Abstract
Objectives
Visuospatial deficits have been occasionally reported but never systematically studied in atypical parkinsonian syndromes. The interpretation of existing studies is complicated by the possible influence of motor and frontal executive deficits. Moreover, no attempt has been made to distinguish visuoperceptual from visuospatial tasks. The aim of the present study was to assess visuoperceptual and visuospatial abilities in three atypical parkinsonian syndromes while minimising the influence of confounding variables.
Methods
Twenty patients with multiple system atrophy (MSA), 43 with progressive supranuclear palsy (PSP), and 25 with corticobasal degeneration (CBD) as well as 30 healthy age matched controls were examined with the Visual Object and Space Perception Battery (VOSP).
Results
Visuospatial functions were intact in MSA patients. PSP patients showed mild deficits related to general cognitive decline and the severity of oculomotor symptoms. The CBD group showed the most pronounced deficits, with spatial tasks more impaired than object based tasks. Performance on object based, but not spatial, tasks was related to general cognitive status. The extent of the visuospatial impairment could not be predicted from disease duration or severity.
Conclusion
Visuospatial functions are not consistently impaired in atypical parkinsonian syndromes. The degree and pattern of impairment varies across the diseases, suggesting that the observed deficits could have a different neural basis in each condition. The distinction between the object based (“ventral stream”) and the space oriented (“dorsal stream”) processing might be useful in the interpretation of visuospatial deficits in parkinsonian syndromes, especially in CBD.
Keywords: progressive supranuclear palsy, multiple system atrophy, corticobasal degeneration, visuospatial functions, cognitive assessment, dorsal and ventral stream
In contrast to the extensive studies of visual cognition in Parkinson's disease and dementia with Lewy bodies,1 little is known about this domain in the atypical parkinsonian syndromes: progressive supranuclear palsy (PSP), multiple system atrophy (MSA), and corticobasal degeneration (CBD). In MSA, some studies reported no significant visuospatial abnormalities,2 while others suggested a decline with the disease progression.3 In PSP, early studies based on the analysis of a small number of patients reported deficits in visual search, scanning, and attention.4,5,6 A recent study comparing PSP with Alzheimer's disease (AD) patients found the prevalence of visuospatial deficits in PSP (60%) to be substantially higher than in AD (38%).7 CBD is often associated with difficulties in visuospatial processing8,9,10 but their frequency and pattern remain unclear.
The assessment of visuospatial functions in atypical parkinsonian syndromes has to overcome a wide range of confounding variables. Rigidity, bradykinesia, tremor, micrographia, ataxia, and apraxia make tasks involving drawing or copying difficult. The psychomotor slowing has an impact on timed tasks, dysarthria, and speech apraxia on verbal responses. Deficits in attention, task switching, and working memory influence the results of any complex testing. While no single test can eliminate all the confounding variables, the Visual Object and Space Perception Battery (VOSP)11 offers a set of tasks that minimise the influence of motor, attentional, mnestic, and executive functions. It also distinguishes between object and space processing, a distinction of considerable anatomical interest since object processing is associated with temporal (“ventral stream”) and space processing with parietal (“dorsal stream”) lobe function.12
Methods
We examined 88 consecutive patients with atypical parkinsonian syndromes (20 with the clinical diagnosis of MSA,13 43 of PSP,14 and 25 of CBD15) and 30 age matched controls recruited through the Medical Research Council (MRC) volunteer panel. The global cognitive status was assessed with Mini‐Mental State Examination (MMSE).16 As PSP and MSA are both associated with imbalance, we assessed their severity using the five level gait/balance staging component from the PSP rating scale.17 In CBD imbalance occurs later, so we based our severity judgement on a four level scale assessing extrapyramidal features and apraxia (1, involvement of one limb; 2, two limbs; 3, all limbs; 4, wheelchair bound). The eye movement abnormalities in PSP were assessed as following: 1, vertical gaze slowing; 2, vertical gaze limitation; 3, vertical gaze paresis, horizontal gaze involvement; 4, complete paresis of vertical and horizontal gaze.18
All patients and controls were administered the VOSP.11 It consists of a visual screening tests and four tests of object recognition and space. The stimuli are presented as black and white pictures and drawings; the required response is single word naming or pointing to the correct item. No drawing or copying is required. We selected three object based (incomplete letters, silhouette naming, and object decision) and three spatial (dot counting, number location, and cube analysis) tasks. We omitted the progressive silhouettes, a test based on two items only and the position discrimination, a subtest with a chance level of 50%, leading to a performance at the ceiling level in the majority of patients. The data were analysed using SPSS (SPSS Inc, Chicago, IL, USA).
Results
There were no significant age differences between the three disease groups and controls: MSA 65.9 (SD 8.2), PSP 69.1 (SD 5.6), CBD 67.7 (SD 7.3), controls 68.2 (SD 7.2). The average disease duration in the MSA group (5.1 (SD 2.8)) was significantly longer than in PSP (3.5 (SD 2.3)) and CBD (3.7 (SD 1.7)) (p<0.05), but the disease severity of MSA and PSP was identical (2.9 (SD 1.0)). In the CBD group, where a different scale was used, the disease severity was 2.3 (SD 0.8). Expressed as the percentage of the highest score the severity of all three diseases was between 57.5 and 58%.
A series of one‐way ANOVAs revealed significant group effects for all subtests (table 1) but with variable patterns of intergroup differences on post hoc pairwise comparisons. There was no significant difference between the controls and the MSA patients on any of the subtests. The PSP patients were impaired in comparison to controls and MSA patients on “silhouette naming” and “number location” only. In addition, they were impaired in comparison to controls on “dot counting”. The CBD group was worse than controls and the MSA group on every subtest except “object decision” and worse than the PSP group on “incomplete letters”, “dot counting”, and “cube analysis”.
Table 1 Group analysis of VOSP subtests (mean and standard deviation) in the three patient groups and in the control group.
| Subtest (max score) | Controls | MSA | PSP | CBD | F |
|---|---|---|---|---|---|
| Incomplete letters (20) | 19.2 (0.8) | 19.4 (0.9) | 17.9 (3.2) | 14.9 (6.5)*†‡ | 7.91 |
| Silhouette naming (30) | 21.4 (2.9) | 20.9 (3.6) | 17.2 (5.7)*† | 14.2 (6.4)*† | 11.45 |
| Object decision (20) | 16.9 (2.3) | 18.6 (2.2) | 17.1 (2.6) | 15.4 (4.3)† | 4.43 |
| Dot counting (10) | 9.9 (0.3) | 9.8 (0.4) | 8.6 (2.2)* | 6.9 (3.1)*†‡ | 13.03 |
| Number location (10) | 8.9 (2.8) | 9.6 (1.1) | 7.1 (2.7)*† | 5.5 (3.9)*† | 8.67 |
| Cube analysis (10) | 9.3 (1.5) | 8.9 (1.8) | 8.0 (2.1) | 5.1 (3.4)*†‡ | 17.4 |
*Impaired relative to controls.
†Impaired relative to MSA patients.
‡Impaired relative to PSP patients.
In all six subtests the differences between the groups are significant with a p<0.01.
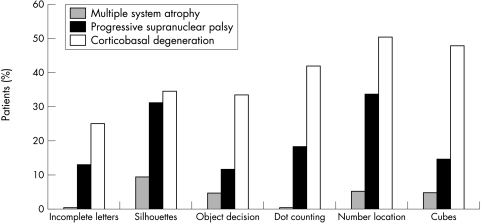
We then categorised the individual patients' scores as impaired, or preserved, relative to the published cut‐off scores for VOSP subtests. The differences between the groups are illustrated in figure 1. In the MSA group the proportion of impaired patients varied between 0 and 5% and never exceeded 10%. The PSP group showed a higher proportion of impaired patients overall, but only for “silhouette naming” and “number location” did this exceed 33%. In the CBD group the percentage of patients impaired varied between 28% and 52% percent, with the spatial tests more often impaired (44%–52%) than the object based (28–38%).
Figure 1 Percentage of patients impaired on VOSP subtests.
Finally, we examined the relation between the clinical data and VOSP performance in the PSP and CBD groups (MSA patients, not impaired in 85% of cases, were not analysed further). In the PSP group a significant correlation was found between the degree of VOSP impairment (number of subtests failed) and the MMSE (rho = 0.480, p = 0.01), disease duration (rho = 0.271, p = 0.04), and severity of eye movement abnormalities (rho = 0.550, p<0.001). In contrast, disease severity did not correlate with VOSP results. MMSE correlated with impairment on both object based (rho = 0.314, p = 0.02) and space based (rho = 0.484, p = 0.001) tests, although the latter correlation was stronger. The degree of eye movement abnormalities also correlated with the performance on object (rho = 0.378, p = 0.006) and space based (rho = 0.330, p = 0.002) tasks. In the CBD group only MMSE (rho = 0.531, p = 0.005), but not disease duration or severity correlated with the VOSP score. However, when examined separately, only the object (rho = 0.603, p = 0.01) but not the space based tasks correlated with MMSE. All other correlations were non‐significant.
Discussion
Our results demonstrate that visuospatial deficits are differentially distributed in atypical parkinsonian syndromes. No visuospatial impairment was detected in the MSA group, which is particularly remarkable given that our sample included patients with long disease duration, high levels of physical disability, and evidence of general cognitive dysfunction (MMSE<23). In neuroanatomical terms our data suggest that extensive basal ganglia pathology, as documented in MSA,19 is not always associated with visuospatial impairment.
The impairment in the PSP group was practically confined to two subtests: “silhouette naming” and “number location”. The first of them was the only subtest requiring naming. As the distorted figures are not immediately recognisable, the usual strategy used by most controls is to produce several possible answers and then select the most likely one. The subtest can, therefore, be influenced by a reduced verbal output and generation of concepts, documented in PSP patients through their pervasive reduction in verbal fluency.7,10 This interpretation is supported by the good performance of the PSP group on “object decision”, a task assessing a similar aspect of visual object processing, but not requiring a verbal response. The other impaired subtest, “number location”, was the only subtest requiring a vertical shift of attention—difficult for PSP patients because of the vertical gaze palsy and the visual scanning deficits preceding it.6 Apart from these two tasks the PSP patients performed remarkably well, including difficult tasks (“cube analysis') sensitive to visuospatial dysfunction in AD.20 The extent of impairment was related to the MMSE, disease duration, and degree of oculomotor impairment but not to overall disease severity. The observed deficits are likely, therefore, to reflect more general cognitive decline, related to the severity of the oculomotor but not motor symptoms.18
By far the greatest impairment was observed in CBD patients. This cannot be sufficiently explained by a general cognitive decline alone: many VOSP impaired patients had normal MMSE. In contrast, normal VOSP results have been documented in the context of a severe dementia21 suggesting that the two tests can dissociate. Moreover, whereas in the PSP group the spatial and object based tasks were equally affected and both correlated with the MMSE score, the spatial deficits in the CBD group were more pronounced and did not correlate with the MMSE. We suggest, therefore, that this pattern reflects an early involvement of the “dorsal stream” with its anatomical substrate in parietal lobe pathology.22 The visuospatial deficits might be further aggravated by impairment in number knowledge.23 Both types of deficits have been associated with parietal lobe dysfunction24 and further studies are needed to determine the exact relation between them.
Acknowledgements
We would like to thank Marion Wilkinson and Margaret Tillson for their help in different stages of the preparation of the manuscript and Angela O'Sullivan for her constant support for patients and their families. Most of this work would not have been possible without the continuous generous support of the PSP Association for the PSP and CBD and Sarah Mathieson Trust for the MSA research.
Abbreviations
AD - Alzheimer's disease
CBD - corticobasal degeneration
MMSE - Mini‐Mental State Examination
MSA - multiple system atrophy
PSP - progressive supranuclear palsy
VOSP - Visual Object and Space Perception Battery
Footnotes
Competing interests: none declared
References
- 1.Mosimann U P, Mather G, Wesnes K A.et al Visual perception in Parkinson disease dementia and dementia with Lewry bodies. Neurology 2004632091–2096. [DOI] [PubMed] [Google Scholar]
- 2.Pillon B, Gouider‐Khouja N, Deweer B.et al Neuropsychological pattern of striatonigral degeneration: comparison with Parkinson's disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 199558174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soliveri P, Monza D, Paridi D.et al Neuropsychological follow up in patients with Parkinson's disease, striatonigral degeneration‐type multisystem atrophy, and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 200069313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisk J D, Goodale M A, Burkhart G.et al Progressive supranuclear palsy: The relationship between ocular motor dysfunction and psychological test performance. Neurology 198232698–705. [DOI] [PubMed] [Google Scholar]
- 5.Kimura D, Barnett H J, Burkhart G. The psychological test patten in progressive supranuclear palsy. Neuropsychologia 198119301–306. [DOI] [PubMed] [Google Scholar]
- 6.Rafal B D, Posner M I, Friedman J H.et al Orienting of visual attention in progressive supranuclear palsy. Brain 1988111267–280. [DOI] [PubMed] [Google Scholar]
- 7.Bak T H, Crawford L M, Hearn V C.et al Subcortical dementia revisited: similarities and differences in cognitive function between progressive supranuclear palsy (PSP), corticobasal degeneration (CBD) and multiple system atyrophy (MSA). Neurocase 200511268–273. [DOI] [PubMed] [Google Scholar]
- 8.Halpern C, McMillan C, Moore P.et al Calculation impairment in neurodegenerative diseases. J Neurol Sci 200320831–38. [DOI] [PubMed] [Google Scholar]
- 9.Mathuranath P S, Xuereb J H, Bak T.et al Corticobasal ganglionic degeneration and/or frontotemporal dementia? A report of two overlap cases and review of literature. J Neurol Neurosurg Psychiatry 200068304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soliveri P, Monza D, Paridi D.et al Cognitive and magnetic resonance imaging aspects of corticobasal degeneration and progressive supranuclear palsy. Neurology 199953502–507. [DOI] [PubMed] [Google Scholar]
- 11.Warrington E K, James M.The Visual Object and Space Perception Battery. Bury St Edmunds: Thames Valley Test Company, 1991
- 12.Goodale M A, Milner A D. Separate visual pathways for perception and action. Trends Neurosci 19921520–25. [DOI] [PubMed] [Google Scholar]
- 13.Gilman S, Low P A, Quinn N P.et al Concensus statement on the diagnosis of multiple system atrophy. J Auton Nerv Syst 199874189–192. [PubMed] [Google Scholar]
- 14.Litvan I, Agid Y, Jankovic J.et al Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele‐Richardson‐Olzewski syndrome). Neurology 199646922–930. [DOI] [PubMed] [Google Scholar]
- 15.Riley D E, Lang A E. Clinical diagnostic criteria. In: Litvan I, Goetz CG, Lang AE, eds. Corticobasal degeneration. Philadelphia: Lippincott, Williams & Wilkins, 200029–34.
- 16.Folstein M F, Folstein S E, McHugh P R. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 17.Golbe L, Lepore F, Johnson W.et al Inter‐rater reliability of the progressive supranuclear palsy scale. Neurology 199952A227 [Google Scholar]
- 18.Esmonde T, Giles E, Gibson M.et al Neuropsychological performance, disease severity and depression in progressive supranuclear palsy. J Neurol 1996243638–643. [DOI] [PubMed] [Google Scholar]
- 19.Papp M I, Lantos P L. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain 1994117235–243. [DOI] [PubMed] [Google Scholar]
- 20.Caine D, Hodges J R. Heterogeneity of semantic and visuospatial deficits in early Alzheimer's disease. Neuropsychology 200115155–164. [PubMed] [Google Scholar]
- 21.Bak T H, O'Donovan D G, Xuereb J H.et al Selective impairment of verb processing associated with pathological changes in the Brodmann areas 44 and 45 in the motor neurone disease/dementia/aphasia syndrome. Brain 2001124103–124. [DOI] [PubMed] [Google Scholar]
- 22.Tang‐Wai D F, Josephs K A, Boeve B F.et al Pathologically confirmed corticobasal degeneration presenting with visuospatial dysfunction. Neurology 2003611134–1135. [DOI] [PubMed] [Google Scholar]
- 23.Halpern C H, Glosser G, Clark R.et al Dissociation of numbers and objects in corticobasal degeneration and semantic dementia. Neurology 2004621163–1169. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard E M, Piazza M, Pinel P.et al Interactions between number and space in parietal cortex. Nat Rev Neurosci 20056435–448. [DOI] [PubMed] [Google Scholar]