Abstract
Sneezing is a rarely explored symptom in neurological practice. In the cat, a sneeze evoking centre is located in the medulla. The existence of a sneezing centre has not been confirmed in humans. A case with abnormal sneezing secondary to a strategic infarct in the right latero‐medullary region is presented. A 66 year old man suddenly presented paroxysmal sneezing followed by ataxia, right sided motor and sensory symptoms, and hoarseness. The application of stimuli to the right nasal fossa did not evoke sneezing nor the wish to sneeze. The same stimuli to the contralateral nasal fossa evoked normal sneezing. The preservation of the superficial sensitivity of the nasal fossa indicates that the lesion was localised in the hypothetical human sneezing centre, very close to the spinal trigeminal tract and nucleus. This centre appears to be bilateral and functionally independent on both sides.
Keywords: sneezing, brain stem, trigeminal nucleus
Sneezing, in contrast to other protective reflexes such vomiting, is not a common symptom in neurological practice. In most cases, patients complain of hyperactivity of the reflex, usually because of nasal irritation, prompting an evaluation by ENT or allergy physicians. Some cases of abnormal sneezing may be of psychogenic origin, especially in young adolescent girls.1,2 However, hypoactivity of the sneezing reflex, or difficulty in provoking sneezing or the urge to sneeze, is an infrequent neurological symptom.
Sneezing abnormalities are usually caused by irritation of the trigeminal nerve terminals in the nasal mucosa. In the last few years, some clinical reports of sneezing abnormalities secondary to neurological diseases have been published and reviewed.1,2,3,4,5,6 Although there is information from animal studies, the neurology of the sneeze reflex in humans, which basically has two phases—an initial spasmodic inspiratory phase followed by a nasal and oral expiratory phase—is still poorly understood. Case reports may provide valuable data about the pathophysiology and central pathways involved with this symptom.
Case report
The patient was a 66 year old male fisherman. He was known to have diabetes mellitus and reported mild non‐treated hypertension. He stopped smoking 10 years earlier.
Previously well, he suddenly presented intense, violent, and repetitive sneezing while on a fishing trip,. After approximately 20 sneezes over an interval of three to four minutes, he developed ataxia with a right steering gait, clumsiness in the right limbs, right facial drooping, dysphonia, and right facial paraesthesiae.
He was admitted to hospital. Blood pressure was 160/90 mm Hg. Cardiac and carotid auscultations were normal. The neurological examination showed a normal mental state, dysphonia, right partial Horner syndrome with miosis, slight right facial palsy, right limb ataxia, and slight superficial hypoaesthesia in the right malar area. The corneal reflex and sensitivity was normal. Gait was abnormal with steering to the right. The rest of the examination was normal.
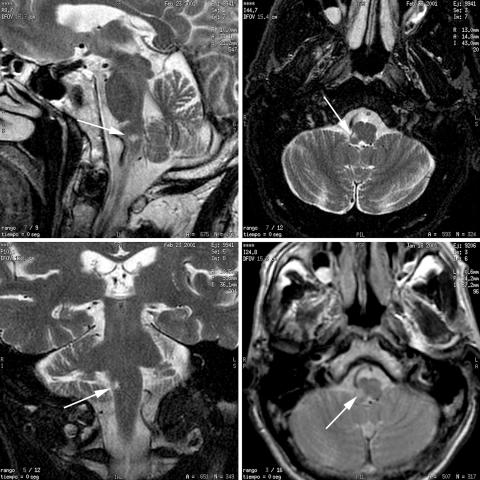
The electrocardiogram showed sinus rhythm at 80 beats/min. The magnetic resonance imaging (MRI) of the brain is shown in fig 1. An angiogram of the brain and cervical blood vessels was normal.
Figure 1 Magnetic resonance imaging of the patient's brain (T2 weighted images). Sagittal and coronal sections (left panels), and axial sections (right panels) showing a hyperintense signal proximal to the interpolaris‐caudalis area of the trigeminal spinal tract and nucleus (arrows).
The facial sensory symptoms disappeared after a few days and permission was obtained from the patient to perform stimulation tests. These were done one week after the stroke with fine cotton tips and capsaicin diluted to 25 μg/ml.
Stimulation of the right nasal fossa with a cotton tip did not provoke the sneeze reflex or the urge to sneeze. However, he conserved touch sensibility and felt the cotton stimulus in this area. Careful application of topical capsaicin diluted to 25 μg/ml to the right anterior nasal fossa provoked an uncomfortable burning sensation and local rhinorrhoea; however, sneezing was not precipitated.
Stimulation of the left nasal fossa with careful repetitive cotton tip touches provoked both the urge to sneeze and some normal sneezes. Stimulation of the same area after application of one drop of topical capsaicin diluted to 25 μg/ml provoked local intense pain, nasal secretion, and repetitive sneezing. The pain subsided after topical application of lignocaine (lidocaine).
Approximately four months after the stroke, he was totally asymptomatic and was able to sneeze normally.
Discussion
The rare presentation of paroxysmal and violent sneezing as the initial symptom of an acute lateral medullary infarct in this patient allowed us to carry out a pathophysiological study of this symptom.
Once the neurological deficits had occurred, the patient developed an inability to sneeze either spontaneously or after stimulation of the distal right nasal fossa by mechanical stimuli (cotton tip). Chemical stimuli (capsaicin) applied to the ipsilateral anterior nasal fossa did not evoke sneezing or the urge to sneeze. However, capsaicin provoked rhinorrhoea and a burning sensation. Application of the same stimuli to the contralateral distal nasal mucosa produced normal sneezing.
Capsaicin, the active ingredient obtained from hot chilli peppers, stimulates the nasal small unmyelinated C‐fibre afferent nerves to release various tachykinins. These nerves, with their somata in the trigeminal ganglion, transmit the information to the central nervous system through the trigeminal dorsal horn in the medulla and lead to sneezing and a sense of pain.7 Although various peptides and tachykinins may be involved, it appears that the capsaicin induced release of substance P8,10 is the most potent trigger of the sneezing response. Capsaicin also precipitates sneezing through a local axon reflex.9
The sneezing reflex may be divided in two phases.3 The first is a nasal or sensitive phase, following stimulation of the nasal mucosa by chemical or physical irritants. The afferent pathways are through the olfactory and ethmoidal nerves, which converge in the putative “sneezing centre” in the medulla. From this point, preganglionic fibres emit impulses by through of the superficial petrosal and sphenopalatine ganglion stimulating the blood vessels and glands, giving rise to nasal secretion and oedema. This results in an increase in trigeminal stimulation with summation of the input to the sneezing centre, where integration occurs. Upon reaching a threshold, the second phase—the efferent or respiratory phase—begins once a critical number of inspiratory and expiratory neurones has been recruited. This consists of eye closing, deep inspiration, and then a forced expiration with initial closing of the glottis, and increasing intrapulmonary pressure. The sudden dilatation of the glottis gives rise to an explosive exit of air through the mouth and nose, washing out mucosal debris and irritants.
The sneezing reflex may be modulated by cortical and voluntary mechanisms.11 The nasal phase may be precipitated by various mechanisms including stimulation of trigeminal fibres in the ophthalmic division, exposure to bright or blue light,12,13 and male orgasm.14,15
Reflexes analagous to sneezing such as coughing, as well as respiration, are probably mediated by the same inspiratory and expiratory neurones but are activated by different nuclei in the brain stem.16 These neurones project to various brain stem nuclei though the vagus, phrenic, and intercostals nerves. Both coughing and sneezing can be suppressed by spinal section.3,16
In the cat, the stimulation of a strategic continuous strip located in the ventromedial part of the spinal trigeminal nucleus, in close proximity to the bilateral pontino‐medular lateral reticular formation, precipitates sneezing. This area is thought to be the sneeze centre in this animal model.17,18,19 Support for the existence and location of a “sneezing centre” also comes from the presence of c‐fos‐like reactivity in specific areas in the cat brain stem when the nasal mucosa is stimulated by air puff.19 In the human, neither the existence nor the location of a sneezing centre has been confirmed up to now, although in the last few years various reports have suggested that structures located in the medulla participate in the human sneeze reflex.3,4,5,6
Touch sensitive pathways from the face and nasal fossa are transmitted through large diameter myelinated fibres to the principal trigeminal sensory nucleus in the brain stem. When activated, free nerve endings—the nociceptor sense organs—result in the excitation of small diameter unmyelinated C afferent nerve fibres and provide nociceptive information to the trigeminal spinal tract nucleus. This nucleus, a long sausage like structure stretching from mid‐pons to the C2–3 segment, comprises three subnuclei: oralis, interpolaris, and caudalis. The subnucleus caudalis seems to be the principal brain stem relay site of nociceptive information in the fifth cranial nerve.
In the patient presented here, the preservation of touch and pain sensitivity from both sides of the face and nasal mucosa indicates the integrity of the trigeminal afferent sensitive pathways and suggests that the lesion causing the sneezing abnormality is located in, or in close proximity to, the hypothetical sneezing area or centre. The neuroimaging results indicate that this area is located proximal to the interpolaris‐caudalis area of the trigeminal spinal tract and nucleus (fig 1). The radiological lesion is ipsilateral to the clinical deficit, and it is probable that a similar structure may exist in the contralateral medulla. As local nasal disease was excluded, the inability to provoke sneezing stimulating the right nasal fossa, but not the left nasal fossa, supports the hypothesis that these two sneezing areas in the medulla are functionally independent, in contrast to recently published studies.4 Asymmetry of the central pathways involved in sneezing has also been inferred from animal studies. These have shown that mechanical stimulation of either nostril activates nasal muscles asymmetrically, the response being greater in the contralateral than in the ipsilateral side, diverting expired air through the ipsilateral side and thus removing the irritant substances from the nose. This asymmetrical response of the nasal muscles is reflexively mediated through trigeminal afferents.20
The repetitive sneezes heralding the stroke were probably related to ischaemic irritation of the sneeze reflex related structures in the medulla, and arterial wall abnormalities were excluded.
To conclude, this case provides evidence for the existence of a human sneeze area or centre located in the rostral medulla.
Footnotes
Competing interests: none declared
References
- 1.Co S. Intractable sneezing: case report and literature review. Arch Neurol 197936111–112. [DOI] [PubMed] [Google Scholar]
- 2.Lin T J, Maccia C A, Turnier C G. Psychogenic intractable sneezing: case reports and a review of treatment options. Ann Allergy Asthma Immunol 200391575–578. [DOI] [PubMed] [Google Scholar]
- 3.Martin R A, Handel S F, Aldama A E. Inability to sneeze as a manifestation of medullary neoplasm. Neurology 1991411675–1676. [DOI] [PubMed] [Google Scholar]
- 4.Hersch M. Loss of ability to sneeze in lateral medullary syndrome. Neurology 200054520–521. [DOI] [PubMed] [Google Scholar]
- 5.Bernat J L, Suranyi L. Loss of ability to sneeze in lateral medullary syndrome. Neurology 200055604. [DOI] [PubMed] [Google Scholar]
- 6.Schattner A. Ominous sneezing. Am J Med 1999106598. [PubMed] [Google Scholar]
- 7.Geppetti P, Fusco B M, Marabini S.et al Secretion, pain and sneezing induced by the application of capsaicin to the nasal mucosa in man. Br J Pharmacol 198893509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitajiri M, Kubo N, Ikeda H.et al Effects of topical capsaicin on autonomic nerves in experimentally‐induced nasal hypersensitivity. An immunocytochemical study. Acta Otolaryngol Suppl 199350088–91. [DOI] [PubMed] [Google Scholar]
- 9.Canning B J. Neurology of allergic inflammation and rhinitis. Curr Allergy Asthma Rep 20022210–215. [DOI] [PubMed] [Google Scholar]
- 10.Imamura T, Kambara T. Substance P as a potent stimulator of sneeze responses in experimental allergic rhinitis of guinea pigs. Agents Actions 199237245–249. [DOI] [PubMed] [Google Scholar]
- 11.Suranyi L. Localization of the “sneeze center”. Neurology 200157161. [DOI] [PubMed] [Google Scholar]
- 12.Everett H C. Sneezing in response to light. Neurology 196414483–490. [DOI] [PubMed] [Google Scholar]
- 13.Lang D M, Howland W C. Solar sneeze reflex. JAMA 19872571330–1331. [DOI] [PubMed] [Google Scholar]
- 14.Stromberg B V. Sneezing: its physiology and management. Eye Ear Nose Throat Mon 19755449–53. [PubMed] [Google Scholar]
- 15.Korpas J, Tomori Z.Cough and other respiratory reflexes. Basel: Karger, 1979218–223.
- 16.Wallois F, Bodineau L, Macron J M.et al Role of respiratory and non‐respiratory neurons in the region of the NTS in the elaboration of the sneeze reflex in cat. Brain Res 199776871–85. [DOI] [PubMed] [Google Scholar]
- 17.Nonaka S, Unno T, Ohta Y.et al Sneeze‐evoking region within the brainstem. Brain Res 1990511265–270. [DOI] [PubMed] [Google Scholar]
- 18.Batsel H L, Lines A J. Neural mechanisms of sneeze. Am J Physiol 1975229770–776. [DOI] [PubMed] [Google Scholar]
- 19.Wall P D, Taub A. Four aspects of trigeminal nucleus and a paradox. J Neurophysiol 196225110–126. [DOI] [PubMed] [Google Scholar]
- 20.Sekizawa S I, Ishikawa T, Sant'Ambrogio G. Asymmetry in reflex responses of nasal muscles in anesthetized guinea pigs. J Appl Physiol 199885123–128. [DOI] [PubMed] [Google Scholar]