis of anosognosia
For this comparison, a care giver–patient discrepancy was considered significant whenever the difference on the respective AQ‐D item was at least two points (ie, the patient scoring a deficit as never present and the care giver scoring the same deficit as often or always present, or the patient scoring a deficit as rarely present and the care giver scoring the deficit as always present). We considered that a difference of 1 point would have poor specificity for anosognosia and would not be clinically relevant. We next calculated receiver–operating characteristic statistics, with the examiner's clinical diagnosis of anosognosia (with or without) as the classification variable and the number of items on the anosognosia‐iADL factor with a noticeable care giver or patient discrepancy as the criterion. The area under the receiver–operating characteristics curve was 0.95 (95% confidence interval (CI) 0.88 to 0.98), showing high accuracy. A score ⩾4 had a specificity of 97% (91% to 99%) and a sensitivity of 81% (63% to 93%) for the clinical diagnosis of anosognosia.
We next examined the frequency of anosognosia at each stage of Alzheimer's disease. On the basis of the above findings, anosognosia was defined as a 2‐point differential (care giver score minus patient score) on four or more items of the anosognosia iADL domain. The hypothesis of unequal frequency of anosognosia based on the severity of Alzheimer's disease was statistically substantiated (χ2 = 89.9, df = 3, p<0.0001). The frequency of anosognosia was 0 in healthy controls (n = 32), 10% (n = 22) in the stage of very mild Alzheimer's disease (Clinical Dementia Rating 0.5), 31% (n = 98) in the stage of mild Alzheimer's disease (Clinical Dementia Rating 1), 50% (n = 85) in the stage of moderate Alzheimer's disease (Clinical Dementia Rating 2), and 57% (n = 28) in the stage of severe Alzheimer's disease (Clinical Dementia Rating 3).
Early symptoms of anosognosia in Alzheimer's disease
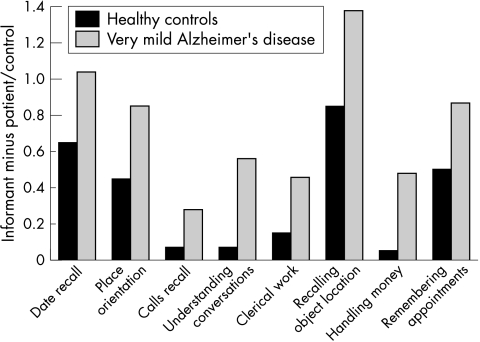
To examine anosognosia in the earliest stages of Alzheimer's disease, we compared differential AQ‐D scores of healthy controls with patients with very mild Alzheimer's disease and a MMSE score above 23 points. A multivariate analysis of variance for the AQ‐D ADL section was statistically significant (Wilks' λ = 0.81, Rao's r = 2.11, df = 22, 211, p<0.01). On individual comparisons, fig 1 shows that patients with very mild Alzheimer's disease had worse scores (ie, less awareness of deficits) than the healthy controls on the following items: date recall (p<0.01), orientation in new places (p<0.01), remembering telephone calls (p<0.001), understanding conversations (p<0.0001), remembering where belongings were left (p<0.001), handling money (p<0.001), remembering appointments (p<0.01), understanding the plot of a movie (p<0.01) and doing clerical work (p<0.01).
Figure 1 Bar graph showing the informant minus the patient or control scores for the activities of daily living items that showed significant differences between patients and controls. AD, Alzheimer's disease.
Clinical correlates of anosognosia
To determine the relative importance of age, cognitive deficits, depression and disinhibition in the mechanism of anosognosia, we calculated a stepwise regression analysis with AQ‐D scores (care giver score minus patient score) as the dependent variable, and age, MMSE, Hamilton Depression Rating Scale and Disinhibition Scale (total scores) as the independent variables. The overall regression was significant (R2 = 0.37, F (4, 388) = 38.6, p<0.0001) and the Disinhibition Scale (R2 change = 0.28, p<0.0001; more severe anosognosia correlating with more severe disinhibition), MMSE scores (R2 change = 0.03, p<0.001) and age (R2 change = 0.01, p<0.01) accounted for a significant part of the variance.
We next examined whether anosognosia in people with very mild Alzheimer's disease was associated with specific neuropsychological deficits. We limited this analysis to patients with very mild Alzheimer's disease to examine the earliest cognitive deficits potentially associated with incipient anosognosia, as well as to avoid floor effects on neuropsychological tests in later stages of dementia. Complete neuropsychological results were available for 173 (80%) of the 219 patients (27 patients had one or more missing values and had to be excluded from statistical analysis, whereas the remaining 19 patients could not be scheduled for the assessment). A multivariate analysis of variance, with group (anosognosia (n = 16) v no anosognosia (n = 157)) as the independent variable, the neuropsychological tests as the dependent variables, and age and MMSE scores as covariates was significant (Wilks' λ = 0.91, Rao's R = 2.10, df = 7, 163, p<0.05). On individual comparisons, patients with anosognosia had significantly lower scores than patients without anosognosia on the Buschke Selective Reminding Test (ie, anterograde verbal memory; F (1, 171) = 6.30, p<0.05), and the Token Test (ie, verbal comprehension; F (1, 171) = 7.50, p<0.01; table 4).
Table 4 Neuropsychological findings for patients with very mild dementia, with or without anosognosia as diagnosed with standardised criteria.
| No of patients n = 173 (100%) | Without anosognosia n = 157 (69%) | With anosognosia n = 16 (31) |
|---|---|---|
| Age in years | 69.1 (8.1) | 69.0 (6.9) |
| Education in years | 11.8 (5.6) | 11.4 (5.8) |
| MMSE score | 27.0 (2.4) | 26.2 (2.7) |
| Boston Naming Test score | 17.4 (2.4) | 16.0 (3.2) |
| Controlled Oral Word Association Test score | 31.2 (11.0) | 25.8 (12.8) |
| Buschke Selective Reminding Test score | 66.9 (17.0) | 56.3 (18.5) |
| Digits Span (Forward) score | 5.4 (1.0) | 5.1 (1.1) |
| Digits Span (Backwards) score | 4.0 (1.0) | 3.6 (1.0) |
| Token Test score | 22.6 (2.9) | 19.3 (5.6) |
Values are mean (SD). MMSE, Mini‐Mental State Examination.
Finally, we examined associations between the four domains of anosognosia and severity of dementia. We calculated a multivariate analysis of variance with Clinical Dementia Rating stage as the between‐group factor and differential scores (care giver score minus patient score) on each of the four anosognosia domains. We found a significant overall effect (Wilks' λ = 0.82, Rao's R = 12.7, df = 12, 1958, p<0.0001; table 5). Patients with moderate or severe Alzheimer's disease showed significantly more severe anosognosia for iADL than patients with very mild or mild Alzheimer's disease (moderate or severe Alzheimer's disease v very mild Alzheimer's disease, p<0.0001; moderate Alzheimer's disease v mild Alzheimer's disease, p<0.01; severe Alzheimer's disease v mild Alzheimer's disease, p<0.001). On the other hand, patients with severe Alzheimer's disease showed more severe anosognosia for bADL than patients with moderate Alzheimer's disease (p<0.0001). Taken together, these findings suggest a ceiling effect for iADL at the stage of moderate Alzheimer's disease, but no ceiling effect for bADL. Patients with mild Alzheimer's disease (p<0.01), moderate Alzheimer's disease (p<0.0001) or severe Alzheimer's disease (p<0.01) showed significantly more severe anosognosia for depression than patients with very mild Alzheimer's disease. On the other hand, we found no marked between‐group differences for the domain of disinhibition.
Table 5 Scores of anosognosia for patients at different stages of Alzheimer's disease.
| Stages of Alzheimer's disease | ||||
|---|---|---|---|---|
| Very mild Alzheimer's disease | Mild Alzheimer's disease | Moderate Alzheimer's disease | Severe Alzheimer's disease | |
| Anosognosia for iADL | 0.20 (0.59) | 0.58 (0.74) | 0.88 (0.84) | 1.19 (1.06) |
| Anosognosia for bADL | 0.0 (0.36) | 0.18 (0.53) | 0.33 (0.62) | 0.86 (1.02) |
| Anosognosia for depression | 0.21 (0.61) | 0.45 (0.69) | 0.67 (0.77) | 0.70 (0.87) |
| Anosognosia for disinhibition | 0.10 (0.44) | 0.17 (0.47) | 0.19 (0.53) | 0.18 (0.65) |
Values are mean (SD). bADL, basic activities of daily living; iADL, instrumental activities of daily living.
Increased awareness of deficits on iADL
Finally, we examined whether patients with Alzheimer's disease may overestimate their cognitive deficits or behavioural changes. To be consistent with the strategy used to diagnose anosognosia, we considered overestimation to be significant whenever we observed a discrepancy of two points or more (patient rating more severe deficits than the respective care giver) on at least four of the items on the iADL domain for anosognosia. Only 24 (3%) of the 750 patients met the criteria for significant overestimation: 5 patients had very mild, 11 had mild, 7 had moderate and 1 had severe Alzheimer's disease. The hypothesis of unequal frequency of overestimation of deficits based on the presence of major or minor depression was statistically substantiated: 10 (42%) of the 24 patients with overestimation had major depression and another 5 (21%) had minor depression, as compared with major depression in 150 (21%) of the 726 patients and minor depression in 180 (25%) patients without overestimation (χ2 = 6.20, df = 2, p<0.05).
Discussion
We examined the phenomenon of anosognosia in a large series of patients with Alzheimer's disease, and the important findings are listed below.
Deficits were underestimated in ADL and poor insight into behavioural changes clustered into specific domains of anosognosia for iADL and bADL, depressive symptoms and disinhibited behaviour.
The information provided by care givers had a considerably stronger correlation with cognitive functioning of the patients' than the information provided by the patients themselves.
Specific criteria for anosognosia in Alzheimer's disease were defined based on principal component analysis, and their validity was demonstrated.
Anosognosia for deficits in ADL were already present at the stage of very mild dementia, as manifested by poor insight into problems with date recall, orientation in new places, remembering telephone calls, understanding conversations, remembering where belongings were left, handling money, remembering appointments, understanding the plot of a movie and doing clerical work.
Anosognosia in people with very mild dementia was significantly associated with anterograde memory and verbal comprehension deficits.
Functional deficits were overestimated in only 3% of the patients, and were markedly associated with major depression.
Before further comments, several limitations of our study should be discussed. Firstly, we do not have neuropathological confirmation of the diagnosis of Alzheimer's disease, and whether some of our patients had other neurodegenerative conditions such as frontotemporal dementia cannot be ruled out. Secondly, we did not measure the effect of anosognosia on functioning of patients', and the severity criterion for most psychiatric disorders could not be ascertained. Nevertheless, we found in a recent study that patients with Alzheimer's disease who have anosognosia are exposed to dangerous situations more often than those without anosognosia,35 suggesting that anosognosia is a clinically relevant condition.
Anosognosia in people with Alzheimer's disease refers to the lack of awareness of deficits on iADL or bADL, behavioural changes and mood problems. Anosognosia is not an “all or none” phenomenon, and awareness of deficits may range from an expression of deep concern about the progressive cognitive decline to the overt denial or minimisation of impairments. Poor awareness about cognitive deficits and behavioural changes is a frequent finding in clinical practice, but the diagnosis of anosognosia is usually made either subjectively or with non‐standardised methods. Several limitations to the accurate diagnosis of anosognosia in people with Alzheimer's disease should be mentioned. Firstly, awareness of deficits is conceptually related to the complexity of the patient's activities. People with intellectually demanding activities may become aware of their impairments much sooner than those with relatively simpler routines. Secondly, whereas the term self‐awareness has the connotation of a private introspective activity, becoming aware of our own functional deficits mostly occurs in the context of everyday life. Therefore, the most accurate instrument to rate anosognosia should be based on each person's pattern of everyday activities, interests, mood and behaviour. Nevertheless, such a strategy may be difficult to implement in busy clinics, it may not prove reliable and results may be difficult to compare. We defined anosognosia as partial or complete loss of awareness about deficits on routine ADL. We assessed this dimension with the AQ‐D, which consists of questions about performance on ADL and changes in mood, emotions and behaviours. A principal component analysis of the scale produced four factors, which were construed as anosognosia for deficits in iADL, anosognosia for deficits on bADL, anosognosia for depression and anosognosia for disinhibition. In a previous study on a much smaller sample, we found two factors for anosognosia in people with Alzheimer's disease—namely unawareness of deficits in ADL and unawareness of mood and behavioural changes. This study expands those findings to different levels of ADL, and to specific mood and behavioural domains.
We next defined standardised criteria for the diagnosis of anosognosia in Alzheimer's disease by using the items included in the iADL factor, given that this factor accounted for most of the variance and also rates the earliest functional deficits in Alzheimer's disease. A care giver to patient discrepancy was considered to be relevant whenever the difference was of at least two points (eg, a deficit reported by the patient as being never present and the care giver reporting the deficit as often or always present). We used receiver–operating characteristic statistics to determine the optimal cut‐off on the AQ‐D to diagnose anosognosia. By using the clinical diagnosis of anosognosia as the gold standard, we found that a cut‐off score of 4 (ie, a noticeable care giver to patient discrepancy on at least four items of the iADL domain) yielded the optimal combination of sensitivity (81%) and specificity (97%).
Another important finding of this study was that the frequency of anosognosia increased with the severity of Alzheimer's disease, suggesting a close association between anosognosia, cognitive decline and more severe changes in mood and behaviour. We found a salient association between anosognosia and more severe deficits on verbal memory and verbal comprehension, but not with tests of verbal fluency, working memory and constructional praxis. Thus, our findings do not support the hypothesis of a specific profile of cognitive deficits underlying anosognosia in people with Alzheimer's disease. Moreover, most of the patients with moderate dementia, and about half of those with severe dementia had no anosognosia, showing that cognitive deficits are not sufficient to cause anosognosia in Alzheimer's disease. Furthermore, our finding that anosognosia was already evident in the earliest stages of Alzheimer's disease also shows that prominent cognitive deficits are not necessary to cause anosognosia.
In this study, we found a marked association between anosognosia and disinhibition in all four stages of Alzheimer's disease, suggesting that anosognosia may be a symptom of a wider emotional or behavioural disorder that starts early in the illness. This hypothesis will have to be further examined with more appropriate longitudinal studies.
The question now arises of the reliability of the information given by the care giver. We found that the ratings given by the care givers' on the AQ‐D accounted for 34% of the variance with the patients' MMSE scores, as compared with 5% of the variance with the patients' own ratings. The strong reliability of the information provided by care givers of patients with dementia has already been shown by other investigators.36,37,38 As all but four of our patients were living in their homes along with a care giver (the spouse, a sibling or a son or daughter) who completed the AQ‐D, whether our findings should generalise to patients living in nursing homes or with less informed care givers will need to be examined further.
In conclusion, anosognosia in Alzheimer's disease is manifested as poor awareness of deficits in iADL and bADL, depressive changes and behavioural disinhibition. The frequency of anosognosia increases markedly with the severity of dementia, but is already present in at least 10% of the patients with very mild dementia. We also showed the validity of a specific set of criteria to diagnose anosognosia in Alzheimer's disease. These criteria have practical relevance for the management of patients with Alzheimer's disease and contribute to the early diagnosis of this condition.
Acknowledgements
This study was partially supported by grants from the University of Western Australia, the Raine Medical Research Foundation, and the National Health and Medical Research Council. We thank Dr Eran Chemerinski, Dr Gustavo Petracca, Dr Laura Garau and Dr Agustina Cao for data collection.
Abbreviations
ADL - activities of daily living, bADL, basic activities of daily living
iADL - instrumental activities for daily living
AQ‐D - Anosognosia Questionnaire for Dementia
DSM‐IV - Diagnostic and Statistical Manual of Mental Disorders (4th edn)
MMSE - Mini‐Mental State Examination Score
Footnotes
Competing interests: None declared.
References
- 1.Babinski J. Reflexes de defense. Brain 192245149–184. [Google Scholar]
- 2.Starkstein S E, Chemerinski E, Sabe L.et al Prospective longitudinal study of depression and anosognosia in Alzheimer's disease. Br J Psychiatry 199717147–52. [DOI] [PubMed] [Google Scholar]
- 3.Ott B R, Lafleche G, Whelihan W M.et al Impaired awareness of deficits in Alzheimer disease. Alzheimer Dis Assoc Disord 19961068–76. [DOI] [PubMed] [Google Scholar]
- 4.Clare L. The construction of awareness in early‐stage Alzheimer's disease: a review of concepts and models. Br J Clin Psychol 200443155–175. [DOI] [PubMed] [Google Scholar]
- 5.Clare L. Awareness in early‐stage Alzheimer's disease: a review of methods and evidence. Br J Clin Psychol 200443177–196. [DOI] [PubMed] [Google Scholar]
- 6.Zanetti O, Vallotti B, Frisoni G B.et al Insight in dementia: when does it occur? Evidence for a nonlinear relationship between insight and cognitive status. J Gerontol Ser B‐Psychol Sci Soc Sci 199954P100–P106. [DOI] [PubMed] [Google Scholar]
- 7.Verhey F R, Rozendaal N, Ponds R W H M.et al Dementia, awareness and depression. Int J Geriatr Psychiatry 19938851–856. [Google Scholar]
- 8.Seltzer B, Vasterling J J, Yoder J A.et al Awareness of deficit in Alzheimer's disease: relation to caregiver burden. Gerontologist 19973720–24. [DOI] [PubMed] [Google Scholar]
- 9.Reed B R, Jagust W J, Coulter L. Anosognosia in Alzheimer's disease: relationships to depression, cognitive function, and cerebral perfusion. J Clin Exp Neuropsychol 199315231–244. [DOI] [PubMed] [Google Scholar]
- 10.Lopez O L, Becker J T, Somsak D.et al Awareness of cognitive deficits and anosognosia in probable Alzheimer's disease. Eur Neurol 199434277–282. [DOI] [PubMed] [Google Scholar]
- 11.Auchus A P, Goldstein F C, Green J.et al Unawareness of cognitive impairments in Alzheimer's disease. Neuropsychiatry Neuropsychol Behav Neurol 1994725–29. [Google Scholar]
- 12.Wagner M T, Spangenberg K B, Bachman D L.et al Unawareness of cognitive deficit in Alzheimer disease and related dementias [see comment]. Alzheimer Dis Assoc Disord 199711125–131. [DOI] [PubMed] [Google Scholar]
- 13.Dalla Barba G, Parlato V, Iavarone A.et al Anosognosia, intrusions and ‘frontal' functions in Alzheimer's disease and depression. Neuropsychologia 199533247–259. [DOI] [PubMed] [Google Scholar]
- 14.Anderson S W, Tranel D. Awareness of disease states following cerebral infarction, dementia and head trauma: standardised assessment. Clin Neuropsychol 19893327–339. [Google Scholar]
- 15.Barrett A M, Eslinger P J, Ballentine N H.et al Unawareness of cognitive deficit (cognitive anosognosia) in probable AD and control subjects. Neurology 200564693–699. [DOI] [PubMed] [Google Scholar]
- 16.Correa D D, Graves R E, Costa L. Awareness of memory deficits in Alzheimer's disease patients and memory‐impaired older adults. Aging Neuropsychol Cogn 19963215–228. [Google Scholar]
- 17.Mangone C A, Hier D B, Gorelick P B.et al Impaired insight in Alzheimer's disease. J Geriatr Psychiatry Neurol 19914189–193. [DOI] [PubMed] [Google Scholar]
- 18.Migliorelli R, Teson A, Sabe L.et al Anosognosia in Alzheimer's disease: a study of associated factors. J Neuropsychiatry Clin Neurosci 19957338–344. [DOI] [PubMed] [Google Scholar]
- 19.Snow A L, Norris M P, Doody R.et al Dementia Deficits Scale. Rating self‐awareness of deficits. Alzheimer Dis Assoc Disord 20041822–31. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 21.Hachinski V C, Iliff L D, Zilhka E.et al Cerebral blood flow in dementia. Arch Neurol 197532632–637. [DOI] [PubMed] [Google Scholar]
- 22.Spitzer R L, Williams J B, Gibbon M.et al The Structured Clinical Interview for DSM‐III‐R (SCID). I: history, rationale, and description, Arch Gen Psychiatry 199249624–629. [DOI] [PubMed] [Google Scholar]
- 23.Folstein M F, Folstein S E, McHugh P R. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 19602356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes C P, Berg L, Danziger W L.et al A new clinical scale for the staging of dementia. Br J Psychiatry 1982140566–572. [DOI] [PubMed] [Google Scholar]
- 26.Starkstein S E, Garau M L, Cao A. Prevalence and clinical correlates of disinhibition in dementia. Cogn Behav Neurol 200417139–147. [DOI] [PubMed] [Google Scholar]
- 27.Granger C V, Hamilton B B, Kayton R.Guide for use of the uniform data set for medical rehabilitation. Buffalo: Uniform Data System for Medical Rehabilitation, 1986
- 28.Starkstein S E, Migliorelli R, Manes F.et al The prevalence and clinical correlates of apathy and irritability in Alzheimer's disease. Eur J Neurol 19952540–546. [DOI] [PubMed] [Google Scholar]
- 29.Migliorelli R, Petracca G, Teson A.et al Neuropsychiatric and neuropsychological correlates of delusions in Alzheimer's disease. Psychol Med 199525505–513. [DOI] [PubMed] [Google Scholar]
- 30.Migliorelli R, Teson A, Sabe L.et al Prevalence and correlates of dysthymia and major depression among patients with Alzheimer's disease. Am J Psychiatry 199515237–44. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan E F, Goodglass H, Weintraub S.The Boston Naming Test. Philadelphia, PA: Lea and Febiger, 1993
- 32.De Renzi E, Faglini P. Development of a shortened version of the Token Test. Cortex 19781441–49. [DOI] [PubMed] [Google Scholar]
- 33.Buschke H, Fuld P A. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology 1974241019–1025. [DOI] [PubMed] [Google Scholar]
- 34.Wechsler D.Wechsler Adult Intelligence Test manual. New York: The Psychological Corporation, 1955
- 35.Starkstein S E, Jorge R, Mizrahi R.et al Insight and danger in Alzheimer's disease. Eur J Neurol In press [DOI] [PubMed]
- 36.Jorm A F. Assessment of cognitive impairment and dementia using informant reports. Clin Psychol Rev 19961651–73. [Google Scholar]
- 37.Cacchione P Z, Powlishta K K, Grant E A.et al Accuracy of collateral source reports in very mild to mild dementia of the Alzheimer type [see comment]. J Am Geriatr Soc 200351819–823. [DOI] [PubMed] [Google Scholar]
- 38.Tierney M C, Szalai J P, Snow W G.et al The prediction of Alzheimer disease. The role of patient and informant perceptions of cognitive deficits. Arch Neurol 199653423–427. [DOI] [PubMed] [Google Scholar]