Abstract
Background
Falls and fractures contribute to morbidity and mortality in bradykinetic rigid syndromes.
Methods
The authors performed a retrospective case notes review at the Queen Square Brain Bank for Neurological Disorders and systematically explored the relation between clinical features and falls and fractures in 782 pathologically diagnosed cases (474 with Parkinson's disease (PD); 127 progressive supranuclear palsy (PSP); 91 multiple system atrophy (MSA); 46 dementia with Lewy bodies (DLB); 27 vascular parkinsonism; nine Alzheimer's disease; eight corticobasal degeneration).
Results
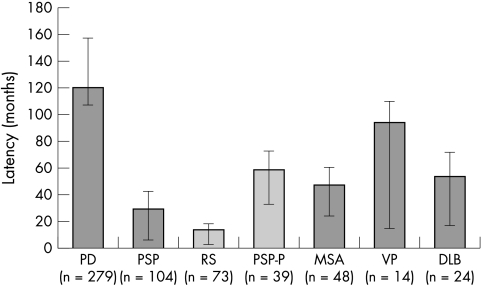
Falls were recorded in 606 (77.5%) and fractures in 134 (17.1%). In PD, female gender, symmetrical onset, postural instability, and autonomic instability all independently predicted time to first fall. In PD, PSP, and MSA latency to first fall was shortest in those with older age of onset of disease. Median latency from disease onset to first fall was shortest in Richardson's syndrome (12 months), MSA (42), and PSP‐parkinsonism (47), and longest in PD (108). In all patients fractures of the hip were more than twice as common as wrist and forearm fractures. Fractures of the skull, ribs, and vertebrae occurred more frequently in PSP than in other diseases.
Conclusion
Measures to prevent the morbidity associated with falls and fractures in bradykinetic rigid syndromes may be best directed at patients with the risk factors identified in this study.
Keywords: Parkinson's disease, bradykinetic rigid syndromes, falls, fractures
The diagnoses of Parkinson's disease (PD) and progressive supranuclear palsy (PSP) are contingent on the presence of postural instability and falls.1,2 In these and other bradykinetic rigid syndromes, falls occur frequently and are associated with a poorer quality of life.3,4 The risk of bone fracture increases with a history of falls, impairment of mobility, low body mass index, and low bone mineral density.5,6 In patients with disturbances of gait due to parkinsonism, fractures are more common than in those with gait disturbances due to other neurological conditions such as peripheral neuropathies.7 In PD the femur is the most commonly fractured bone8,9,10 but in the community the forearm is more frequently broken. This difference is thought to be due to the manner in which patients with PD fall.11,12 Despite the greater frequency of falls in atypical parkinsonism, falls and their relation to bone fractures have not been systematically analysed in progressive supranuclear palsy (PSP), multiple system atrophy (MSA), corticobasal degeneration, or vascular parkinsonism (VP).10,13 Falling within the first year of disease is required by the National Institute for Neurological Disorders and Stroke (NINDS) diagnostic criteria for a diagnosis of probable PSP, although in practice only 61% of patients with this diagnosis had fallen that early on.2,14 Together with a supranuclear gaze palsy, nuchal dystonia, and early frontostriatal cognitive deficits, falls complete the cardinal clinical quartet of PSP first described by Richardson.15 We have recently defined a subgroup of patients designated PSP‐parkinsonism (PSP‐P), in whom the PSP tau pathology more frequently causes early asymmetrical levodopa responsive bradykinesia and in whom falls, cognitive changes, and supranuclear gaze palsy occur later than in classic Richardson's syndrome (RS).14 A number of factors contribute to falls in other bradykinetic rigid syndromes. Gait unsteadiness and cognitive impairment contribute to falls in corticobasal degeneration (CBD), dementia with Lewy bodies (DLB) and in those with pathological changes diagnostic of Alzheimer's disease (AD) who present with parkinsonism. In MSA, on the other hand, orthostatic hypotension and cerebellar or parkinsonian gait disorder are important precipitants.16
We have retrospectively analysed data from a large number of pathologically diagnosed PD, PSP, MSA, CBD, DLB, VP, and AD patients and have investigated the relation between pathological diagnosis and the bones fractured. We have also quantified the temporal evolution of falls in each disease group and analysed the relation between time from disease onset to first fall (latency) and disease.
Subjects and methods
Subjects
A retrospective analysis was performed using the clinical files of 782 patients with a lifetime diagnosis of a bradykinetic rigid syndrome or parkinsonism with a pathologically confirmed diagnosis (PD, n = 474; PSP, n = 127; MSA, n = 91; DLB, n = 46; VP, n = 27; AD, n = 9; CBD, n = 8) archived at the Queen Square Brain Bank for Neurological Disorders. Patients were recruited from around the United Kingdom and died between 1988 and 2003. A few cases (2% of total) could not be included in the analysis because of insufficient clinical data (10 PD, three PSP, one MSA, one AD, one VP). The PSP group were further divided according to the number of clinical features reported associated with each clinical phenotype: RS (supranuclear gaze palsy, abnormality of saccadic eye movements, cognitive changes in first two years), PSP‐P (tremor and non‐axial dystonia in the first two years, asymmetric onset, and response to levodopa).
Data collection
A systematic chart review was performed; specifically the case notes of the family doctor were scrutinised together with all the correspondence between the medical specialist and family doctor. Hospital inpatient notes, inpatient consultations, and emergency room admission notes were also reviewed. The clinical features were recorded as present or absent early in the disease (within two years of first symptom onset) and at anytime during the disease. These features included: age of onset (age at the time of the first reported symptom considered to be attributable to disease), disease duration (time from onset until death), falls, time to first fall (time from onset to fall), bones fractured (confirmed radiologically), bradykinesia, cognitive dysfunction (reported by family, patient or physician, not attributed to affective disorder), symmetry of disease at onset, tremor, rigidity, postural reflexes (if reported by physician), supranuclear gaze palsy, impaired saccadic or pursuit eye movements, non‐axial dystonia, pyramidal signs, autonomic dysfunction (abnormal autonomic function tests or any two of: urinary urgency, frequency, and nocturia without hesitancy; chronic constipation; postural hypotension; sweating abnormalities; erectile dysfunction), dyskinesias, and response to levodopa (graded by patient or clinician as nil/slight, moderate, good, excellent) were recorded. Standardised forms were not used by the doctors recording the antemortem medical history. We recorded symptoms as being absent if not reported, and clinical signs were recorded as missing if they were not specifically mentioned in the notes. Where conflicting clinical features were reported the findings of the neurologist were used.
Statistics
The report and timing of any falls from the time of disease onset until death and/or fractures was the primary outcome measure. Descriptive statistics were used to analyse the latency to first fall and frequency of different skeletal bones fractured in each diagnostic group. Clinical characteristics in different diseases were compared. We investigated the associations between bradykinesia, rigidity, tremor, cognitive dysfunction, speech disturbance, dysphagia, dystonia, dyskinesias, autonomic dysfunction, gender, symmetry of clinical features, visual hallucinations, and age at disease onset with falls and fractures in all diseases. Univariable analyses using χ2 for categorical and two tailed t test or the Mann Whitney U test, as appropriate, for continuous variables were applied. In PSP univariable analysis was performed on falls in the first two years (that is, early falls, rather than any falls) because of the high frequency of falls. In PSP, RS, and PSP‐P the effect of falls on prognosis was assessed by calculating the mean disease duration in patients who fell in the first two years of disease and comparing it to those who fell after two years. Kaplan‐Meier plots were used to graphically assess survival. Cox multiple stepwise regression analysis was performed in all diseases for latency to first fall, using clinical factors present around disease onset (within the first two years) and gender as categorical covariates and age at onset as a continuous variable. This work was approved by the local ethics committee.
Results
Among the 782 cases the incidence of falls from disease onset to death was 77.5%. It was highest in CBD (100%) and PSP (97.5%) and lowest in MSA (77.6%) and PD (73.3%) (table 1).
Table 1 Characteristics of patients according to pathological diagnosis.
| PD | PSP | MSA | DLB | CBD | VP | AD | |
|---|---|---|---|---|---|---|---|
| Number | 474 | 127 | 91 | 46 | 8 | 27 | 9 |
| Mean age at onset, years (range) | 60.4 (28.6–84.2) | 65.7 (40.1–87.5) | 56.4 (33.5–79) | 66.7 (43.0–82.6) | 66.2 (55.6–76.1) | 69.6 (39.5–81.3) | 69.4 (59.6–84.8) |
| Mean age at death, years (range) | 76.4 (49.8–93.4) | 73.7 (45.4–95.8) | 64.4 (39.4–86.0) | 74.9 (54.9–88.3) | 73.0 (65.8–81.6) | 82.5 (72.4–93.6) | 80.2 (71.0–94.6) |
| Women (%) | 39.9 | 37.3 | 53.8 | 20 | 20 | 48 | 44.4 |
| Fallers (%) | 73.3 | 98.3 | 77.6 | 58.1 | 100 | 73.9 | 85.7 |
| Median time to first fall, months (range) | 108 (0–384) | 16.8 (0–244) | 42 (0–165) | 54 (0–158) | 30 (0–90) | 40.8 (0–304) | 66.6 (36–228) |
| Fractures (%) | 16.9 | 28.6 | 11 | 4.3 | 0 | 11.1 | 22.2 |
Falls occurred earlier in those with PSP (median 17 months; range 0–244 months) than all other diseases (fig 1). Median time to first fall was significantly shorter in RS (12; range 0–95) compared to both PSP‐P (47; 0–228, p<0.001), PD (108; 0–384, p<0.001), and MSA (42; 0–165). In PD univariable analysis showed an association between the occurrence of falls and the late clinical features of cognitive dysfunction, speech disturbance, dysphagia, autonomic dysfunction, and hallucinations and a negative association between the falls and early and late tremor (table 2).
Figure 1 Latency to first fall according to disease, showing interquartile range.
Table 2 Clinical features in fallers and non‐fallers: percentage, χ2.
| Parkinson's disease | PSP | MSA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fallers | Non‐fallers | p Value | Early fallers | Early non‐fallers | p Value | Fallers | Non‐fallers | p Value | |
| Number | 313 | 114 | 75 | 49 | 66 | 19 | |||
| Gender (% female) | 43.1 | 27.2 | 0.002 | 36 | 40.8 | NS | 59 | 36.8 | NS |
| Early clinical features | |||||||||
| Cognitive dysfunction | 0 | 0 | NS | 70.7 | 28.6 | <0.001 | 4.8 | 0 | NS |
| Tremor | 72.4 | 86.2 | 0.004 | 6.8 | 35.4 | <0.001 | 42.2 | 42.1 | NS |
| Axial rigidity | 5.6 | 8.1 | NS | 54.7 | 32.5 | 0.024 | 16.7 | 5.9 | NS |
| Limb rigidity | 75 | 81.9 | NS | 63.9 | 61.4 | NS | 61.1 | 68.9 | NS |
| Asymmetric onset | 84.8 | 88.2 | NS | 34.8 | 83.1 | <0.001 | 60.3 | 50 | NS |
| Postural instability | 8.7 | 1.9 | 0.017 | 91.9 | 41.9 | <0.001 | 28.6 | 21.1 | NS |
| Vertical gaze palsy | 6.2 | 0 | NS | 54.9 | 11.5 | <0.001 | |||
| Abnormal saccades | 12 | 0 | NS | 83.3 | 45.0 | 0.002 | |||
| Speech disturbance | 9.6 | 6.5 | NS | 66.7 | 41.7 | 0.006 | 19.3 | 38.9 | NS |
| Dysphagia | 0.7 | 2.9 | NS | 26.7 | 12.5 | NS | 4.9 | 17.6 | NS |
| Pyramidal signs | 3.3 | 0 | NS | 12.3 | 15.2 | NS | 17.4 | 16.7 | NS |
| Dyskinesias | 0.7 | 0.9 | NS | 0 | 0 | NS | 0 | 1.6 | NS |
| Autonomic dysfunction | 5.7 | 3.6 | NS | 1.4 | 2.0 | NS | 55.6 | 58.9 | NS |
| Late clinical features | |||||||||
| Cognitive dysfunction | 71.7 | 49.5 | <0.001 | 31.6 | 12.5 | NS | |||
| Tremor | 85.7 | 93.6 | 0.018 | 60.3 | 68.4 | NS | |||
| Axial rigidity | 30.1 | 22.3 | NS | 47.5 | 41.2 | NS | |||
| Limb rigidity | 98 | 98.2 | NS | 96.7 | 77.8 | 0.008 | |||
| Postural instability | 45.6 | 83.8 | <0.001 | 94.7 | 88.2 | NS | |||
| Speech disturbance | 78.8 | 59 | <0.001 | 98.4 | 82.4 | 0.007 | |||
| Dysphagia | 59.7 | 46.9 | 0.033 | 94.9 | 68.8 | 0.003 | |||
| Dyskinesias | 58.1 | 53.3 | NS | 22.2 | 22.2 | NS | |||
| Autonomic dysfunction | 46.9 | 21.5 | <0.001 | 88.9 | 100 | NS | |||
| Pyramidal signs | 5.4 | 9.4 | NS | 66.7 | 33.3 | 0.018 | |||
| Hallucinations | 53.3 | 37.5 | 0.004 | 10.6 | 5.9 | NS | |||
NS, not significant.
In PSP, early falls (within the first two years of disease) were associated with symmetrical disease onset and early cognitive dysfunction, axial rigidity, postural instability, eye movement abnormalities, and speech disturbance (occurring within two years of disease onset). In common with PD, in those with early falls tremor was less frequent. In MSA falls at any time were associated with the late clinical features of limb rigidity, speech disturbance, dysphagia, and pyramidal tract signs. There were no significant associations between falls and clinical features in VP, DLB, CBD, and AD; this may be due to the small sample sizes for these diseases.
Multivariate analyses were performed using the Cox multiple stepwise regression model on early clinical features, age, and gender identified several clinical factors which independently influence time to first fall (table 3).
Table 3 Factors affecting time to first fall in PD, PSP and MSA: independent predictors from Cox multiple regression analysis on early clinical features, age and sex.
| PD | HR | 95% CI | p Value | PSP | HR | 95% CI | p Value | MSA | HR | 95% CI | p Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age of onset | 1.07 | 1.05–1.08 | <0.001 | Age of onset | 1.05 | 1.01–1.09 | 0.007 | Age of onset | 1.04 | 1.02–1.08 | 0.003 |
| Symmetrical onset | 1.86 | 1.25–2.77 | 0.002 | Early postural instability | 3.4 | 1.7–6.8 | 0.001 | Early postural instability | 2.08 | 1.07–3.94 | 0.03 |
| Early autonomic dysfunction | 2.56 | 1.32–4.98 | 0.005 | ||||||||
| Female gender | 1.39 | 1.05–1.83 | 0.021 |
In PD female gender, older age, symmetrical onset, and autonomic dysfunction were all independent predictors of earlier falls. Age of onset was also an important independent factor predicting time to first fall in PSP and MSA as was postural instability.
Almost all patients with PSP had falls at some point in their clinical history; patients with falls in the first two years had mean life expectancy 3.3 years lower than other PSP patients (table 4).
Table 4 Disease duration (time from onset until death) and age at onset in early (<2 years) and late (>2 years) fallers in PSP, RS, and PSP‐P (Mann‐Whitney U test).
| Falls <2 years | Falls >2 years | p Value | ||
|---|---|---|---|---|
| PSP | Duration, years | 6.4 | 9.7 | <0.001 |
| Onset, years | 66.7 | 64.7 | NS | |
| RS | Duration, years | 6.2 | 6.8 | NS |
| Onset, years | 66.1 | 64.6 | NS | |
| PSP‐P | Duration, years | 7.1 | 11.0 | 0.005 |
| Onset, years | 69.1 | 64.7 | NS |
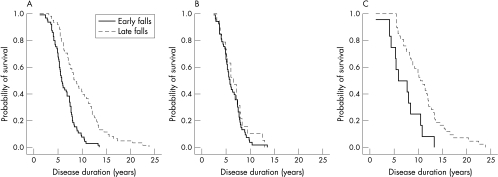
This difference was greater in the PSP‐P group, and there was no difference in the RS group. This is illustrated graphically by the Kaplan‐Meier plot (fig 2).
Figure 2 Kaplan‐Meier plot illustrates the effect of early or late falls upon survival in 124 cases of (A) PSP in total, and separated into subgroups; (B) RS (72 cases); and (C) PSP‐P (54 cases).
There were 166 fractures recorded in 134 (17.1%) patients. Eighty patients (16.9%) with PD sustained a total of 96 fractures, 36 (28.6%) patients with PSP sustained 52 fractures, and 10 (11%) patients with MSA sustained a fracture.
Hip fractures comprised 46.9% of all fractures in PD and one third of fractures occurred in the upper limb. In PSP the proportion of hip fractures was less than in PD (30.7% v 46.9%, p = 0.053 t test), and the proportion of “other upper limb fractures” (including the clavicle, scapula, and bones in the hand) was higher than in PD (p = 0.109, t test) (table 5). Comparing all patients (with and without fractures), multiple fractures were significantly more common in PSP than in both PD (6.4% v 1.9%, p = 0.007 t test) and MSA (6.4% v 0%, p = 0.014 t test). Skull fractures (3.2% v 0.8%, p = 0.43 t test) and truncal fractures (5.6% v 1.9%, p = 0.24 t test) were both more common in PSP than in PD. There were no substantial or significant differences in fracture site between the two subgroups of PSP.
Table 5 Distribution of fracture location in bradykinetic rigid syndromes: number (% of all fractures).
| PD | PSP | RS | PSP‐P | MSA | VP | DLB | AD | |
|---|---|---|---|---|---|---|---|---|
| Forearm | 17 (17.7) | 7 (13.5) | 5 (16.7) | 2 (9.1) | 3 (30) | 1 (33.3) | ||
| Humerus | 10 (10.4) | 5 (9.6) | 2 (6.6) | 3 (13.7) | 1 (10) | 1 (33.3) | ||
| Other upper limb | 6 (6.3) | 8 (15.4) | 5 (16.7) | 3 (13.7) | 0 | 1 (33.3) | 1 (50) | |
| Hip | 45 (46.9) | 16 (30.7) | 10 (30.0) | 7 (31.8) | 4 (40) | 2 (100) | ||
| Distal leg | 5 (5.2) | 4 (7.7) | 3 (10) | 1 (4.5) | 0 | 1 (50) | ||
| Pelvis | 1 (1.0) | 1 (1.9) | 0 | 1 (4.5) | 0 | |||
| Trunk | 8 (8.3) | 7 (13.5) | 3 (10) | 4 (18.2) | 1 (10) | |||
| Skull | 4 (4.2) | 4 (7.7) | 3 (10) | 1 (4.5) | 1 (10) | |||
| Multiple | 9 | 8 | 7 | 5 | 0 | |||
| Total fractures | 96 (100) | 52 (100) | 30 (100) | 22 (100) | 10 (100) | 3 (100) | 2 (100) | 2 (100) |
The clinical features that were significantly associated with fractures in PD were early falls (9.3% with fractures v 3.2% no fractures, χ2 p = 0.024), late falls (100% v 66.7%, χ2 p<0.001), and late postural instability (88.4% v 71%, χ2 p = 0.003)); in PD fractures were also more frequent in women (28.6% v 11.3%, χ2 p<0.001). In PSP early bradykinesia (77.8% with fractures v 92% without fractures, χ2 p = 0.027), early limb rigidity (47% v 68.7%, χ2 p = 0.028), and early pyramidal signs (2.5% v 18.8%, χ2, p = 0.02) occurred less frequently in those with fractures. Sex distribution was not significantly different between fracture and non‐fracture groups in PSP (34% women and 44% women respectively, χ2 p = 0.275). In contrast, women were overrepresented in the 10 MSA patients with fractures (90% v 49.4%, χ2 p = 0.015). None of the three VP patients with fractures had either bradykinesia (0% v 71% in VP patients without fractures, χ2 p = 0.017) or limb rigidity (0% v 85% , χ2 p = 0.02).
Discussion
Using the “gold standard” of pathological diagnosis and retrospective case notes review methodology we have identified several important factors related to falling which help to distinguish the most common neurodegenerative, bradykinetic rigid syndromes. Falls occur within the first three years of disease onset in a majority of patients with PSP, within four years in MSA, five for those with DLB, and a median of nine years for those with PD. In this study, the incidence of falls and fractures was high (77.6% and 17.1%). 73% of patients with PD had falls and 16.9% sustained fractures. A previous prospective survey of PD and elderly patients predicted a higher incidence of fractures caused from falls in PD.11 Studies have estimated an incidence of falls as high as 69% in PD and 32% in elderly patients.17,18 Falls and fractures are likely to be underestimated in the present study because it relies entirely on the accuracy and completeness of clinical notes. Patients with other bradykinetic rigid syndromes, including PSP, CBD, and MSA, fall more frequently and earlier in the disease than in PD, and it has been suggested that these patients have a higher incidence of fractures.13 We have found that more than three quarters of patients with PSP (97%), MSA (77%), and CBD (100%) fell during their disease, though only 72% of patients with VP and 58% of patients with DLB fell. The incidence of fractures was higher in PSP than in PD, but lower in MSA, CBD, VP, DLB, and AD, partially reflecting the differences in disease duration.
Falls in PD
In PD fallers, we have confirmed the previously reported high incidence of disturbances of higher cortical functions,18,19 as indicated by our clinical parameters of cognitive dysfunction and hallucinations, and axial symptoms, such as speech disturbance and dysphagia. We also found autonomic dysfunction and absence of tremor in PD were more frequent in those who fell. A lower incidence of tremor in PD fallers has previously been reported.18,20 Several authors have argued that a bradykinetic‐rigid or non‐tremor dominant PD phenotype exists, where tremor is minimal or absent, depression is more frequent, and prognosis is poorer.21,22,23,24 Our results suggest that falling is more likely to occur in these patients. A number of standardised measures of disease severity include falls and postural instability as indicators of advanced disease.25,26 Our data were unable to identify any correlation between time to first fall and prognosis as measured by disease duration until death.
Age of onset, gender, symmetrical disease onset, and autonomic dysfunction were identified as independent significant factors contributing to latency to first fall in PD. Both older age and female gender are well recognised risk factors for falls in the general population17,27,28 and have been identified as risk factors for falls in some, but not all reports in PD.10,29,30 We have shown that, in this population, falls occur more frequently in women and they tend to fall significantly earlier in the disease than men.
Falls in PSP
In PSP early falls were associated with symmetrical disease onset, early cognitive dysfunction, axial rigidity, dysphagia, and eye movement abnormalities. These clinical factors reflect the importance of axial symptoms in balance and gait disturbance. Prognosis in PSP has previously been related to early falls, speech and swallowing problems, and time to PEG tube insertion.31 There was a significant difference in disease duration between early fallers and late fallers in PSP and PSP‐P. This difference was not found in RS. The Kaplan‐Meier curves suggest that early falls in PSP predict mortality. In the general population the risk of falling increases with age and so too in PSP the age of onset had the greatest effect on the latency to first fall in the multiple regression analysis. Postural instability was also a significant factor, although given the retrospective nature of the study, it is possible that the recording of this clinical feature was more likely in those who had fallen than those who had not.
Patients were selected on the basis that they died during a specified period of time. If the diagnosis is becoming increasingly or decreasingly common over time, or if the criteria for diagnosis is changing subtlety over time, then this may cause some bias in the type of patients included in our study. For instance, people diagnosed more recently need to die sooner after diagnosis to be included in this study.
Falls in MSA
Clinical factors that were more common in falls in MSA were similar to those in PD, in particular axial symptoms and signs (early axial rigidity, speech disturbance, and dysphagia) and early pyramidal tract signs. Less than 15% of our cases presented with prominent cerebellar symptoms, the MSA‐C clinical phenotype, perhaps explaining the lack of association with cerebellar signs and falls. Interestingly autonomic dysfunction, which was present in more than 50% of patients in the first two years of disease, was not significantly associated with falling. The clinical features which significantly and independently influenced latency to first fall were the same as PSP: age of disease onset and postural instability.
Fractures
More than one quarter of patients with PSP (28.6%) fractured at least one bone and the frequency of fractures was significantly higher than in the other diseases (Fisher's exact test, p<0.02). The clinical features that were more frequent in those with fractures and PSP were also different to other diseases. Whereas in PD and MSA and control populations women were more at risk12 and falls and postural instability were strongly associated with fractures, in PSP there was no association with female gender. In PSP only the absence of early bradykinesia, limb rigidity, and pyramidal signs were significantly associated with fractures. We speculate that when early bradykinesia, limb rigidity, and pyramidal signs are present patients are less likely to attempt to independently mobilise. In these patients there are fewer opportunities of succumbing to the poor balance and “motor recklessness” of PSP and fractures are less frequent. Among the elderly in residential care facilities men fall more than women but fractures are more frequent in women.32 In contrast, men with PSP had a similar propensity to fracture bones, perhaps reflecting the type of falls associated with the disease and the frontal cognitive deficits in these patients. A similar pattern was seen in patients with VP, who were likely to have had frontal cognitive impairment.
The proportion of hip fractures was higher in PD than in PSP and MSA (table 4). There was a significantly smaller proportion of fractures in the distal leg in MSA than in PD and PSP. We found that fractures of the trunk (ribs, vertebrae, and sternum) were proportionally more in PSP and MSA than in PD. In contrast to previous reports10 rib and vertebral fractures were proportionally less than hip and proximal upper limb fractures in PSP. These studies have different methodology and the discrepancy may be explained if rib and vertebral fractures occur earlier in the disease, and hip and proximal upper limb fractures later. In other respects, the location of fractures was similar between disease groups.
Nordell et al reported that in a community dwelling population of 65–74 year olds fractures of the upper extremities, and in particular the distal radius, were most common12 and fractures of the proximal femur or hip were half as common as radial fractures. In all bradykinetic rigid syndromes diagnosed in our study this proportion is reversed, reflecting a common disturbance of postural reflexes. Bradykinesia prolongs reaction times following a postural challenge and thereby limiting the protective responses, including outstretching of the arm, thus limiting fractures of the distal upper limb.
Conclusion
Falls and fractures are frequently associated with bradykinetic rigid syndromes. Patients with PD, PSP, MSA, DLB, and VP should be assessed for the independent risk factors identified in this study. The disease burden associated with disturbances of posture, resulting in falls and fractures, appears to be highest in PSP. The significant difference between RS and PSP‐P in latency to first fall supports a division of these clinical syndromes. Furthermore, the presence of early supranuclear gaze palsy, cognitive change, and a lack of response to levodopa predicts early falls and bone fractures.14 Patients with RS, and those with other bradykinetic rigid syndromes with symmetrical disease onset, postural instability, autonomic dysfunction, and cognitive disturbance, and in particular women, may benefit from early assessment for osteoporosis, treatment of decreased bone mineral density, and physiotherapy intervention to limit falling related morbidity.5
Acknowledgements
We would like to thank Dr Andrew Evans for advice on the design of this analysis and Susan Stoneham for her help in data retrieval. Without the kind donations from the patients registered with the QSBB this study would not have been possible.
Abbreviations
AD - Alzheimer's disease
CBD - corticobasal degeneration
DLB - dementia with Lewy bodies
MSA - multiple system atrophy
PD - Parkinson's disease
PSP - progressive supranuclear palsy
PSP‐P - PSP‐parkinsonism
RS - Richardson's syndrome
VP - vascular parkinsonism
Footnotes
Competing interests: none declared
References
- 1.Hughes A J, Ben‐Shlomo Y, Daniel S E.et al What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology 1992421142–1146. [DOI] [PubMed] [Google Scholar]
- 2.Litvan I, Mangone C A, McKee A.et al Natural history of progressive supranuclear palsy (Steele‐Richardson‐Olszewski syndrome) and clinical predictors of survival: a clinicopathological study. J Neurol Neurosurg Psychiatry 199660615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrag A, Jahanshahi M, Quinn N P. What contributes to depression in Parkinson's disease? Psychol Med 20013165–73. [DOI] [PubMed] [Google Scholar]
- 4.Bloem B R, Hausdorff J M, Visser J E.et al Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord 200419871–884. [DOI] [PubMed] [Google Scholar]
- 5.Sato Y, Kaji M, Tsuru T.et al Risk factors for hip fracture among elderly patients with Parkinson's disease. J Neurol Sci 200118289–93. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan S L, Myers E R, Kiel D P.et al Fall direction, bone mineral density, and function: risk factors for hip fracture in frail nursing home elderly. Am J Med 1998104539–545. [DOI] [PubMed] [Google Scholar]
- 7.Syrjala P, Luukinen H, Pyhtinen J.et al Neurological diseases and accidental falls of the aged. J Neurol 20032501063–1069. [DOI] [PubMed] [Google Scholar]
- 8.Johnell O, Melton L J, III, Atkinson E J.et al Fracture risk in patients with parkinsonism: a population‐based study in Olmsted County, Minnesota. Age Ageing 19922132–38. [DOI] [PubMed] [Google Scholar]
- 9.Grisso J A, Kelsey J L, Strom B L.et al Risk factors for falls as a cause of hip fracture in women. The Northeast Hip Fracture Study Group. N Engl J Med 19913241326–1331. [DOI] [PubMed] [Google Scholar]
- 10.Wielinski C L, Erickson‐Davis C, Wichmann R.et al Falls and injuries resulting from falls among patients with Parkinson's disease and other parkinsonian syndromes. Mov Disord 200420410–415. [DOI] [PubMed] [Google Scholar]
- 11.Genever R W, Downes T W, Medcalf P. Fracture rates in Parkinson's disease compared with age‐ and gender‐matched controls: a retrospective cohort study. Age Ageing 20053421–24. [DOI] [PubMed] [Google Scholar]
- 12.Nordell E, Jarnlo G B, Jetsen C.et al Accidental falls and related fractures in 65–74 year olds: a retrospective study of 332 patients. Acta Orthop Scand 200071175–179. [DOI] [PubMed] [Google Scholar]
- 13.Wenning G K, Ebersbach G, Verny M.et al Progression of falls in postmortem‐confirmed parkinsonian disorders. Mov Disord 199914947–950. [DOI] [PubMed] [Google Scholar]
- 14.Williams D R, de Silva R, Paviour D C.et al Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP‐parkinsonism. Brain 20051281247–1258. [DOI] [PubMed] [Google Scholar]
- 15.Richardson J C, Steele J C, Olszewski J. Supranuclear ophthalmoplegia, pseudobulbar palsy, nuchal dystonia and dementia. Transactions of the American Neurological Association 196325–29. [PubMed]
- 16.Wenning G K, Ben‐Shlomo Y, Magalhaes M.et al Clinicopathological study of 35 cases of multiple system atrophy. J Neurol Neurosurg Psychiatry 199558160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tromp A M, Smit J H, Deeg D J.et al Predictors for falls and fractures in the Longitudinal Aging Study Amsterdam. J Bone Miner Res 1998131932–1939. [DOI] [PubMed] [Google Scholar]
- 18.Wood B H, Bilclough J A, Bowron A.et al Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry 200272721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchese R, Bove M, Abbruzzese G. Effect of cognitive and motor tasks on postural stability in Parkinson's disease: a posturographic study. Mov Disord 200318652–658. [DOI] [PubMed] [Google Scholar]
- 20.Koller W C, Glatt S, Vetere‐Overfield B.et al Falls and Parkinson's disease. Clin Neuropharmacol 19891298–105. [DOI] [PubMed] [Google Scholar]
- 21.Zetusky W J, Jankovic J, Pirozzolo F J. The heterogeneity of Parkinson's disease: clinical and prognostic implications. Neurology 198535522–526. [DOI] [PubMed] [Google Scholar]
- 22.Lewis S J, Foltynie T, Blackwell A D.et al Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry 200576343–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortimer J A, Pirozzolo F J, Hansch E C.et al Relationship of motor symptoms to intellectual deficits in Parkinson disease. Neurology 198232133–137. [DOI] [PubMed] [Google Scholar]
- 24.Huber S J, Paulson G W, Shuttleworth E C. Relationship of motor symptoms, intellectual impairment, and depression in Parkinson's disease. J Neurol Neurosurg Psychiatry 198851855–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoehn M M, Yahr M D. Parkinsonism: onset, progression and mortality. Neurology 196717427–442. [DOI] [PubMed] [Google Scholar]
- 26.Martinez‐Martin P, Gil‐Nagel A, Gracia L M.et al Unified Parkinson's Disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov Disord 1994976–83. [DOI] [PubMed] [Google Scholar]
- 27.Lord S R, Ward J A, Williams P.et al An epidemiological study of falls in older community‐dwelling women: the Randwick falls and fractures study. Aust J Public Health 199317240–245. [DOI] [PubMed] [Google Scholar]
- 28.Campbell A J, Borrie M J, Spears G F. Risk factors for falls in a community‐based prospective study of people 70 years and older. J Gerontol 198944M112–M117. [DOI] [PubMed] [Google Scholar]
- 29.Ashburn A, Stack E, Pickering R M.et al Predicting fallers in a community‐based sample of people with Parkinson's disease. Gerontology 200147277–281. [DOI] [PubMed] [Google Scholar]
- 30.Bloem B R, Grimbergen Y A, Cramer M.et al Prospective assessment of falls in Parkinson's disease. J Neurol 2001248950–958. [DOI] [PubMed] [Google Scholar]
- 31.Nath U, Ben‐Shlomo Y, Thomson R G.et al Clinical features and natural history of progressive supranuclear palsy: a clinical cohort study. Neurology 200360910–916. [DOI] [PubMed] [Google Scholar]
- 32.Sadigh S, Reimers A, Andersson R.et al Falls and fall‐related injuries among the elderly: a survey of residential‐care facilities in a Swedish municipality. J Community Health 200429129–140. [DOI] [PubMed] [Google Scholar]