Abstract
Background
Hippocampal atrophy on magnetic resonance imaging (MRI) is an early characteristic of Alzheimer's disease. However, hippocampal atrophy may also occur in other dementias, such as frontotemporal lobar degeneration (FTLD).
Objective
To investigate hippocampal atrophy on MRI in FTLD and its three clinical subtypes, in comparison with Alzheimer's disease, using volumetry and a visual rating scale.
Methods
42 patients with FTLD (17 frontotemporal dementia, 13 semantic dementia, and 12 progressive non‐fluent aphasia), 103 patients with Alzheimer's disease, and 73 controls were included. Hippocampal volumetry and the easily applicable medial temporal lobe atrophy (MTA) rating scale were applied to assess hippocampal atrophy.
Results
Multivariate analysis of variance for repeated measures showed an effect of diagnostic group on hippocampal volume. There was a significant diagnosis by side (left v right) interaction. Both FTLD and Alzheimer's disease showed hippocampal atrophy compared with controls. Results of the visual MTA rating scale confirmed these findings. Within the FTLD subtypes there were marked differences in hippocampal atrophy. Frontotemporal dementia and semantic dementia showed bilateral hippocampal atrophy, and in semantic dementia the left hippocampus was smaller than in Alzheimer's disease. No significant hippocampal atrophy was detected in non‐fluent progressive aphasia.
Conclusions
Hippocampal atrophy is not only a characteristic of Alzheimer's disease but also occurs in FTLD. The three clinical subtypes of FTLD show different patterns of hippocampal atrophy.
Keywords: hippocampus, Alzheimer's disease, frontotemporal lobar degeneration, MRI
Individuals with frontotemporal lobar degeneration (FTLD) present with alterations in personality and cognitive dysfunction.1 Three clinical subtypes can be distinguished by their neurobehavioural profile: frontotemporal dementia (FTD), semantic dementia (SD), and progressive non‐fluent aphasia (PA).1 These subtypes have overlapping neuropathological substrates and all result in progressive degeneration of the frontotemporal lobes.1
Subjects with Alzheimer's disease present with episodic memory impairment.2 On MRI, hippocampal atrophy is an early marker of Alzheimer's disease, differentiating affected individuals from controls.3,4,5,6,7,8,9 However, hippocampal atrophy may also occur in FTLD. Although previous studies have investigated hippocampal atrophy in FTD and SD,10,11,12,13 none of these studies included all three FTLD subtypes.
Our aim was to investigate hippocampal atrophy on magnetic resonance imaging (MRI) in FTLD and its three clinical subtypes, in comparison with Alzheimer's disease. We used both hippocampal volumetry and an easily applicable visual rating scale for medial temporal lobe atrophy (MTA).8
Methods
Subjects
In all, 145 subjects were included from two centres. At the University of Leipzig eight FTLD patients (4 FTD, 1 SD, 3 PA) and 44 Alzheimer patients were recruited. From the Alzheimer Centre of the VU Medical Centre in Amsterdam 34 FTLD patients (13 FTD, 12 SD, 9 PA) and 59 Alzheimer patients were included. Forty two controls were recruited from the LEILA75+ study14 and among patients' spouses in Leipzig, and 31 controls were recruited through advertisements and among patients' spouses and friends in Amsterdam.
Subjects underwent a standard battery of examinations, including history taking, medical and neurological examination, laboratory tests, and psychometric evaluation. Brain MRI was acquired between 0 and 3 months after initial evaluation. Dementia severity was assessed with the Clinical Dementia Rating scale (CDR).15 Diagnoses of FTLD and Alzheimer's disease were made by multidisciplinary teams, based on clinical criteria.1,2 Although brain MRI contributed to the diagnostic process, it should be noted that hippocampal volumes and MTA scores were not used. All subjects provided written informed consent for their clinical data being used for research.
MRI
In Leipzig, participants were scanned on a 1.5 Tesla scanner (Siemens Vision, Erlangen, Germany), while in Amsterdam scanning was done on a 1.0 T scanner (Siemens Magnetom Impact Expert, Erlangen, Germany). A three dimensional T1 weighted MPRAGE sequence was acquired (parameters: Leipzig: time of repetition (TR) = 11.4 ms, time of echo (TE) = 4.4 ms, transverse orientation, matrix 256×256, voxel size 0.90×0.90×1.5 mm; Amsterdam: TR = 15 ms, TE = 7 ms, coronal orientation, matrix 256×256, voxel size 0.98×0.98×1.49 mm.)
Hippocampal volumes were analysed using in‐house software of the Max‐Planck‐Institute of Human Cognitive and Brain Sciences.16 Volumetric datasets were aligned with the stereotactical coordinate system, using the anterior and posterior commissure as reference points, scaled to an isotropical voxel resolution of 1 mm. Six hippocampal cross sections were segmented manually in the coronal plane. Hippocampal measures started behind the amygdala at the slice in which the area of the hippocampal head appeared maximal and were continued posteriorly at 3 mm intervals.17 Manual outlining of the hippocampus was shown to have a high inter‐rater reliability (intraclass correlation 0.996).17
Visual rating of hippocampal atrophy was done on coronal T1 weighted images. The MTA scale ranges from 0 (no atrophy) to 4 (severe atrophy).8 The intrarater agreement was good, as determined on 20 MRI scans (κ = 0.68). Raters were blinded to the clinical information and each others results. To correct for head size, the mid‐sagittal intracranial area (ICA) was manually outlined following Pantel's technique.18
Statistical analysis
Group differences in hippocampal volumes were examined using multivariate analysis of variance (MANOVA) for repeated measures, with diagnostic group as between‐subjects variable, side (left v right) as within‐subjects variable, and age, sex, ICA, and type of scanner as covariates. To evaluate group differences of left and right hippocampal volumes separately, additional analyses of variance (ANOVAs) with post hoc Bonferroni tests were undertaken, including the same covariates. Group differences in MTA scores were tested using Kruskal–Wallis tests with post hoc Mann–Whitney U tests. To correct for multiple comparisons the significance level for this analysis was set at p<0.01.
Results
Group characteristics are presented in table 1. Age differed between the groups (F[2, 215] = 18.8, p<0.001). Alzheimer's disease patients were older than FTLD patients and younger than controls. FTLD patients had a longer disease duration than Alzheimer patients. FTLD patients had a larger ICA than Alzheimer patients or controls (F[2, 215] = 5.1, p<0.01). There were no differences between groups in distribution of sex or CDR score.
Table 1 Demographic, clinical, and magnetic resonance imaging characteristics by diagnostic group.
| Controls (n = 73) | FTLD (n = 42) | AD (n = 103) | ||||
|---|---|---|---|---|---|---|
| Age (y) | 75 (8) | 65 (7)**/*** | 71 (9)* | |||
| Sex (M:F) | 34:39 | 26:16 | 43:60 | |||
| CDR†‡ | – | 1.0 (0.5 to 3.0) | 1.0 (0.5 to 3.0) | |||
| Disease duration (y)†§ | – | 6 (1 to 13)*** | 3 (1 to 13) | |||
| ICA (cm2) | 146 (12) | 151 (11)*/*** | 145 (11) | |||
| Left hippocampus (cm3) | 1.69 (0.21) | 1.43 (0.24)** | 1.37 (0.24)** | |||
| Right hippocampus (cm3) | 1.75 (0.19) | 1.61 (0.28)** | 1.46 (0.24)** | |||
| Left MTA¶ | 1.34 (0.78) | 2.41 (0.94)** | 2.38 (0.84)** | |||
| Right MTA¶ | 1.37 (0.77) | 2.07 (0.92)** | 2.39 (0.87)** |
Values are mean (SD) or median (range). Raw hippocampal volumes are presented, but these were subsequently corrected for age, sex, intracranial area, and type of scanner. Analysis of variance (ANOVA) with post hoc Bonferroni tests was used unless stated otherwise.
*p<0.05 v controls; **p<0.01 v controls; ***p<0.01 v AD.
†Mann–Whitney U test.
‡CDR score was missing for one FTLD and one AD patient.
§Data on disease duration were missing for five AD patients.
¶Kruskal–Wallis test with post hoc Mann–Whitney U tests.
AD, Alzheimer's disease; CDR, Clinical Dementia Rating scale; FTLD, frontotemporal lobar degeneration; ICA, intracranial area; MTA, medial temporal lobe atrophy.
Group differences in hippocampal volume were examined using MANOVA for repeated measures. The main effect of diagnosis was significant (F[2, 211] = 49.6, p<0.001), whereas the main effect of side (left v right) was not (F[1, 211] = 3.3, p = 0.07). In addition, there was a significant diagnosis by side interaction (F[2,211] = 8.2, p<0.001) (table 1).
ANOVAs with post hoc Bonferroni tests were used to evaluate group differences in left and right hippocampal volumes separately (left, F[2,211] = 48.0, p<0.001; right, F[2,211] = 39.7, p<0.001) (table 1). Alzheimer and FTLD patients had smaller hippocampal volumes bilaterally compared with controls (p<0.001). The differences in hippocampal volumes between Alzheimer's disease and FTLD were not significant.
Group differences in MTA scores were analysed using Kruskal–Wallis tests with post hoc Mann–Whitney U tests. This analysis yielded comparable results, FTLD and Alzheimer groups having more MTA bilaterally than controls (p<0.001). FTLD and Alzheimer's disease patients had comparable MTA scores bilaterally (left, p = 0.91; right, p = 0.04).
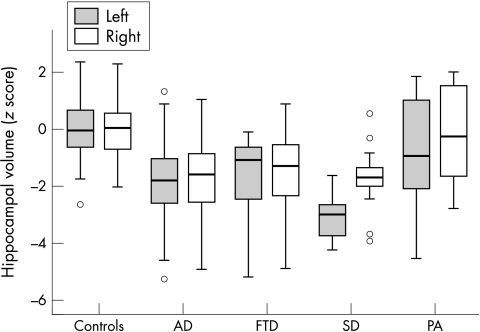
In an additional analysis hippocampal volumes in the three FTLD subtypes were evaluated separately. ANOVAs using the five diagnostic groups with age, sex, ICA, and type of scanner as covariates were carried out, applying post hoc Bonferroni tests (fig 1). As observed before, bilateral hippocampal volumes differed according to group (left, F[4,209] = 29.5, p<0.001; right, F[4,209] = 23.0, p<0.001). Different distributions of hippocampal atrophy compared with Alzheimer's disease and controls were found in the three clinical FTLD subtypes. First, FTD patients showed bilateral hippocampal atrophy similar to patients with Alzheimer's disease (both sides: FTD<controls, p<0.001; FTD = AD, p = 1.00). Second, SD patients showed bilateral hippocampal atrophy as well (both sides: SD<controls, p<0.001). Moreover, the left hippocampus was affected to a greater extent than in Alzheimer patients (left: SD<AD, p = 0.03; right: SD = AD, p = 1.00), reflecting asymmetry in SD. Finally, PA patients had a left hippocampal volume intermediate to that seen in controls and Alzheimer patients (PA = controls, p = 0.12; PA = AD, p = 0.26), while the right hippocampus was spared (PA = controls, p = 1.00; PA>AD, p = 0.001). In a direct comparison between FTLD subgroups, left hippocampal volume in SD was reduced compared with PA, whereas left hippocampal volume in FTD was intermediate to SD and PA (FTD = SD, p = 0.16; FTD = PA, p = 0.79; SD<PA, p = 0.001). Right hippocampal volumes were not significantly different between the three subgroups (FTD = SD, p = 1.00; FTD = PA, p = 0.06; SD = PA, p = 0.08).
Figure 1 Boxplot of hippocampal volumes (z scores) by diagnostic group. AD, Alzheimer's disease; FTD, frontotemporal dementia; PA, progressive non‐fluent aphasia; SD, semantic dementia. Based on our control population we corrected for the influence of possible confounding variables on hippocampal volumes. In the control population multiple linear regression was carried out with age, sex, ICA, and type of scanner as independent and hippocampal volume (left and right separately) as dependent variables. On the basis of the resulting model, an expected hippocampal volume for each subject was calculated. This volume was subtracted from the measured volume. The residue of the hippocampal volume was divided by the standard deviation of the residue in the reference population to give a z score. A z score below 0 indicates a below average volume.
Discussion
The main findings of this study were twofold. First, we have confirmed that hippocampal atrophy occurs in FTLD, and is not restricted to Alzheimer's disease. Second, there are differences in hippocampal atrophy in the three clinical FTLD subtypes: FTD showed bilateral hippocampal atrophy comparable to Alzheimer's disease; in SD bilateral hippocampal atrophy was observed, with left sided hippocampal atrophy exceeding that in Alzheimer's disease; while in PA hippocampal atrophy was not consistently observed.
Our finding that FTLD and Alzheimer's disease both show hippocampal atrophy is consistent with previous studies.10,11,12,13 Beyond corroborating earlier findings we provide for the first time data on hippocampal atrophy in all three clinical subgroups of FTLD, including PA. Noteworthy is the fact that a proportion of PA patients shows no hippocampal atrophy. A possible explanation may be that neuropathology in PA, especially in the early stages, is mainly concentrated around the Sylvian fissure.19
Differentiation between FTLD and Alzheimer's disease is essential in terms of clinical management and pharmacotherapy. Both diseases are diagnosed using clinical criteria,1,2 but there is considerable clinical overlap.20 We have shown that the presence of hippocampal atrophy on MRI does not exclude a diagnosis of FTLD. Hippocampal atrophy in FTLD may be attributable to co‐existing Alzheimer pathology or reflect a different disease. A recent neuropathological study reported consistent involvement of the hippocampus in the early stages of FTLD, without Alzheimer's disease neuropathology.21
A limitation of this study is the use of a clinical diagnosis as the gold standard, without neuropathological verification. However, careful follow up of patients without a change in their clinical diagnosis may serve as nearly a gold standard in clinical studies.22 The selection of patients from two centres may have introduced bias, although we corrected for this using type of scanner as a covariate. Also, our method of hippocampal volumetry, segmenting only six cross sections, underestimates absolute volumes; however, this method was shown to be reproducible and sensitive in a previous paper.17 One of the strengths of our study is the relatively large group of FTLD patients which enabled us to investigate the three clinical subtypes. Furthermore, as hippocampal volumetry is labour intensive and not suitable for routine clinical practice, we also used the easily applicable MTA score. In accordance with earlier studies, results of both methods were comparable,23,24,25 indicating that the use of this simple scale in daily clinical practice is possible.
In conclusion, hippocampal atrophy is not restricted to Alzheimer's disease but occurs in FTLD as well. The three clinical subtypes of FTLD show different patterns of hippocampal atrophy compared with Alzheimer's disease and controls.
Acknowledgements
This study was supported by a grant from Internationale Stichting Alzheimer Onderzoek (nr: 02502/028809) and the Interdisciplinary Centre for Clinical Research (IZKF) at the University of Leipzig (01KS9504/1, project C8).
Abbreviations
AD - Alzheimer's disease
CDR - Clinical Dementia Rating scale
FTD - frontotemporal dementia
FTLD - frontotemporal lobar degeneration
ICA - intracranial area
MTA - medial temporal lobe atrophy
PA - progressive non‐fluent aphasia
SD - semantic dementia
Footnotes
Competing interests: none declared. PS is associate editor of the Journal of Neurology, Neurosurgery and Psychiatry. He had no involvement in the review process.
References
- 1.Neary D, Snowden J S, Gustafson L.et al Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998511546–1554. [DOI] [PubMed] [Google Scholar]
- 2.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 3.Jack C R, Dickson D W, Parisi J E.et al Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 200258750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack C R, Petersen R C, O'Brien P C.et al MR‐based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology 199242183–188. [DOI] [PubMed] [Google Scholar]
- 5.Jack C R, Petersen R C, Xu Y C.et al Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology 199749786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaye J A, Swihart T, Howieson D.et al Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology 1997481297–1304. [DOI] [PubMed] [Google Scholar]
- 7.Laakso M P, Soininen H, Partanen K.et al Volumes of hippocampus, amygdala and frontal lobes in the MRI‐based diagnosis of early Alzheimer's disease: correlation with memory functions. J Neural Transm Park Dis Dement Sect 1995973–86. [DOI] [PubMed] [Google Scholar]
- 8.Scheltens P, Leys D, Barkhof F.et al Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 199255967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Leon M J, Golomb J, George A E.et al The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. Am J Neuroradiol 199314897–906. [PMC free article] [PubMed] [Google Scholar]
- 10.Frisoni GB Laakso M P, Beltramello A.et al Hippocampal and entorhinal cortex atrophy in frontotemporal dementia and Alzheimer's disease. Neurology 19995291–100. [DOI] [PubMed] [Google Scholar]
- 11.Galton C J, Gomez‐Anson B, Antoun N.et al Temporal lobe rating scale: application to Alzheimer's disease and frontotemporal dementia. J Neurol Neurosurg Psychiatry 200170165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galton C J, Patterson K, Graham K.et al Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology 200157216–225. [DOI] [PubMed] [Google Scholar]
- 13.Chan D, Fox N C, Scahill R I.et al Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol 200149433–442. [PubMed] [Google Scholar]
- 14.Riedel‐Heller S G, Schork A, Matschinger H.et al Recruitment procedures and their impact on the prevalence of dementia. Results from the Leipzig Longitudinal Study of the Aged (LEILA75+). Neuroepidemiology 200019130–140. [DOI] [PubMed] [Google Scholar]
- 15.Morris J C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993432412–2414. [DOI] [PubMed] [Google Scholar]
- 16.Mon A. Morphometry for neurobiological applications ( https://wiki.cbs.mpg.de/cgi‐bin/twiki/view )
- 17.Wolf H, Grunwald M, Kruggel F.et al Hippocampal volume discriminates between normal cognition; questionable and mild dementia in the elderly. Neurobiol Aging 200122177–186. [DOI] [PubMed] [Google Scholar]
- 18.Pantel J, Schroder J, Jauss M.et al Topography of callosal atrophy reflects distribution of regional cerebral volume reduction in Alzheimer's disease. Psychiatry Res 199990181–192. [DOI] [PubMed] [Google Scholar]
- 19.Mesulam M M. Primary progressive aphasia. Ann Neurol 200149421–423. [PubMed] [Google Scholar]
- 20.Pijnenburg Y A, Gillissen F, Jonker C.et al Initial complaints in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord 200417302–306. [DOI] [PubMed] [Google Scholar]
- 21.Broe M, Hodges J R, Schofield E.et al Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology 2003601005–1011. [DOI] [PubMed] [Google Scholar]
- 22.Qizilbash N.Evidence‐based dementia practice. Oxford: Blackwell Science, 2002
- 23.Visser P J, Verhey F R, Hofman P A.et al Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry 200272491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermersch P, Leys D, Scheltens P.et al Visual rating of hippocampal atrophy: correlation with volumetry. J Neurol Neurosurg Psychiatry 1994571015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wahlund L O, Julin P, Lindqvist J.et al Visual assessment of medical temporal lobe atrophy in demented and healthy control subjects: correlation with volumetry. Psychiatry Res 199990193–199. [DOI] [PubMed] [Google Scholar]