Abstract
A late onset axonal Charcot‐Marie‐Tooth phenotype is described, resulting from a novel mutation in the myelin protein zero (MPZ) gene. Comparative computer modelling of the three dimensional structure of the MPZ protein predicts that this mutation does not cause a significant structural change. The primary axonal disease process in these patients points to a function of MPZ in maintenance of the myelinated axons, apart from securing stability of the myelin layer.
Keywords: Charcot‐Marie‐Tooth disease, myelin protein zero gene, MPZ
Charcot‐Marie‐Tooth disease (CMT) is clinically and genetically heterogeneous. Patients are categorised in the demyelinating (CMT1) or the axonal form (CMT2). Patients with CMT1 have motor nerve conduction velocities (MNCV) of the median nerve below 38 m/s, whereas those with CMT2 have median nerve MNCV faster than 38 m/s. Mutations in two Schwann cell expressed myelin genes—the myelin protein zero gene (MPZ) and the Connexin 32 gene (CX32)—may show mainly axonal changes both electrophysiologically and pathologically.1 A similar discrepancy between genotype and expected phenotype may occur with mutations in the neurofilament light gene (NEFL), a gene expressed in neurones, as NEFL mutations are often associated with nerve conduction velocities in the demyelinating range.
MPZ is the major protein component of peripheral nervous system myelin, composed of an extracellular, an intracellular, and a transmembrane domain. The extracellular domain is an immunoglobulin‐like domain which plays an important role in compaction and maintenance of the peripheral myelin.2MPZ mutations are associated with CMT1, Dejerine‐Sottas syndrome (DSS), congenital hypomyelination syndrome (CH), and CMT2.1
We report a novel point mutation in the extracellular domain of the MPZ gene in two families with axonal CMT.
Methods
Patients
In a cohort of 20 families with an axonal CMT phenotype, one family with a new MPZ mutation was identified. This mutation was present in several asymptomatic adult patients. Subsequently, the same mutation was found in a second CMT2 family. We obtained medical records of relatives with a CMT phenotype of the first family and undertook DNA analysis, which confirmed the MPZ mutation in the extended family.
Molecular genetic analysis
The genes for PMP22, MPZ, and CX‐32 were analysed for mutations by single strand confirmation polymorphism analysis (SSCP). The whole coding region of these genes was polymerase chain reaction (PCR) amplified from genomic DNA and screened for altered mobility on SSCP gels. Gels with 0% and 10% glycerol were used for analysis. Fragments with aberrant migration were sequenced with BigDye terminator chemistry.
Sural nerve biopsy
Sural nerve biopsy specimens from two affected family members (one from each family) were available. Sural nerve biopsies were prepared for light and electron microscopic examination using standard techniques. The cluster ratio, defined as the number of clusters per 1000 myelinated fibres, was assessed by counting the number of clusters (three or more closely packed myelinated fibres) on the electron microscopic prints, divided by the number of myelinated fibres × 1000 in the same prints.
Results
Patients
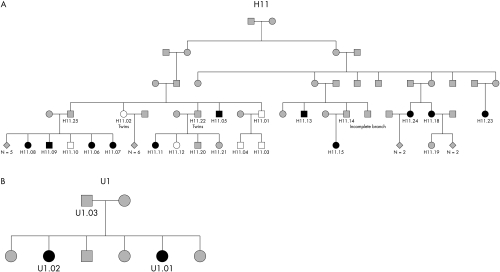
Clinical and electrophysiological data are listed in table 1. The pedigrees are shown in fig 1. Apart from H11.22 (father of H11.11), the Tyr82His mutation was confirmed in all patients. The phenotype is mild in most affected individuals and apart from a 34 year old female patient (H11.15) family members younger than 41 years were asymptomatic. Note that patient H11.11 did not have any complaints such as weakness, walking difficulty, or sensory symptoms and is therefore marked as asymptomatic, although clinical examination revealed minor weakness of toe flexor and extensor muscles and distal sensory abnormalities. Only one patient (H11.05) used a cane and boots for walking. Foot deformities, including pes cavus, pes planus, or claw toes, were found in six patients. Pes cavus was present in one asymptomatic patient only. There was no deafness co‐segregating with the disease. Pupillary function was normal and lancinating pain was not mentioned.
Table 1 Clinical and electrophysiological findings of the affected patients from the two families with a Tyr82His mutation in the MPZ gene.
| Age (y) (sex) | Age at onset (y) | Initial symptoms | Weakness/deformity | Sensory signs | Reflexes | NCV motor | NCV sensory | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Ulnar | Peroneal L/R | Median | Ulnar | Sural | |||||||
| H11.05 | 63 (M) | 46 | Back pain | ULd 4 LL 0–3/pes planus | UL+ LL+ * | Areflexia | 34 | 50 | NR/NR | NR | 48 | NR |
| H11.06 | 32 (F) | nr | AS | –/pes cavus | – | Normal | 46 | 50 | 41/31 | 46 | 48 | 37 |
| H11.07 | 26 (F) | nr | AS | – | – | Normal | 55 | 56 | 44/44 | 54 | 55 | 39 |
| H11.08 | 40 (F) | nr | AS | –/pes planus | – | Normal | 55 | 59 | 34/41 | 50 | 53 | 36 |
| H11.09 | 35 (M) | nr | AS | – | – | Normal | 50 | 54 | 38/37 | 47 | 50 | 35 |
| H11.11 | 31 (F) | nr | AS | UL ‐ LLd 4/pes planus | UL ‐ LL + | Reduced ankle | 48 | 48 | 25/28† | 45 | 41 | 32 |
| H11.13 | 73 (M) | 45 | NE | Steppage gait | NE | NE | NE | NE | NE | NE | NE | NE |
| H11.15 | 34 (F) | ? | Legs: S; pain feet | – | – | Normal | NE | 54 | 34/36 | NE | 65 | 39 |
| H11.18 | 43 (F) | 35 | Legs: WD; S | UL ‐ LLd 4 | UL ‐ LL + * | Absent ankle | 50 | 60 | NR/NR | NR | ||
| H11.22 | 51(M) | 45 | Legs: WD; M and S | ULd 4 LL 0–3 | UL ‐ LL + * | Absent ankle | 39 | 48 | ||||
| H11.23 | 53 (F) | ? | Legs: WD | UL 4 LLd 4 | UL ‐ LL + | Absent ankle, knee | 34 | 48 | NE | NE | NE | NE |
| H11.24 | 61 (F) | 55 | Legs: WD; pain | UL ‐ LLd 4/pes planus | UL ‐ LL + * | Absent ankle | 42 | 57 | NE/40† | NE | NE | 51 |
| U1.01 | 44 (F) | 36 | Legs S | –/claw toes | UL ‐ LL + | Reduced ankle | 49 | 57 | 32/34 | NE | 48 | |
| U1.02 | 51 (F) | 47 | Legs S | – | UL ‐ LL + * | Absent ankle, knee | 45 | 52 | 20†/41† | NE | 46 | 55 |
NCV, figures in bold: associated with decreased compound muscle action potential (CMAP) or sensory nerve action potential (SNAP).
†CMAP amplitude <1 mV.
H11.22 (father of H11.11) is included in the table because he was clearly affected and an obligate gene carrier. However, DNA was not available. H11.14, a 64 year old man, also an obligate carrier (father of H11.15), had CMT symptoms from age 45 years, but also a history of alcohol abuse and was therefore excluded. H11.15 complained of pain in her feet and back and altered sensation under her feet, but she had no signs of Charcot‐Marie‐Tooth disease (CMT). Electrophysiological examination showed slightly reduced motor nerve conduction velocities in the legs and a decreased abductor hallucis brevis compound muscle action potential.
Additional family members from whom DNA was not available who are not included in the table: a 66 year old woman (H11.16) with a mainly sensory neuropathy in the legs, who stated that symptoms began after aorto‐iliac segment vascular surgery, and her 40 year old daughter (H11.17) who was asymptomatic but had minor sensory signs in the feet, atrophy of both extensor digitorum brevis muscles and minor electrophysiological abnormalities. H11.25 (father of H11.07 and H11.08) had CMT symptoms starting in the sixth decade, but died from amyotrophic lateral sclerosis. In family U1 the father was believed to be affected as he experienced difficulty walking from the age of 50; a medical record was not available.
AS, asymptomatic; (F), female; L, left; M, motor; (M), male; NE, not examined; R, right; S, sensory; LL, lower limbs; NCV, nerve conduction velocity (for median and ulnar nerve, calculated between elbow and wrist; for peroneal nerve, between fibular head and ankle; for sural nerve, from calf to lateral malleolus); nr, not relevant; NR, no response; UL, upper limbs; WD, walking difficulty; y, years
Motor signs: d, distal; MRC gradings; –, absent.
Sensory signs: *including abnormal pain sensation; +, present; –, absent.
Figure 1 Pedigrees H11 (incomplete pedigree, only relevant individuals are shown) and U1. A more extensive pedigree H11 is provided as online supplementary material. Squares: males; circles: females. Black, mutation present; white, mutation absent; grey, mutation unknown (not tested).
The mean median nerve motor conduction velocity (MNCV) was 46 m/s and the mean ulnar nerve MNCV was 54 m/s. Median nerve MNCV was slower in symptomatic patients (mean 42 m/s) than in asymptomatic patients (mean 51 m/s) and was in the demyelinating range in two. Needle EMG of leg muscles showed spontaneous muscle fibre activity in three symptomatic patients, reinnervation signs in all but one patient (H11.08), and a reduced recruitment pattern in seven patients.
Molecular genetic analysis
An abnormal migration pattern was observed for the PCR fragment of exon 3 of the MPZ gene. The sequence of the fragment containing exon 3 on the MPZ gene was determined by dideoxy sequencing and a c.T244C substitution was found, resulting in a p.Tyr82His substitution. This DNA sequence alteration was not found in over 200 unrelated control chromosomes tested.
Computer model prediction by SWISS‐Model, based on the information of the crystal structure of MPZ protein, showed that the Tyr82His mutation does not cause a significant change in the tertiary structure of the MPZ protein.3
Sural nerve biopsy
Sural nerve biopsies were undertaken in patient H11.05 at the age of 46 years and in patient U01.02 at the age of 52. Both showed a marked loss of myelinated fibres (48% and 53%, respectively, of age matched control values), predominantly large diameter myelinated fibres (fibres >8 μm amounted to 15% and 4%, respectively, of age matched control values). Several clusters of regenerated myelinated axons were present. Cluster ratios were 29.7 and 50.2, respectively. No active axonal degeneration was observed, nor were signs of segmental demyelination, remyelination, or onion bulb formation. Electron microscopy revealed no structural abnormalities of myelin, Schwann cells, unmyelinated axons, or axon cytoskeleton.
Discussion
Up to now, over 100 different mutations in the MPZ gene have been identified, from which approximately 10–15% show an axonal phenotype (Inherited Neuropathies Mutation Database: http://www.molgen.ua.ac.be/CMTMutations/default.cfm). How mutations in the MPZ gene cause mainly axonal injury is as yet not understood. We describe two families with a late onset axonal CMT phenotype caused by a Tyr82His mutation in the MPZ gene. Associated features such as deafness, lancinating pains, and pupillary abnormalities that were described in several axonal MPZ gene mutations were not present in our patients.4,5 Sensory symptoms seem to be more prominent in late onset axonal CMT caused by MPZ mutations.6 In our patients symptoms at onset were often sensory, but positive sensory features were not prominent. In all reports regarding an axonal phenotype associated with MPZ mutations, disease onset is in or beyond the fourth decade, as in our families.1,6 Nearly all family members below age 40 in our study are in the presymptomatic stage. Electrophysiological examination revealed only minor abnormalities in these asymptomatic individuals and therefore cannot reliably be used for case ascertainment in this stage of the disease. The rare occurrence of pes cavus is in line with an actual disease onset beyond the first years of life. The mild phenotype, together with the lack of pes cavus in most patients with this mutation, may cause confusion with acquired neuropathy if the hereditary nature of the disease is not recognised.
The Tyr82His mutation in the MPZ gene has not been reported previously. However, mutations of tyrosine 82 have been described in CMT1 and DSS patients with onset in (early) childhood and very slow MNCVs.7,8,9 In these cases, the tyrosine 82 was substituted for by cystein. The difference in phenotype can be attributed to the nature of the mutation. The replacement of tyrosine by cysteine in the extracellular part of the protein will have a large impact on the tertiary structure and thus on myelin structure and function owing to the introduction of novel disulphide bridges. In case of a tyrosine to histidine mutation, modelling studies predicted that our Tyr→His mutation does not cause a significant structural change, which is in line with the lack of primary demyelinating features in our patients. Instead, nerve conduction studies were compatible with a diagnosis of CMT2 in most patients. The electrophysiological findings in our patients and the absence of a primary demyelinating process in two nerve biopsies indicate that axonal degeneration and regeneration is the primary pathophysiological basis of the CMT phenotype caused by the Tyr82His mutation. This suggests a function of MPZ in the maintenance of myelinated axons, apart from securing stability of the myelin layer.
Acknowledgements
We thank J P Vreijling for DNA sequence analysis and J Bekker for the pedigree drawings. This study was supported by funds from the Prinses Beatrix Fonds, The Hague.
Abbreviations
CMT - Charcot‐Marie‐Tooth disease
DSS - Dejerine‐Sottas syndrome
MNCV - motor nerve conduction velocity
SSCP - single strand confirmation polymorphism analysis
Footnotes
Competing interests: none declared
References
- 1.Shy M E, Jani A, Krajewski K.et al Phenotypic clustering in MPZ mutations. Brain 2004127371–384. [DOI] [PubMed] [Google Scholar]
- 2.Lemke G. The molecular genetics of myelination: an update. Glia 19937263–271. [DOI] [PubMed] [Google Scholar]
- 3.Schwede T, Kopp J, Guex N.et al SWISS‐MODEL: an automated protein homology‐modeling server. Nucleic Acids Res 2003313381–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapon F, Latour P, Diraison P.et al Axonal phenotype of Charcot‐Marie‐Tooth disease associated with a mutation in the myelin protein zero gene. J Neurol Neurosurg Psychiatry 199966779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jonghe P, Timmerman V, Ceuterick C.et al The Thr124Met mutation in the peripheral myelin protein zero (MPZ) gene is associated with a clinically distinct Charcot‐Marie‐ Tooth phenotype. Brain 1999122281–290. [DOI] [PubMed] [Google Scholar]
- 6.Auer‐Grumbach M, Strasser‐Fuchs S, Robl T.et al Late onset Charcot‐Marie‐Tooth 2 syndrome caused by two novel mutations in the MPZ gene. Neurology 2003611435–1437. [DOI] [PubMed] [Google Scholar]
- 7.Himoro M, Yoshikawa H, Matsui T.et al New mutation of the myelin P0 gene in a pedigree of Charcot‐Marie‐Tooth neuropathy 1. Biochem Mol Biol Int 199331169–173. [PubMed] [Google Scholar]
- 8.Silander K, Meretoja P, Juvonen V.et al Spectrum of mutations in Finnish patients with Charcot‐Marie‐Tooth disease and related neuropathies. Hum Mutat 19981259–68. [DOI] [PubMed] [Google Scholar]
- 9.Boerkoel C F, Takashima H, Garcia C A.et al Charcot‐Marie‐Tooth disease and related neuropathies: mutation distribution and genotype‐phenotype correlation. Ann Neurol 200251190–201. [DOI] [PubMed] [Google Scholar]