Abstract
Background
Peripheral neuropathy is present in 65% of patients with end stage kidney disease (ESKD) starting dialysis. Studies of membrane potential and axonal ion channel function may help explain the pathophysiology.
Objectives
To follow changes in median sensory axon excitability in patients with ESKD treated with haemodialysis, and correlate them with clinical rating scales and serum levels of potential neurotoxins.
Methods
Sensory nerve action potentials were recorded from the second digit following stimulation of the median nerve in 12 ESKD patients. Stimulus–response behaviour using two stimulus durations, threshold electrotonus to 100 ms polarising currents, a current–threshold relation, and recovery of excitability following supramaximal stimulation were recorded before, during, and after haemodialysis. Serum concentrations of potential neurotoxins were measured.
Results
Before dialysis, there were changes in nerve excitability consistent with axonal depolarisation: refractoriness was increased; superexcitability and depolarising threshold electrotonus were reduced. Following dialysis there were improvements in all indices, with correlations between excitability abnormalities and serum potassium measurements. Neuropathic symptoms correlated with excitability changes.
Conclusions
Nerves are depolarised before haemodialysis in ESKD patients. The correlation of excitability abnormalities with potassium indicates that the achievement of normokalaemia should be a priority in treating such patients.
Keywords: membrane potential, nerve excitability, threshold electrotonus, uraemic neuropathy
Neuropathy in end stage kidney disease (ESKD) presents as a symmetrical length dependent process with predominant sensory involvement.1 The pathophysiology of uraemic neuropathy has not been established, although its development has been attributed to retention of “middle molecules” (300–12 000 Daltons).2 Although this hypothesis has not been proven, the concept of a dialysable neurotoxin remains prevalent.3
Assessment of nerve excitability provides information about the biophysical properties of nodal and internodal axonal function.4,5,6 Previous studies of motor nerve excitability in ESKD7,8 have shown pre‐dialysis abnormalities consistent with axonal depolarisation, with resolution of these changes following dialysis. Given the predominantly sensory nature of uraemic neuropathy, our aim in the present study was to explore further the pathophysiology of uraemic neuropathy by recording the sensory nerve excitability properties in ESKD patients before, during, and after dialysis, and to correlate excitability changes with clinical features and levels of potential neurotoxins.
Methods
Consecutive patients with ESKD were studied (eight men and four women, aged 17 to 70 years, mean age 53.5 years). All were receiving haemodialysis. None of the patients had a history of other illnesses known to cause neuropathy. Patients gave informed consent to all procedures, which were approved by our regional and institutional review boards.
Neurological history and examination were undertaken before dialysis, and neuropathic symptoms were graded using subsets IA, IIA, and IIB of the neuropathy symptom score (NSS).9 Each symptom received a score of 1, and the number of symptoms present in each subset was summed to give a total neuropathy symptom score (T‐NSS). The maximum possible T‐NSS was 9.
Routine nerve conduction studies (NCS) were undertaken in all patients on the sural, tibial, common peroneal, and superficial radial nerves using a Medelec Synergy system (Oxford Instruments, High Wycombe, UK) and standard techniques.10 The severity of neuropathy in the present study was staged using a modified form of a previously devised system11:
stage 0, no neuropathy (T‐NSS <2 with normal NCS);
stage 1, asymptomatic neuropathy (T‐NSS = 0 with abnormalities on NCS);
stage 2, symptomatic neuropathy (T‐NSS >2 with normal NCS or T‐NSS ⩾1 with abnormal NCS; neuropathic symptoms non‐disabling);
stage 3, disabling neuropathy (T‐NSS ⩾2 with normal NCS or T‐NSS ⩾1 with abnormal NCS; neuropathic symptoms reported to be disabling.
Disability was defined as a curtailment in recreational activities, absence of feeling in the hand or inability to walk independently.11
Excitability properties of median nerve sensory axons were measured before, during, and one hour after a standard five hour haemodialysis session. Serum electrolytes, urea, creatinine, parathyroid hormone, and β2‐microglobulin were measured at the time of the excitability studies. Kt/V, a urea based measure of dialysis adequacy which relates the clearance of urea to its distribution volume,12 was also calculated.
Median nerve sensory excitability studies were carried out using a previously described technique.5 The following excitability parameters were recorded: stimulus–response behaviour using two stimulus durations; threshold electrotonus to 100 ms polarising currents (a marker of internodal axonal membrane function); a current–threshold relation (a measure of an inwardly rectifying K+ conductance); refractoriness (owing to inactivation of nodal transient Na+ channels); superexcitability (dependent on paranodal fast K+ channels); and late subexcitability (determined in part by nodal slow K+ channels).
Abnormalities of nerve conduction and excitability were established by comparing the results with normative data from our unit5,13 and other centres.14,15,16,17 In the case of excitability studies, individual measurements were corrected for age and temperature before statistical tests were applied, using relations established by normative data.5,18 Single comparisons of excitability parameters were analysed using Student's unpaired t tests for comparisons with normative data (n = 50, age range 18 to 61, mean 36 years) and paired t tests for comparisons before and after dialysis. Correlations were analysed using Pearson's correlation coefficient. A probability (p) value of <0.05 was considered statistically significant. Results are expressed as mean (SEM).
Results
The complete excitability protocol could not be completed in two of the 12 ESKD patients because sensory nerve action potentials (SNAP) amplitudes were too small for threshold tracking. All 12 patients reported symptoms of neuropathy (mean T‐NSS, 1.9 (0.2)). Sensory symptoms were present in all patients, predominantly paraesthesiae and numbness, while six experienced motor symptoms. Four patients described pain, a symptom of small fibre dysfunction. According to the neuropathy staging system (above), 8% had no evidence of neuropathy, 58% had stage 2, and 33% had stage 3. In keeping with sensory predisposition of uraemic neuropathy, sural SNAP amplitude13 was the most commonly affected NCS parameter, reduced in 75% of patients (to a mean of 6.0 (1.2) μV), with relative preservation of conduction velocity (mean velocity, 42.6 (0.9) ms−1), consistent with axonal neuropathy.
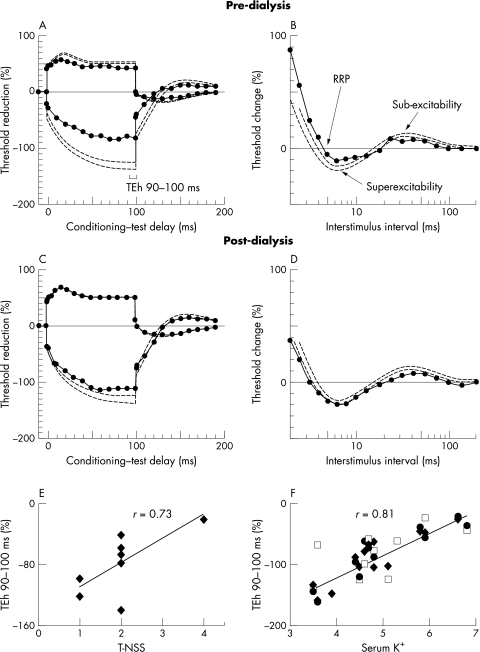
Excitability recordings in patients before dialysis showed significant abnormalities. Maximum SNAP amplitude, measured peak to peak,5 was significantly reduced (p<0.00005)—accompanied by a shift to the left of stimulus–response curves, consistent with axonal depolarisation.19 Depolarising threshold electrotonus (TEd) at the 90–100 ms interval (TEd 90–100) was lower in ESKD patients than in controls (p<0.0005), as was hyperpolarising threshold electrotonus (TEh) at the same time interval (p<0.00005), leading to a “fanned in” appearance of the threshold curve (fig 1A).19,20,21 Changes in threshold electrotonus were also noted in TEd 10–20 ms (p<0.00005), TEd 40–60 ms (p<0.0005), and TEh 10–20 ms (p<0.00005). During the recovery cycle (fig 1B), refractoriness was significantly greater in ESKD patients (p<0.005) and this was accompanied by a prolongation of the relative refractory period (p<0.05). Superexcitability and late subexcitability were both reduced in pre‐dialysis recordings (p<0.05 and 0.005, respectively). There was no significant difference in strength–duration properties between EKSD patients and controls.
Figure 1 Comparison of pre‐dialysis threshold electrotonus (A) and recovery cycle (B) in patients with end stage kidney disease (ESKD) (continuous lines with circles) with post‐dialysis recordings (C, D). Broken lines represent 95% confidence intervals for normal controls. The conditioning–test interval corresponding to hyperpolarising threshold electrotonus at 90–100 ms (TEh 90–100 ms) is depicted in (A). Pre‐dialysis traces demonstrate “fanning in” of threshold electrotonus, a prolonged relative refractory period, and reduced superexcitability and late subexcitability. Abnormalities had largely resolved by one hour post‐dialysis. (E) Relation between pre‐dialysis TEh 90–100 ms and total neuropathy symptom score for sensory nerves in ESKD patients. (F) Pre‐dialysis TEh 90–100 ms and K+ for pooled data from median sensory studies (empty square) and lower limb motor studies8 (recorded from tibialis anterior, filled diamond, and extensor digitorum brevis, filled circle). The correlations represented in (E) and (F) were significant at the 0.05 level. TEh, hyperpolarising threshold electrotonus; RRP, relative refractory period; T‐NSS, total neuropathy symptom score.
Excitability recordings undertaken one hour after completion of dialysis showed significant improvement in excitability parameters (table 1; fig 1 panels C and D). Persistent abnormalities were, however, noted in TEd 40–60 ms (p = 0.06), TEh 10–20 ms (p<0.005), TEh 90–100 ms (p<0.05), and late subexcitability (p<0.0005), indicating that haemodialysis failed to restore nerve excitability to normal completely.
Table 1 Excitability data for end stage kidney disease patients and controls.
| Pre‐dialysis | During dialysis | Post‐dialysis | Normal controls | |
|---|---|---|---|---|
| TEd 90–100 ms | 41.1 (2.6)%*** | 49.3 (2.6)%*** | 50.7 (2.1)% | 50.6 (0.9)% |
| TEh 90–100 ms | −82.7 (11.5)%*** | −110.4 (11.5)%*** | −111.4 (9.5)% | −131.9 (3.2)% |
| TEd 40–60 ms | 43.6 (3.2)%*** | 50.9 (2.1)%*** | 48.5 (1.3)% | 51.4 (0.7)% |
| TEd 10–20 ms | 55.0 (3.7)%*** | 60.8 (1.2)%*** | 65.2 (1.5)% | 65.9 (0.5)% |
| TEh 10–20 ms | −51.3 (6.4)%*** | −56.4 (7.6)%*** | −72.4 (5.0)% | −84.6 (1.2)% |
| RRP | 4.2 (0.4) ms*** | 4.5 (0.8) ms*** | 2.7 (0.1) ms | 3.6 (0.1) ms |
| Superexcitability | −13.1 (2.3)%*** | −16.2 (2.3)%*** | −18.2 (2.3)% | −17.2 (0.7)% |
| Late subexcitability | 7.4 (1.5%)*** | 6.9 (2.0%)** | 6.1 (1.2)% | 12.0 (0.5)% |
Mean excitability values before, during and after dialysis, with normal control data.5 Data are shown for threshold electrotonus and recovery cycle parameters. TEd and TEh are represented for different time intervals as stated.
Data are expressed as mean (SEM). Pre‐dialysis and during dialysis measures were compared with post‐dialysis values using Student's paired t test.
*p<0.05; **p<0.005; ***p<0.0005.
RRP, relative refractory period; TEd, depolarising threshold electrotonus; TEh, hyperpolarising threshold electrotonus.
A significant correlation was noted between T‐NSS and both pre‐dialysis subexcitability (r = 0.79, p<0.01) and TEh 90–100 ms (r = 0.73, p<0.01), a sensitive indicator of membrane potential (fig 1E), suggesting that patients with greater neuropathic symptoms showed more pronounced changes in membrane excitability before dialysis. Correlation analysis was also undertaken to investigate the relation between neurophysiological variables and the levels of potential neurotoxins. There was close correlation in pre‐dialysis recordings between serum K+ and both threshold electrotonus and late subexcitability. The correlation with TEd 90–100 ms was further strengthened when post‐dialysis data were included in the analysis (r = 0.73, p<0.01). When sensory data from the present study were pooled with results obtained previously on pre‐dialysis changes in lower limb motor nerves,8 there remained a close correlation between K+ and both TEh 90–100 ms (r = 0.81, p<0.05; fig 1F) and TEd 90–100 ms (r = 0.76, p<0.05). There was no correlation between parameters from standard NCS with symptom severity or serum K+.
The only other potential toxin which correlated well with pre‐dialysis excitability parameters was urea (TEd 40–60 ms, r = 0.86, p<0.01; TEd 90–100 ms, r = 0.76, p<0.05). Urea concentration, however, also correlated well with pre‐dialysis K+ (r = 0.90, p<0.01). No significant correlation was noted between Kt/V, a urea based measure of dialysis adequacy, and excitability parameters. Critically, all patients had Kt/V greater than 1.2, in keeping with current guidelines of dialysis adequacy.12
Sensory excitability parameters from the present study were compared with results of a previous study of median motor nerve excitability.7 While no differences were noted between sensory and motor axons with respect to parameters of threshold electrotonus, refractoriness, or superexcitability, late subexcitability was significantly lower in motor axons than sensory axons (motor studies, 4.5 (1.8)%; sensory studies, 9.1 (1.2)%, p<0.05), although the mean pre‐dialysis serum K+ was almost identical in the two groups (motor studies, 5.0 (0.2) mmol/l; sensory studies, 4.9 (0.3) mmol/l)).
Discussion
The present study confirms that sensory axons are depolarised in ESKD patients before dialysis. Specifically, there was fanning in of threshold electrotonus with increases in refractoriness and the duration of the relative refractory period, and attenuation of superexcitability, all consistent with membrane depolarisation.21 Correlation was noted between clinical symptoms and excitability parameters. Patients with a greater number of neuropathic symptoms showed more severe changes in these excitability parameters. These findings suggest a direct relation between excitability abnormalities and neuropathic symptoms, and mirror the results for lower limb motor nerves.8
Of the potential neurotoxins, K+ and urea correlated most closely with excitability parameters. The correlations with threshold electrotonus indices were further strengthened following inclusion of pre‐dialysis excitability results from lower limb motor nerves.8 Given the dependence of membrane potential on the concentration gradient for K+,3 alterations in K+ concentration would be likely to produce abnormalities of membrane potential. In contrast, there is no evidence to suggest that urea exerts any effect on membrane potential.
While the serum K+ abnormalities noted in the present study improved following haemodialysis, such improvement is likely to be transient. Following dialysis, there is rebound of K+ in ESKD patients, which typically occurs within six hours of dialysis owing to re‐equilibration between the intracellular and the extracellular compartments.22 Such prolonged hyperkalaemia may cause disruption of normal ionic gradients essential for axonal survival, thereby leading to accumulation of Ca+ within the cell and subsequent axonal degeneration.23
The paradoxical reduction in late subexcitability rather than the expected increase with membrane depolarisation19 mirrors the findings noted in median motor axons in ESKD patients.7 Late subexcitability, which is determined by activation of nodal slow K+ channels and the difference between membrane potential (Er) and potassium equilibrium potential (Ek), increases with axonal depolarisation if extracellular K+ remains unchanged.19 Raised extracellular K+, as may occur in ESKD patients, will lead to a reduction in (Er−Ek) and thereby decreased subexcitability. There was a significantly greater reduction in late subexcitability in median nerve motor axons7 than in sensory axons, despite similar pre‐dialysis mean K+ concentrations. This suggests that raised K+ has greater effects on motor axons than on sensory axons, a difference that has been noted in previous in vitro studies.24,25 This apparently paradoxical reduction in subexcitability suggests that hyperkalaemia is driving axonal depolarisation. Overall, the present study on sensory axons in ESKD, combined with previous studies in motor axons,7,8 provides clear evidence that the achievement of normokalaemia3 rather than just the avoidance of hyperkalaemia should become a priority in the treatment of patients with ESKD to avoid derangement in nerve excitability, especially in those patients with incipient neuropathy.
Acknowledgements
AK was supported by the Australian Association of Neurologists Research Fellowship and the National Health and Medical Research Council of Australia. Grant support from the Brain Foundation, the Sylvia and Charles Viertel Charitable Foundation, and the Ramaciotti Foundation is gratefully acknowledged.
Abbreviations
ESKD - end stage kidney disease
Kt/V - ratio of clearance of urea to distribution volume
NCS - nerve conduction study
NSS - neuropathy symptom score
RRP - relative refractory period
SNAP - sensory nerve action potential
TEd - depolarising threshold electrotonus
TEh - hyperpolarising threshold electrotonus
T‐NSS - total neuropathy symptom score
Footnotes
Competing interests: none declared
References
- 1.Bolton C F. Peripheral neuropathies associated with chronic renal failure. Can J Neurol Sci 1980789–96. [DOI] [PubMed] [Google Scholar]
- 2.Babb A L, Popovich R P, Christopher T G.et al The genesis of the square meter‐hour hypothesis. Trans Am Soc Artif Intern Org 19711781–91. [PubMed] [Google Scholar]
- 3.Bostock H, Walters R J, Andersen K V.et al Has potassium been prematurely discarded as a contributing factor to the development of uraemic neuropathy? Nephrol Dial Transplant 2004191054–1057. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan A V, Lin C S‐Y, Kiernan M C. Nerve excitability properties in lower limb motor axons: evidence for a length‐dependent gradient. Muscle Nerve 200429645–655. [DOI] [PubMed] [Google Scholar]
- 5.Kiernan M C, Lin C S, Andersen K V.et al Clinical evaluation of excitability measures in sensory nerve. Muscle Nerve 200124883–892. [DOI] [PubMed] [Google Scholar]
- 6.Kiernan M C, Burke D, Andersen K V.et al Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve 200023399–409. [DOI] [PubMed] [Google Scholar]
- 7.Kiernan M C, Walters R J, Andersen K V.et al Nerve excitability changes in chronic renal failure indicate membrane depolarization due to hyperkalaemia. Brain 20021251366–1378. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan A V, Phoon R K, Pussell B A.et al Altered motor nerve excitability in end‐stage kidney disease. Brain 20051282164–2174. [DOI] [PubMed] [Google Scholar]
- 9.Dyck P J, Sherman W R, Hallcher L M.et al Human diabetic endoneurial sorbitol, fructose, and myo‐inositol related to sural nerve morphometry. Ann Neurol 19808590–596. [DOI] [PubMed] [Google Scholar]
- 10.Kimura J.Electrodiagnosis in diseases of nerve and muscle. Philadelphia: FA Davis, 1983
- 11.Dyck P J. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle Nerve 19881121–32. [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation NKF‐DOQI clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis 199730(suppl 2)S15–S66. [DOI] [PubMed] [Google Scholar]
- 13.Burke D, Skuse N F, Lethlean A K. Sensory conduction of the sural nerve in polyneuropathy. J Neurol Neurosurg Psychiatry 197437647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buschbacher R M. Tibial nerve motor conduction to the abductor hallucis. Am J Phys Med Rehabil 199978S15–S20. [DOI] [PubMed] [Google Scholar]
- 15.Puksa L, Stalberg E, Falck B. Reference values of F wave parameters in healthy subjects. Clin Neurophysiol 20031141079–1090. [DOI] [PubMed] [Google Scholar]
- 16.Ma D M, Kim S H, Spielholz N.et al Sensory conduction study of distal radial nerve. Arch Phys Med Rehabil 198162562–564. [PubMed] [Google Scholar]
- 17.Ma D M, Liveson J A.Nerve conduction handbook. Philadelphia: FA Davis, 1983
- 18.Kiernan M C, Cikurel K, Bostock H. Effects of temperature on the excitability properties of human motor axons. Brain 2001124816–825. [DOI] [PubMed] [Google Scholar]
- 19.Kiernan M C, Bostock H. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain 20001232542–2551. [DOI] [PubMed] [Google Scholar]
- 20.Bostock H, Baker M. Evidence for two types of potassium channel in human motor axons in vivo. Brain Res 1988462354–358. [DOI] [PubMed] [Google Scholar]
- 21.Kaji R. Physiology of conduction block in multifocal motor neuropathy and other demyelinating neuropathies. Muscle Nerve 200327285–296. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed J, Weisberg L S. Hyperkalemia in dialysis patients. Semin Dialysis 200114348–356. [DOI] [PubMed] [Google Scholar]
- 23.Stys P K. General mechanisms of axonal damage and its prevention. J Neurol Sci 20052333–13. [DOI] [PubMed] [Google Scholar]
- 24.Neumcke B, Schwarz W, Stampfli R. Slow actions of hyperpolarization on sodium channels in the membrane of myelinated nerve. Biochim Biophys Acta 1979558113–118. [DOI] [PubMed] [Google Scholar]
- 25.Palti Y, Moran N, Stampfli R. Potassium currents and conductance. Comparison between motor and sensory myelinated fibers. Biophys J 198032955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]