Abstract
Mild cognitive impairment (MCI) is an operational definition for a cognitive decline in individuals with a greater risk of developing dementia. The amnestic subtype of MCI is of particular interest because these individuals most likely progress to Alzheimer's disease (AD). Currently hypothesised therapeutic approaches in MCI are mainly based on AD treatment strategies. Long term secondary prevention randomised clinical trials have been completed in amnestic MCI populations, encompassing agents with various mechanisms of action: acetylcholinesterase inhibitors (donepezil, rivastigmine, galantamine), antioxidants (vitamin E), anti‐inflammatories (rofecoxib), and nootropics (piracetam). The design of clinical trials in MCI is influenced by study objectives and definition of primary end points: time to clinical diagnosis of dementia, and AD in particular, or symptom progression. As none of the drugs previously shown to have clinical efficacy in AD trials or benefit in everyday practice have met the primary objectives of the respective trials, design of future clinical trials in MCI should be further developed particularly as regards the selection of more homogeneous samples at entry, optimal treatment duration, and multidimensional and reliable outcomes.
Keywords: acetylcholinesterase inhibitors, anti‐inflammatories, antioxidants, clinical trials, mild cognitive impairment, piracetam
Aging brings physical and intellectual limitations and is a major risk factor for a number of chronic somatic and neurodegenerative diseases. It is well known that there is wide variability among older individuals with respect to physical as well as cognitive aging. Scientific advances in understanding the factors that contribute to optimal aging, the characteristics of the normal aging process and the pathological conditions that occur more frequently with increasing age, such as dementia, have resulted in a broad social and medical initiative towards the prevention of dementia, its early recognition, and therapeutic interventions. In this context, one of the challenges for clinical investigators is to clearly distinguish between benign forms of cognitive dysfunction associated with increasing age and cognitive decline associated with incipient dementing disease. The latter condition is known in the literature as mild cognitive impairment (MCI) and is hypothetically an important treatment target in order to stabilise symptoms or delay progression to dementia. This paper reviews the design and results of recently completed clinical trials in MCI. Information has been obtained from earlier reviews on ongoing trials in MCI from Schneider1 and Petersen,2 the NIH website on drug trials (www.clinicaltrials.gov), papers presented at the last Alzheimer's Disease and Related Disorders Conference in Philadelphia 2004,3,4,5,6,7 as well as via correspondence with representatives of the sponsors of the different trials. A standard search strategy for papers was employed using keywords in PubMed.
Refinement of clinical definition, neurobiological correlates, and rationale for treatment
There have been various definitions of MCI in the literature with consequent variability in outcome after follow up of subjects.8 In general, higher “conversion” rates in clinically selected samples, on average 15% per year as compared with 7.5% in community samples, were reported.9 So far, the most popular and widely used clinical criteria are those developed by researchers at the Mayo Clinic.10 In the original version of the criteria, there was an emphasis on memory impairment without clinically manifest dementia and with preserved activities of daily living, which is designated as the amnestic form of MCI and suggested by the authors to be the most common clinical presentation that most likely progresses to Alzheimer's disease (AD).10 With increasing interest in the concept of MCI in clinical practice and increasing awareness of the heterogeneity of its clinical presentation, course, and outcomes, the Mayo Clinic criteria have been further refined by the addition of several MCI subtypes based on different neuropsychological profiles and different postulated aetiologies.11,12,13 Although in the American studies amnestic MCI showed high “conversion” rates to AD, retroactive application of MCI criteria to data from a prospective epidemiological study showed that isolated amnestic MCI is an unstable entity with regard to outcome after a longer follow‐up period.14 In a recent clinical study where diagnosis of MCI was based on psychometric definitions without clinical judgement, Rasquin et al reported that multiple domain MCI had even higher sensitivity than amnestic MCI in identifying subjects at risk of developing AD in a 2 year follow‐up study.15 Therefore, the initial concept of amnestic MCI has undergone further revision by subclassification into pure isolated amnestic MCI with an emphasis on memory deficit only, and amnestic MCI which also allows the presence of other non‐memory deficits.11,16 An additional difficulty in the application of MCI criteria in clinical settings is a requirement for a subjective report of memory problems and preserved activities of daily living. Recent efforts towards a consensus regarding these issues took place at an international expert meeting in Stockholm in 2003. In the latest revision of general clinical criteria for MCI, major requirements are that the individual is neither normal nor demented according to current classification criteria for dementia, cognitive decline (in contrast to impairment) should be reported by the subjects themselves and/or their closest informant and indicated by objective cognitive assessment, and while basic activities of daily living are preserved, a minimal impairment in complex instrumental functions is possible.17
Clinically defined amnestic MCI has a neurobiological profile that suggests a neurodegenerative aetiology and, therefore, potential treatment interventions. Several studies have reported that MCI subjects had intermediate values between normal aging and dementia, and AD in particular, on various psychometric measures,18 on cerebrospinal fluid biomarkers,19 and on neuroimaging investigations showing atrophy in medial temporal lobe structures on MRI, reduced glucose metabolism or cerebral blood flow in temporoparietal cortex measured by PET and SPECT, respectively, or increased slowing in EEG.20 In addition, a higher prevalence of the APOE e4 allele has been reported in this population21 and post‐mortem studies showed that subjects with MCI in close proximity to death have pathological changes characteristic of AD, such as severe neuron loss in the hippocampus and entorhinal cortex,22 β‐amyloid load in the entorhinal cortex,23 and a density of tau positive neurofibrillary tangles in the mesial temporal lobe24 intermediate between that found in healthy subjects and that found in AD patients. The presence of typical AD pathology in these subjects implies that pathological processes in the brain began a long previously and realistic expectations are that with currently available pharmacological interventions we most probably cannot reverse disease process and already existing pathological changes but could eventually modify symptom progression and its clinical expression. However, it should be emphasised MCI cannot be considered an exclusive predictor of AD in individual patients, since not all subjects with MCI have AD and subjects without MCI could have AD.25,26
Due to this inherent heterogeneity of the MCI concept, there is still no definite and precise definition or clinical diagnosis, but working criteria are evolving. Criteria for amnestic MCI showed satisfactory reliability in a multicentre American clinical trial,27 and were in general implemented in all clinical trials discussed in this review.
Putative treatment strategies for MCI
MCI treatment has two aims: improvement of memory loss and prevention of further cognitive decline to clinically manifest AD. Because of the assumed pathophysiological relationship between MCI and AD, hypothesised therapeutic approaches in MCI are mainly based on current and hypothesised treatment strategies for AD: acetylcholinesterase inhibitors (AChEI), antioxidants, nootropics, and anti‐inflammatories.28
Three AChEI (donepezil, rivastigmine, galantamine) are currently established treatments in AD and are considered to be the first choice candidates for the treatment of MCI. Randomised clinical trials (RCT) of up to 6 months' duration of these AChEI have shown positive effects on cognitive measures and measures of global function, and symptomatic improvements for up to 1 year have been reported in patients with mild to moderate AD.29 Possible neuroprotective effects of AChEI have been suggested in a recent study, which showed that the mean annual rate of hippocampal volume loss among patients treated with donepezil was significantly smaller than that among untreated controls.30 Open‐label extension trials relying either on a historical placebo treated cohort or a predicted rate of decline on cognitive measures such as ADAS‐Cog (Alzheimer's Disease Assessment Scale, Cognitive subscale), have suggested that although most patients experience a cognitive decline after 1 year of treatment, benefits are maintained relative to placebo for 3–4 years.31
Antioxidants in the diet have been associated with a reduced risk of AD in observational studies.32 Furthermore, high plasma levels of antioxidant vitamins were related to better memory performance in the elderly.33 In AD clinical trials, antioxidants have shown modest but positive effects on disease progression: selegiline or vitamin E in moderately severe AD in a 2 year trial34 and Gingko biloba in mild stages of the disease in a 52 week trial.35
Anti‐inflammatory drugs have shown prophylactic and possible therapeutic neuroprotective properties in observational as well as in experimental studies: a reduced risk of AD was noted among users of non‐steroidal anti‐inflammatory drugs (NSAID) several years prior to dementia diagnosis and inflammatory processes were reported to have a role in the pathogenetic cascade of AD.36 However, a 1 year randomised, double blind clinical trial with a three group parallel design compared rofecoxib or low dose naproxen with placebo and reported that there was no slowing of cognitive decline in patients with mild to moderate AD.37 Although this first large scale trial does not support the hypothesis that NSAID therapy could slow the progression of AD, only a primary prevention trial in an elderly population without dementia can evaluate the possible prophylactic neuroprotective properties of NSAID.
Nootropics have been present on the market for more than three decades and were probably the first agents indicated for the treatment of dementia related symptoms and age related cognitive impairment. A recent meta‐analysis re‐examined 19 RCT of piracetam that included 1488 older subjects with diverse cognitive impairment ranging from age associated memory impairment38 to dementia, and demonstrated improvements on Clinical Global Impression of Change (CGIC)39 which was a common outcome measure in all studies.40 A 1 year RCT with a high dose of 8 g of piracetam per day in 33 patients with mild to moderate AD showed that the drug was well tolerated and that the treatment group, although not improved in general, had significant positive differences with respect to four memory subtests.41 Possible modes of action of piracetam and similar drugs from the same class are non‐specific. The agent influences neuronal and vascular function, has both central and peripheral effects probably mediated via influence on membrane fluidity that affects neurotransmission non‐selectively, offers neuroprotective benefits, promotes neuroplasticity, and has anticonvulsant and rheological properties.42 Therefore, it is biologically plausible to expect eventual symptomatic and not disease modifying effects.
Interestingly, the results of a recent international survey on issues of diagnosis, therapeutic strategies, and management of MCI showed that 93% of experts in the field from around the world shared the opinion that it will not be possible to develop a single treatment for patients with MCI due to the aetiological heterogeneity of the disease.43
Clinical trials in MCI: methodological issues
During the meeting of the Peripheral and Central Nervous System Drugs Advisory Committee of the Food and Drug Administration (FDA) held in March 2001, several requirements were suggested in the context of the development of drugs intended for the treatment of MCI: (a) valid, reliable, and widely applicable criteria to define aetiologically homogeneous MCI (in terms of future transition to AD); (b) appropriate instruments to measure the clinical effects of the drugs; (c) clinical trials designed to measure both symptomatic and disease modifying effects; and (d) evidence of clinically meaningful effects and magnitude of benefit as compared with harm.44 Although current diagnostic criteria of MCI include neuropsychological assessment of cognitive loss, there is no suggested cut‐off score for memory impairment and the clinician through the examination and interview determines the result and clinical significance of impairment.11 The second requirement for appropriate instruments to measure the clinical effects of the drugs is equally demanding, since outcome measures were designed to assess symptomatic effects on various domains of impairment in AD. Judgment of treatment success is based on known deterioration rates during the natural history of untreated disease as measured by functional measures such as the Clinical Dementia Rating scale (CDR),45 the Global Deterioration Scale (GDS),46 CGIC,47 or psychometric measures such as ADAS‐Cog.48 However, these efficacy measures were derived from AD trials and might be rather insensitive and unreliable in short‐term MCI trials since the decline is slower in the early stages of the disease.
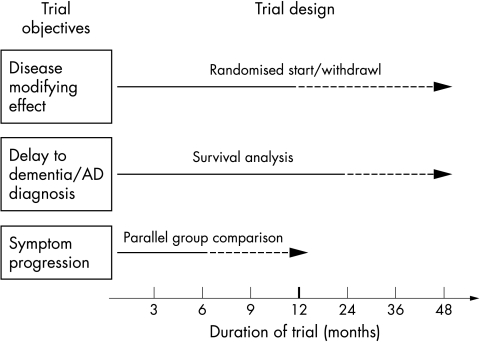
The design of clinical trials in MCI (study sample, duration, primary efficacy measures) is influenced by study objectives: symptomatic versus disease modifying effects, and the consequent definition of primary end points: “conversion” to dementia or symptom progression2 (fig 1). Symptomatic effect implies no effect on transition rate to dementia and positive change on one or both primary efficacy measures, such as the clinical rating scales measuring global change, function, or cognition. Symptom progression as a primary end point based on a continuous measure requires a trial of shorter duration and a smaller sample size, while a dichotomous outcome in studies with “conversion” as a primary outcome requires a large sample size for the power calculation. For example, in the piracetam trial with symptomatic effect as a primary objective, it was calculated, based on the effect size from a previous trial with the same agent,41 that 140 subjects per treatment arm would allow detection with a 90% power of significant difference on a Cognitive Battery Composite Score (CBCS) between placebo and piracetam after a 12 month trial. On the other hand, disease modifying effect implies effect on the transition rate to dementia and a positive change on one or both primary efficacy measures. A survival analysis, a larger sample size of at least 700 individuals, and a trial duration of at least 3 years ensures adequate power to detect small and possible disease modifying effects, given the estimated annual “conversion” rate of 15%. Optimally, both end points, symptom progression and “conversion”, are combined and symptom progression evaluated via annual interim analysis through change in surrogate markers. The assumption with surrogate markers is that they are biologically close to the disease process and correlate to symptom domains, clinical outcome, and neuropathological features of the disease, as indirectly measured by cognitive and behavioural scales or neuroimaging and biochemical markers. In practice, treatment response on surrogate measures, such as change in neuroimaging parameters or biochemical markers, should be predictive of the treatment effect on the important clinical outcomes.49,50 For example, medial temporal lobe atrophy, and hippocampal volume in particular, as measured by MRI, has been consistently reported as a predictor of future development of AD in MCI subjects.51,52
Figure 1 Primary objectives, theoretical designs, and optimal duration of clinical trials in MCI. Solid lines indicate minimum trial duration and dashed lines optimal trial duration. Probable disease modifying effect requires a randomised start or randomised withdrawal design,54,56 where after delayed start or withdrawal of active treatment the placebo group does not converge with the treatment group. The last design is a theoretical and has not been applied in either AD or MCI trials.
Long‐term trials increase the possibility of violation of the protocol as well as participants dropping out for various reasons. The intention‐to‐treat (ITT) principle should be applied to minimise bias in assessment of treatment efficacy in this case.53
Finally, the risk‐benefit ratio is of the utmost importance in the interpretation of the results because eventually future treatment of MCI will also target some non‐diseased elderly subjects.
Table 1 Selected review of short‐ and long‐term completed clinical trials in MCI.
| Donepezil+vitamin E* | Donepezil | Rivastigmine† | Galantamine study 1 (GAL‐INT‐11) | Galantamine study 2 (GAL‐INT‐18) | Rofecoxib | Piracetam | |
|---|---|---|---|---|---|---|---|
| Sponsor | NIA, Pfizer, Eisai, | Pfizer | Novartis | Johnson & Johnson | Johnson & Johnson | Merck | UCB Pharma |
| Sites (start year) | Multicentre, 69 ADCS centres in US and Canada (1999) | Multicentre, US | Multicentre, 69 centres in 14 countries: US, Canada, Europe, Latin America, South Africa (1999) | Multicentre, 8 European countries, Canada, and US (2001) | Multicentre, 4 European countries, Canada, US, Argentina, and Australia (2001) | Multicentre | Multicentre, 69 sites in 16 European countries (2000) |
| Study design | RCT, placebo controlled, double blind, three arms | RCT, placebo controlled, double blind, parallel group | RCT, placebo controlled, double blind, parallel group | RCT, placebo controlled, double blind, parallel group | RCT, placebo controlled, double blind, parallel group | RCT, placebo controlled, double blind, parallel group | RCT, placebo controlled, double blind, three arms |
| Daily dose | Vitamin E (1000 IU/bid), donepezil (10 mg/day) | 5 mg/day first 42 days, thereafter 10 mg/day | 3–12 mg/day | 16 or 24 mg/day, flexible dose | 16 or 24 mg/day, flexible dose | 25 mg/day | 4800 mg/day, 9600 mg/day |
| Trial duration | 3 years | 24 weeks | Planned 3 years, extended to 4 years | 24 months | 24 months | Planned 2–3 years, extended to 4 years | 12 months |
| Enrolment criteria | Amnestic MCI‡, (Del Pg Rec Logical Memory III from WMS‐R), HDRS ⩽12, CDR 0.5 (⩾0.5 memory domain) | Amnestic MCI‡ (Del Pg Rec Logical Memory II from WMS‐R), CDR 0.5 (0.5–1.0 memory domain), MMSE ⩾24, HDRS ⩽12 | Amnestic MCI‡, (NYU Pg Rec Delayed Recall <9), CDR 0.5 (⩾0.5 memory domain), HDRS <13, HDRS item 1 ⩽1 | Amnestic MCI‡, (NYU Pg Rec Delayed Recall ⩽10), CDR 0.5 (⩾0.5 memory domain) | Amnestic MCI‡, (NYU Pg Rec Delayed Recall ⩽10), CDR 0.5 (⩾0.5 memory domain) | Amnestic MCI‡, (AVLT ⩽37), CDR 0.5 (⩾0.5 memory domain), MMSE ⩾24, BDRS ⩽3.5, HDRS ⩽12 | Amnestic MCI‡, CDR 0.5 (⩾0.5 memory domain), WMS‐R Logical Memory immediate Recall <10 or difference between immediate and delayed recall >5, HDRS <18 |
| No of subjects | 769 | 269 | 1018 | 995 | 1062 | 1457 | 675 |
| Age (years) | 55–90 (mean 72.9) | 55–89 (mean 73) | Mean 70.5 | >50 | >50 | ⩾65 | 50–89 (mean 68) |
| ApoE e4 | 58% donepezil | Not available | 41% (determined in 49% of sample) | 26.4% galantamine | 24.4% galantamine | 35% rofecoxib | 43% (determined in 60% of sample) |
| 55% vitamin E | 29.5% placebo | 23.5% placebo | 36% placebo | ||||
| 53% placebo | |||||||
| Primary outcome | Time to clinical diagnosis of AD (NINCDS‐ADRDA criteria) | Symptom change: NYU Pg Test Delayed Recall, CGIC‐MCI | (i) Time to clinical diagnosis of AD (NINCDS‐ADRDA criteria); (ii) change from baseline on cognitive function as measured by a single score summed from weighted scores on a series of individual cognitive tests | Incident dementia (CDR >1.0) at 24, ADAS‐Cog/MCI, CDR‐SB at 12 months | Incident dementia (CDR >1.0) at 24, ADAS‐Cog/MCI, CDR‐SB at 12 months | CDR >1.0 and incident AD (NINCDS‐ADRDA criteria) | Symptom change: CBCS |
| Secondary outcome | MMSE, ADAS‐Cog, NP tests, CGIC, CDR, GDS, QL, ADL, outcome by APOE e4 status | ADAS‐Cog, NYU Pg Immediate Rec, DS‐backw, Symb Dig Modalities, PGA | ADAS‐Cog, MMSE, CDR, GDS, ADCS‐ADL, NPI, QOL‐AD, healthcare utilisation, outcome by APOE e4 status, volumetric MRI, and biomarkers (eg, CSF and blood levels of tau, amyloid‐beta peptide) | ADAS‐Cog/MCI, CDR‐SB, ADCS‐ADL/MCI, DSST, ADAS‐Cog/11, ADAS‐Cog/13, MRI brain and hippocampal atrophy | ADAS‐Cog/MCI, CDR‐SB, ADCS‐ADL/MCI, DSST, ADAS‐Cog/11, and ADAS‐Cog/13 | ADAS‐Cog, MMSE, Selective Reminding Test, CDR, BDRS | CIBIC‐plus, change in separate tests of CBCS, ADL‐MCI, MMSE, BSI, GDS |
| Conversion rates | 16%/year | – | 19.4%/3–4 years | 13% (galantam) | 17% (galantam) | 6.4% (rofecoxib) | – |
| 18% (plac)/2 years | 21% (plac)/2 years | 4.5% (plac)/year | |||||
| Dropout rate | 12%/year | 20% | 43% | Not available | Not available | 45% (rofecoxib) | 27% (4800 mg) |
| 45% (plac) | 21% (9600 mg) | ||||||
| 24% (plac) | |||||||
| Adverse events | 88% (donep) | 96% (rivastig) | 90% (galantam) | 90% (galantam) | 90% (rofecoxib) | 72% (4800 mg) | |
| 73% (plac) | 93% (plac) | 88% (plac) | 86% (plac) | 92% (plac) | 68% (9600 mg) | ||
| 76% (plac) | |||||||
| Results | Significant positive effect on conversion time and cognitive tests during first 18 months. In e4 carriers, positive treatment effect during 36 months. No effect in the vitamin E group | Significant positive effect on ADAS‐Cog in donep groupFE population: immediate and delayed recall tests and DS backw, Symb Dig Modalities PGA. | The study did not achieve its primary objectives | No effect on conversion rate and ADAS‐Cog, positive effect on CDR‐CB, attention (DSST) at month 12, rate of atrophy of whole brain volume (not hippocampal), effect of symptom duration, and baseline severity (NYU Pg Rec) | No effect on conversion rate, ADAS‐Cog, or CDR‐CB, positive effect on attention (DSST) at month 24. Effect of baseline severity (NYU Pg Rec) on conversion at month 24 (9% galantamine, 26% placebo) | No change in primary or secondary efficacy parameters | No change in primary or secondary efficacy parameters |
*Memory Impairment Study (MIS); †the Investigation into the Delay to Diagnosis of AD with Exelon (InDDEx); ‡amnestic MCI, defined according to generally accepted criteria10: by memory complaint, corroborated by an informant, abnormal memory function documented by a variant of a delayed recall test, normal general cognitive function as determined by CDR and MMSE, no or minimal impairment in ADL, and not clinically demented.
ADAS‐Cog, Alzheimer's Disease Assessment Scale, Cognitive subscale; ADCS, Alzheimer Disease Cooperative Study; ADL, Activities of Daily Living scale; AVLT, Auditory Verbal Learning Test; BDRS, Blessed Dementia Rating Scale; CBCS, Cognitive Battery Composite Score; CDR, Clinical Dementia Rating scale; CDR‐SB, Clinical Dementia Rating Scale—Sum of Boxes; CGIC, Clinical Global Impression of Change scale; BSI, Brief Symptoms Inventory; CIBIC‐plus, Clinician's Interview‐Based Impression of Change with caregiver input; CSF, cerebrospinal fluid; Del Pg Rec, delayed paragraph recall; donep, donepezil; DS backw, Digit Symbol backwards; DSST, Digit Symbol Substitution Test; FE, fully available population; galantam, galantamine; GDS, Global Deterioration Scale; HDRS, Hamilton Depression Rating Scale; MMSE, Mini Mental State Examination; MRI, magnetic resonance imaging; NIA, National Institute on Aging; NINCDS‐ADRDA criteria, National Institute of Neurological Disorders and Communicative Disorders‐Alzheimer's Disease and Related Disorders criteria; NP tests, neuropsychological tests; NPI, Neuropsychiatric Inventory; NYU Pg Rec, New York University Paragraph Recall; PET, positron emission tomography; PGA, Patient Global Assessment; plac, placebo; QL, quality of life; QOL‐AD, Quality of Life—Alzheimer's Disease; RCT, randomised clinical trial; rivastig, rivastigmine; Symb Dig Modalities, Symbol Digit Modalities; WMS‐R, Wechsler Memory Scale‐Revised.
Review of clinical trials in MCI
Seven secondary prevention RCT in amnestic MCI have been performed so far, encompassing agents with various mechanisms of action: all three AChEI available on the market (donepezil, rivastigmine, galantamine), antioxidants (vitamin E), anti‐inflammatories (rofecoxib), and nootropics (piracetam). The study design and outcome measures of the respective trials are summarised in table 1. A short 4 week trial with ampakine in MCI subjects is not reviewed in this paper due to its unusual design.
Donepezil and vitamin E
The Memory Impairment Study (MIS) is the first reported large scale trial in amnestic MCI.3,56 The primary outcome measure was time to development of possible or probable AD as defined by NINCDS‐ADRDA criteria.57 The combined group of subjects had a mean age of 72 years and 55% were APOE e4 carriers (53% in the placebo, 58% in the donepezil, and 55% in the vitamin E groups, respectively). After 3 years, 539 (70%) of participants had completed the trial and 214 had progressed to dementia (212 to probable or possible AD); there was an approximately 16% annual progression rate. Patients on donepezil fared slightly better in terms of staying clinically stable during the first half of the trial, when more differences in change from baseline scores on MMSE (Mini‐Mental State Examination), CDR Sum of Boxes, GDS, modified ADAS‐Cog, and cognitive scores were observed between the treatment and placebo groups. However, during the second 18 months all three groups converged and after 36 months there were 73 transitions to AD in the placebo group, 63 in the donepezil group, and 76 in the vitamin E group in total. Proportions of converters relative to the total number of completers for each arm were: 38% in the placebo group, 39% in the donepezil group, and 41% in the vitamin E group. Dropout rate was about 12%/year. At 6, 12, and 18 months there was a higher dropout rate in the donepezil group, probably because the subjects were slightly more impaired at baseline. Ten deaths were reported in the donepezil group (three cardiac arrests), seven in the placebo group, and six in the vitamin E group.3 The APOE e4 allele showed a modifying effect on the rate of progression and the overall result is driven by the e4 positive group: 76% of patients who progressed to AD were APOE e4 carriers. After adjustment for multiple comparisons, hazards ratios (HR) for progression to AD in the total sample on donepezil versus placebo were only significant during the first 12 months, while in APOE e4 carriers HR was significant during the first 24 months and trends were observed throughout the 36 months of trial. In summary, amnestic MCI and the presence of APOE e4 allele were predictive of progression to AD in this study.
Donepezil
The efficacy and tolerability of donepezil in patients with MCI was evaluated in a 24 week multicentre, randomised, double blind placebo controlled parallel group trial.58 Enrolled subjects had a CDR of 0.5 (memory box score 0.5–1.0) and MMSE score ⩾24. At the end of the trial at week 24, a significant improvement was observed in the donepezil group in the ADAS‐Cog total score and the ADAS‐Cog Immediate Word Recall test. In the fully evaluable (study medication compliance at least 80%, and no significant protocol violations at week 24) study population, scores on NYU (New York University) Paragraph Immediate and Delayed Recall tests as well as Digit Span Backwards were also significantly different in favour of donepezil. The CGIC‐MCI improved in both the treatment and placebo groups with no difference between the groups at the end of the trial, and self rated Patient Impression of Change was significantly different in favour of the donepezil group. Adverse events, predominantly gastrointestinal, were reported in 88% of the donepezil group and 73% of the placebo group, occurring at a higher frequency than in AD trials.59
Rivastigmine
The Investigation into the Delay to Diagnosis of AD with Exelon (InDDEx) study is a large multicentre, double blind placebo controlled parallel group trial of rivastigmine 3–12 mg/day. Similarly to the MIS, the entry criteria correspond to those for amnestic MCI.60 Primary outcomes were time to clinical diagnosis of AD (NINCDS‐ADRDA criteria) and change from baseline on cognitive function as measured by change on overall summary score on neurocognitive test battery (a series of individual tests measuring working memory, immediate and delayed recall, cued recall, attention/concentration, language, executive functioning, and praxis). Of the total study sample 49% consented to pharmacogenetic assessment, and approximately 41% of this subsample were e4 allele carriers.60 There was a high prevalence (over 97%) of concurrent medical illness, and more than 90% of patients were receiving medication for concurrent illness. Preliminary results of the per protocol analysis show that study objectives were not satisfied. There was a high dropout rate and only 51% of rivastigmine treated patients and 63% of placebo treated patients completed the trial. The preliminary reported conversion rate during the 3–4 years of the trial at 19.4% was lower than expected: 17.3% of patients in the rivastigmine group and 21.4% in the placebo group progressed to probable or possible AD. Patients who converted to AD tended to be older and less educated, with a lower body mass index, higher baseline scores on GDS and CDR, more pronounced cognitive impairment as measured by the MMSE and NYU Delayed Paragraph Recall, and smaller brain volumes as measured by MRI.
Galantamine
The efficacy and safety of a flexible dose of galantamine in patients with MCI was evaluated in two 24 month multicentre randomised, double blind parallel group placebo controlled studies.61,62 The process of data evaluation is still ongoing but some preliminary results have been reported.4,5,6,7 According to baseline demographics, patients had a mean age of ∼70 years, the duration of cognitive problems was highly variable among individuals, and there was greater impairment in immediate than in delayed recall. Conversion to dementia (CDR ⩾1.0) at month 24 was similar in the two studies: 13% in the galantamine and 18% in the placebo group in study 1, and 17% in the galantamine and 21% in the placebo group in study 2. In neither study was statistically significant treatment effect observed on cognition as assessed by ADAS‐Cog or ADL measures at 24 months. Treatment effect on global functioning was demonstrated in study 1 by significant difference in CDR‐SB scores in favour of galantamine at 12 and 24 months and no difference in study 2 on either of two occasions. Attention assessed by the DSST (Digit Symbol Substitution Test) was significantly improved in the galantamine group at 12 months in study 1 and at 24 months in study 2. Although no detailed results are available, it has been reported that a reduced rate of whole brain atrophy, but not hippocampal atrophy, has been found in patients on galantamine treatment at month 24.7
Pharmacogenomic analyses are still ongoing, but reported preliminary frequencies of the e4 allele in the placebo and galantamine groups are 29.5% and 26.4% for study 1 and 23.5% and 24.4% for study 2, respectively.
An effect of duration of symptoms and baseline severity of cognitive impairment was observed in both studies. In study 2 in the subgroup of patients with an NYU Delayed Recall score of 4–5, 9% and 26% in the galantamine and the placebo groups, respectively, converted to dementia.
In the same study in the subgroup with an NYU Immediate Recall score ⩽1, there was a significant positive effect in the galantamine group on CDR‐SB scores at month 24.
In study 1, superiority of galantamine over placebo in change on ADAS‐Cog/MCI score at 24 months was shown in the subgroup of patients with symptom duration of 2–3 years and worse baseline performance on NYU Delayed Recall (score 2–3) and NYU Immediate Recall (score 2–3).
Greater mortality was observed in the galantamine group in both studies: five patients on placebo and 15 patients on galantamine died, resulting in the relative risk 3.04 (95% CI: 1.26 to 7.32). The investigators did not consider the causes of death to be related to treatment; however, the imbalance in the number of deaths between the treatment and placebo group remains a concern. Further evaluation of the mortality rate in this study will be conducted in a retrieved dropout study, GAL‐COG‐3002.
Rofecoxib
A recently completed randomised, double blind placebo controlled multicentre trial evaluated whether a fixed dose of a COX‐2 inhibitor, rofecoxib (25 mg/day), could delay an AD diagnosis in elderly MCI patients and investigated the long‐term tolerability of this agent.63 The primary outcome was the number of patients with CDR ⩾1.0 and incident AD according to NINCDS‐ADRDA criteria. The two randomised groups were balanced with respect to gender, family history of AD, years of education, age (mean 75 years), and APOE e4 allele (36% in the placebo group were carriers and 35% in the rofecoxib group). The study was terminated after 189 cases of AD were clinically diagnosed because of lower than expected conversion rates and a high dropout rate that compromised the power of the study. The estimated progression to an AD diagnosis was lower than expected at 10–15% (6.4% in the rofecoxib group and 4.5% in the placebo group) giving a rofecoxib:placebo hazard ratio of 1.46 (CI 1.09 to 1.94). However, this treatment difference in favour of placebo was not consistent with results from secondary measures of cognition and function, which did not demonstrate any significant differences between the treatment groups.
In the placebo group, 45% of patients did not complete the study, with 10% of this percentage due to the adverse events. The same 45% rate occurred in the rofecoxib group, with 11% of this percentage due to the adverse events. Relatively few subjects discontinued the study due to drug related adverse events (8% in the rofecoxib group and 5.6% in the placebo group).
Piracetam
The efficacy and tolerability of piracetam in MCI patients was evaluated in a multicentre 12 month trial sponsored by UCB Pharma. The trial objective was symptom progression and the primary efficacy measure was a composite score that contained key outcomes from eight tests: the NYU Paragraph Recall Test (Delayed Recall), ADCS (Alzheimer Disease Cooperative Study) Cancellation Test, Symbol‐Digit Modalities Test, Colour Trails Test (form A), Letter Number Sequence Test from the WMS‐III (Wechsler Memory Scale, Third Edition), Free and Cued Selective Reminding Task, Block Design from the WAIS‐R (Wechsler Adult Intelligence Scale–Revised), and Semantic Category Fluency. All tests were applied at both the selection and baseline visits to document any learning effect. A ceiling effect was observed on the Free and Cued Selective Reminding Task. The sum over the eight standardised variables (the mean of each test score subtracted from the individual score and divided by the pooled standard deviation) was defined as the Cognitive Battery Composite Score (CBCS). All primary and secondary efficacy parameters were assessed at the selection, baseline, interim evaluation, and final evaluation visits. In addition, the study also aimed to describe the relationship between cognitive decline and APOE e4 allele and (in a subpopulation) neuroimaging, CSF, and neurophysiological markers.
All treatment groups were similar in demographic variables with a mean age of 68 years.
Overall, APOE genotype was determined in 405 (60%) of 675 patients: of these 405 patients, 173 (43%) were carriers of the APOE e4 allele, 141 (35%) were heterozygotes, and 32 (8%) were homozygotes.
All analyses were performed on both the ITT and the per protocol (PP) populations. Discontinuation from the study was similar in all treatment groups: 24% in the placebo, 27% in the piracetam 4800 mg, and 21% in the piracetam 9600 mg group. The most frequent reason for discontinuation was an adverse event: 12% in the placebo, 13% in the piracetam 4800 mg, and 8% in the piracetam 9600 mg group. Overall, 72% of subjects reported 1755 adverse events: 76% in the placebo, 72% in the piracetam 4800 mg, and 68% in the piracetam 9600 mg group. All these data confirm the good safety profile of piracetam given at a relatively high dose over a long period (1 year).
No statistically significant differences on any primary or secondary outcome at month 12 were observed for either of two piracetam doses or for placebo. The results were consistent for both PP and ITT populations and the results of analysis of primary and secondary outcomes were consistent with each other.
Summary and concluding remarks
This review shows that none of the listed clinical trials on MCI met their primary objectives. A short 6 month donepezil trial and a 3 year secondary prevention study confirmed previous observations from AD trials on the symptomatic effects of AChEI. Nevertheless, the scientific community has gained both valuable information on the natural course of MCI as defined by currently accepted clinical criteria as well as lessons for the future with regard to the design and methodology of MCI trials.
The question arises whether the study objectives were justified by scientific proof or were based on assumptions. The common assumption is that we know the effects of treatment in this still controversial early stage of the disease and that effects would be clearly demonstrated on cognitive and functional measures used in earlier AD trials.
Despite the consensus on a generally accepted clinical definition of amnestic MCI that isolates individuals at high risk of developing AD within a few years, the “conversion” rate varied considerably among the trials from 4.5% and 6.4%/year in the rofecoxib trial to 16%/year in the MIS (donepezil and vitamin E study). Obviously, different populations of patients, a number of whom in some centres were probably early AD cases, were chosen using very similar entry criteria. In their most recent article, Visser et al64 investigated retrospectively the diagnostic accuracy of MCI criteria used in different MCI trials for predementia Alzheimer's disease in a cohort of non‐demented patients from their clinic. The authors pointed out that there were marked differences in the definition of cognitive impairment. The MIS had a strict cut‐off score for memory impairment and a higher positive predictive value and “conversion” rate in contrast to the GAL‐INT‐11 and rofecoxib studies, which used a more lenient cut‐off score. In the MIS, a considerably higher percentage of patients (55%) were carriers of the APOE e4 allele as compared with those published in a meta‐analysis of 42 case‐control series where reported frequencies of the e4 allele were 32% in sporadic AD cases over 65 years of age and 41% in sporadic AD cases under 65 years of age.65 One possible explanation is that the variation in the APOE e4 prevalence across the studies results from variation in the definition of memory impairment, meaning that more severe impairment in the MIS is clearly associated with higher APOE e4 allele frequency.
Another important methodological concern is the choice of primary and secondary outcome measures. In the current trials these measures were chosen according to previous FDA guidelines for AD trials and should include assessments of global function and cognition with scales well validated in AD patients, such as CGIC and its more popular formats the Clinician's Interview‐Based Impression of Change (CIBIC) and the Clinician's Interview‐Based Impression of Change with caregiver input (CIBIC‐plus), and ADAS‐Cog. Although these scales have been adapted for MCI patients, there is still uncertainty about the expected rate of change during the trial period, as the rate differs from that in AD patients where it has been shown that the rate of cognitive deterioration is strongly related to baseline severity.66 Very few studies have investigated the performance of normal subjects on ADAS‐Cog and the effect of age, gender, and level of education.67 Such normative studies provide a reference for the clinician to distinguish variations in normal cognitive aging from those accelerated by subclinical neurodegenerative disorders as probably occur in most individuals with MCI. With respect to the MCI version of the ADL scale, there is concern that it does not measure high‐order instrumental tasks previously shown to have high predictive validity for future conversion to AD. Low “conversion” rates are also influenced by strict entry criteria in some studies which exclude significant co‐morbidity that probably determines faster progression and “conversion” rates. These limitations might considerably reduce the therapeutic potential of agents with effects on microcirculation, such as piracetam, which have documented efficacy in elderly patients with an aetiologically broad range of cognitive disorders.42 Exclusion of patients with depressive symptoms and silent cerebrovascular pathology on CT or MRI probably means the selection of a very exclusive group not representative of MCI in general or the overall clinical population. It has been observed that placebo treated AD patients in clinical trials declined by 0.55 MMSE points per year as compared with 2.5–3 points per year in natural cohorts.68,69
Differences in study populations raise the question of whether current generally accepted clinical criteria are also generally applicable across different clinical centres in a multicentre international trial. It could be of importance that multicentre cross‐cultural studies report the intersite variability of baseline measures as well as the outcomes. Furthermore, there is evidence that there is ∼20% discordance between clinical diagnosis of probable AD and definite pathological diagnosis.70 Therefore, if a clinical diagnosis of AD can be questioned from a neuropathological perspective, an aetiological diagnosis of MCI based exclusively on clinical grounds should be a matter of even greater concern. Would it help increase sensitivity and specificity and ensure the outcome if inclusion criteria were further enriched by the addition of biological markers, such as genetic and CSF markers or atrophy measures on MRI? In most of the listed trials, instrumental investigations and biochemical and genetic markers were included as exploratory objectives, but none of the trials included them as enrichment criteria.
An useful approach has been suggested recently which combines a number of predictor variables for AD, such as age, MMSE score, degree of functional impairment, neuropsychological test impairment, medial temporal lobe atrophy, and APOE genotype in the Preclinical Alzheimer's Disease scale (PAS).71 Study samples enriched in this way could have a higher diagnostic sensitivity and specificity for prodromal AD,72 which would be very important for the clinician who needs greater certainty as to possible clinical outcome in order to initiate treatment in MCI subjects. Indeed, predementia or prodromal AD could be a better designation for the selection of non‐demented subjects for secondary prevention trials in AD.
While sub‐analysis of the predictors of progression to AD is still ongoing in most of the trials, in particular in APOE e4 allele carriers, it has been reported that the APOE e4 carriers in the MIS progressed faster and showed more sustained response on the donepezil treatment.56 Epidemiological, clinical, and basic science evidence supports a relationship between APOE genotype and risk for AD.73,74,75,76 Furthermore, it has been reported that APOE e4 carriers over the age of 50 demonstrated a modest decline in memory skills over a median period of 33 months prior to the symptomatic onset of MCI.76 In a subpopulation of 494 subjects participating in the InDDEx study and consenting to pharmacogenetic assessment, APOE e4 genotype was associated with greater memory and functional impairment and hippocampal atrophy.60 It could be that in such a heterogeneous condition as MCI, subjects with APOE e4 are more likely to have AD as the underlying pathology, which might also explain the better treatment response. While the post‐mortem study of MCI subjects by DeKosky et al77 showed that choline acetyltransferase activity was up‐regulated in the frontal cortex and hippocampus, this compensatory mechanism might be compromised in MCI patients who are also APOE e4 carriers and who, therefore, might respond better to AChEI therapy. However, observed treatment difference according to APOE e4 status in the MIS was not statistically impressive and taken together with the results from the entire study cohort, could suggest the alternative explanation that the beneficial effects of treatment wear off, as noted in the editorial accompanying the article.78 It should be noted that in AD trials with tacrine, galantamine, and donepezil, APOE e4 allele was not found to be a predictor of more favourable outcome.79,80,81
Evaluation of treatment effects in long‐term trials of donepezil+vitamine E, rivastigmine, and rofecoxib is also compromised by the effect of missing data due to high dropout rates, which varied between 40% and 45% in the case of MCI trials lasting more than 24 months. Although reported adverse events were equally balanced between the active treatment and placebo groups in most studies, in the MIS a higher withdrawal rate was observed in the donepezil group due to the more severely impaired cognitive status at baseline. Analysing a random sample of dropout patients is not an optimal solution to the problem of dropout bias, which compromises understanding of group differences in the clinical trials. A retrieved dropout analysis should be established as a standard method since it minimises dropout bias by assessing as many as possible of the patients who did not completed a trial for various reasons.82
A secondary objective in most of the secondary prevention trials is improvement on the specific tests in the neuropsychological battery covering major cognitive domains. What do small statistical improvements on test scores on an individual neuropsychological test mean for the patient's overall functioning? The clinical relevance of improvements on cognitive tests and global measures of change has been questioned from the perspective of caregivers of AD patients.83 The CBCS used in the piracetam trial as a primary outcome, in general enhances the effects of standardised change in any of eight tests that compose this battery; however, it is difficult to interpret either positive or negative results in terms of clinically meaningful effects. In addition, a few outliers could influence the magnitude of standardised change on CBCS. Correspondence with secondary outcomes encompassing global cognitive and functional measures could support findings in primary efficacy parameters. Still, there remains a concern that a 1 year trial in a population that shows ceiling effects on some of the tests at baseline and does not deteriorate quickly, could not detect any significant changes. Indeed, in the piracetam trial there was a ceiling effect on the Free and Cued Selective Reminding task at baseline and neither the placebo nor the treatment groups deteriorated during the course of the trial.
In summary, experience with the clinical trials in MCI performed so far has shown that even though they were using the same criteria for amnestic MCI, which were created to increase specificity and reduce the heterogeneity of MCI, various studies were recruiting different samples with respect to the “conversion” rates to dementia and other biological characteristics such as APOE genotype. The lack of effects on symptom progression questions not only the clinical efficacy of the evaluated agents in MCI but also the sensitivity of the outcome measures used in the trials and calls for more effective and reliable markers of disease progression. The fact that none of the drugs previously shown to have clinical efficacy in AD trials as well as benefit in everyday practice have met the primary objectives of the respective trials, indicates that the clinical trial design in MCI has to be further developed with special attention being paid to the selection of more homogeneous samples at entry, optimal treatment duration, and multidimensional and reliable outcomes. Both validation of natural cohorts of MCI subjects followed for longer in clinical settings and biological markers are needed. Because of these unresolved issues, it is still premature to conclude that lack of proof of efficacy in the MCI trials performed so far confirms a definite lack of efficacy of the therapeutic agents being evaluated.
Electronic‐database information
The NIH website on drug trials can be found at www.clinicaltrials.gov.
Copyright © 2006 BMJ Publishing Group
Abbreviations
AChEI - acetylcholinesterase inhibitors
AD - Alzheimer's disease
ADAS‐Cog - Alzheimer's Disease Assessment Scale, Cognitive subscale
ADCS - Alzheimer Disease Cooperative Study
CBCS - Cognitive Battery Composite Score
CDR - Clinical Dementia Rating scale
CGIC - Clinical Global Impression of Change
CIBIC - Clinician's Interview‐Based Impression of Change
CIBIC‐plus - Clinician's Interview‐Based Impression of Change with caregiver input
DSST - Digit Symbol Substitution Test
FDA - Food and Drug Administration
GDS - Global Deterioration Scale
HR - hazards ratio
InDDEx - Investigation into the Delay to Diagnosis of AD with Exelon
ITT - intention‐to‐treat
MCI - mild cognitive impairment
MIS - Memory Impairment Study
MMSE - Mini‐Mental State Examination
NYU - New York University
PAS - Preclinical Alzheimer's Disease scale
PP - per protocol
RCT - randomised clinical trial
WAIS‐R - Wechsler Adult Intelligence Scale–Revised
WMS‐III - Wechsler Memory Scale, Third Edition
Footnotes
Competing interests: the authors have not received research grants from any of the pharmaceutical companies marketing anti‐dementia drugs, neither have they have stocks in any of these companies. Dr Bengt Winblad received consultancy fees for advisory board meetings from most companies marketing anti‐dementia drugs. Dr Miia Kivipelto received consulting fees for an advisory board meeting from Pfizer. All authors received fees for lecturing or organising education sponsored by Novartis.
The NIH website on drug trials can be found at www.clinicaltrials.gov.
References
- 1.Schneider L S. Current therapeutic trials. In: Iqbal K, Winblad B, eds. Alzheimer's disease and related disorders. Research advances. Bucharest: “Ana Aslan” International Academy of Aging, 2003639–651.
- 2.Petersen R C. Mild cognitive impairment trials. Nat Rev Drug Discov 20032646–653. [DOI] [PubMed] [Google Scholar]
- 3.Petersen R C, Grundman M, Thomas R.et al Donepezil and vitamin E as treatments for mild cognitive impairment. Neurobiol Aging 200425(Suppl 2)S20 [Google Scholar]
- 4.Gold M, Francke S, Nye J S.et al Impact of APOE genotype on the efficacy of galantamine for the treatment of mild cognitive impairment. Neurobiol Aging 200425(Suppl 2)S521 [Google Scholar]
- 5.Goldstein H R, Gold M. Galantamine in the treatment of patients with mild cognitive impairment: baseline demographics and psychometric testing results. Neurobiol Aging 200425(Suppl 2)S472–S473. [Google Scholar]
- 6.Novak G. Galantamine in the treatment of patients with mild cognitive impairment: effect of diagnostic type at baseline. Neurobiol Aging 200425(Suppl 2)S474 [Google Scholar]
- 7.Scheltens P, Fox N C, Barkhof F.et al Effect of galantamine on brain atrophy as assessed by MRI in patients with mild cognitive impairment. Neurobiol Aging 200425(Suppl 2)S270–S271. [Google Scholar]
- 8.Palmer K, Fratiglioni L, Winblad B. What is mild cognitive impairment? Variations in definitions and evolution of nondemented persons with cognitive impairment. Acta Neurol Scand 2003107(Suppl 179)14–20. [DOI] [PubMed] [Google Scholar]
- 9.Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr 200416129–140. [DOI] [PubMed] [Google Scholar]
- 10.Petersen R C, Smith G E, Waring S C.et al Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 199956303–308. [DOI] [PubMed] [Google Scholar]
- 11.Petersen R C. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004256183–194. [DOI] [PubMed] [Google Scholar]
- 12.Gauthier S, Touchon J. Subclassification of mild cognitive impairment in research and clinical practice. In: Gauthier S, Scheltens P, Cummings J, eds. Alzheimer's disease and related disorders. London, UK: Martin Dunitz, 200461–69.
- 13.Gauthier S, Reisberg B, Zaudig M.et al Mild cognitive impairment. Lancet 2005. (in press) [DOI] [PubMed]
- 14.Ganguli M, Dodge H H, Shen C.et al Mild cognitive impairment, amnestic type. An epidemiologic study. Neurology 200463115–121. [DOI] [PubMed] [Google Scholar]
- 15.Rasquin S M C, Lodder J, Visser P J.et al Predictive accuracy of MCI subtypes for Alzheimer's disease and vascular dementia in subjects with mild cognitive impairment: a 2‐year follow‐up study. Dement Geriatr Cogn Disord 200519113–119. [DOI] [PubMed] [Google Scholar]
- 16.Golomb J, Kluger A, Ferris S H. Mild cognitive impairment: historical development and summary of research. Dialogues Clin Neurosci 20046351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winblad B, Palmer K, Kivipelto M.et al Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004256240–246. [DOI] [PubMed] [Google Scholar]
- 18.Arnaiz E, Almkvist O. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease. Acta Neurol Scand 2003107(Suppl 179)34–41. [PubMed] [Google Scholar]
- 19.Blennow K. CSF biomarkers for mild cognitive impairment. J Intern Med 2004256224–234. [DOI] [PubMed] [Google Scholar]
- 20.Wolf H, Jelic V, Gertz H‐J.et al A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand 2003107(Suppl 179)52–76. [DOI] [PubMed] [Google Scholar]
- 21.DeCarli C, Miller B L, Swan G E.et al Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol 200158643–647. [DOI] [PubMed] [Google Scholar]
- 22.Kordower J H, Chu Y, Stebbins G T.et al Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol 200149202–213. [PubMed] [Google Scholar]
- 23.Mufson E J, Ma S Y, Dills J.et al Loss of basal forebrain P75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer's disease. J Comp Neurol 2002443(2)136–153. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell T W, Mufson E J, Schneider J A.et al Parahippocampal tau pathology in healthy aging, mild cognitive impairment and early Alzheimer's disease. Ann Neurol 200251182–189. [DOI] [PubMed] [Google Scholar]
- 25.Knopman D S, Parisi J E, Salviati A.et al Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 2003621087–1095. [DOI] [PubMed] [Google Scholar]
- 26.Snowdown D A. Healthy aging and dementia: findings from the Nun Study. Ann Intern Med 2003139450–454. [DOI] [PubMed] [Google Scholar]
- 27.Grundman M, Petersen R C, Ferris S H.et al Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol 20046159–66. [DOI] [PubMed] [Google Scholar]
- 28.Jelic V, Winblad B. Treatment of mild cognitive impairment: rationale, present and future strategies. Acta Neurol Scand 2003107(Suppl 179)83–93. [DOI] [PubMed] [Google Scholar]
- 29.Winblad B, Engedal K, Soininen H.et al A 1‐year, randomized, placebo‐controlled study of donepezil in patients with mild to moderate AD. Neurology 200157489–495. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto M, Kazui H, Matsumoto K.et al Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer's disease? Am J Psychiatry 2005162676–682. [DOI] [PubMed] [Google Scholar]
- 31.Winblad B, Jelic V. Long‐term treatment of Alzheimer disease: efficacy and safety of acetylcholinesterase inhibitors. Alzheimer Dis Assoc Disord 200418(Suppl 1)S2–S8. [DOI] [PubMed] [Google Scholar]
- 32.Engelhart M J, Geerlings M I, Ruitenberg A.et al Dietary intake of antioxidants and risk of Alzheimer's disease. JAMA 2002287323–329. [DOI] [PubMed] [Google Scholar]
- 33.Perrig W J, Perrig P, Stähelin H B. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc 199745718–724. [DOI] [PubMed] [Google Scholar]
- 34.Sano M, Ernesto C, Thomas R G.et al A controlled trial of selegiline, alpha‐tocopherol, or both as treatment for Alzheimer's disease. N Engl J Med 19973361216–1222. [DOI] [PubMed] [Google Scholar]
- 35.Le Bars P L, Velasco F M, Ferguson J M.et al Influence of the severity of cognitive impairment on the effect of the Ginko biloba extract Egb 761 in Alzheimer's disease. Neuropsychobiology 20024519–26. [DOI] [PubMed] [Google Scholar]
- 36.Akiyama H, Berger S, Barnum S.et al Inflammation and Alzheimer's disease. Neurobiol Aging 200021(3)383–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aisen P S, Schafer K A, Grundman M.et al Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 2003289(21)2819–2826. [DOI] [PubMed] [Google Scholar]
- 38.Crook T, Bartus R T, Ferris S H.et al Age‐associated memory impairment: proposed diagnostic criteria and measures of clinical change. Report of a National Institute of Mental Health work group. Dev Neuropsychol 19862261–276. [Google Scholar]
- 39.Schneider L S, Olin J T. Clinical global impressions in clinical trials. Int Psychogeriatr 19978277–290. [DOI] [PubMed] [Google Scholar]
- 40.Waegemans T, Wilsher C R, Danniau A.et al Clinical efficacy of piracetam in cognitive impairment: a meta‐analysis. Dement Geriatr Cogn Disord 200213(4)217–224. [DOI] [PubMed] [Google Scholar]
- 41.Croisile B, Trillet M, Fondarai M.et al Long‐term and high‐dose piracetam treatment of Alzheimer's disease. Neurology 199343301–305. [DOI] [PubMed] [Google Scholar]
- 42.Winblad B. Piracetam: a review of pharmacological properties and clinical uses. CNS Drug Rev 200511169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubois B, Verstichel P. Issues in diagnosis, therapeutic strategies and management of MCI disease in 2003. Results of an international survey. MCI Forum 200329–11. [Google Scholar]
- 44. FDA Memorandum: Issues paper on mild cognitive impairment, Peripheral and Central Nervous System Advisory Committee Meeting, March 13, 2001. Available at http://www.fda.gov/ohrms/dockets/ac/01/briefing/3724b1_01_MCIposition.pdf
- 45.Morris J C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993432412–2414. [DOI] [PubMed] [Google Scholar]
- 46.Reisberg B, Ferris S H, de Leon M J.et al The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 19821391136–1139. [DOI] [PubMed] [Google Scholar]
- 47.Schneider L S, Olin J T, Doody R S.et al Validity and reliability of the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 199711(Suppl 2)S22–S32. [DOI] [PubMed] [Google Scholar]
- 48.Mohs R C, Knopman D, Petersen R C.et al Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord 199711(Suppl 2)13–21. [PubMed] [Google Scholar]
- 49.Winblad B, Brodaty H, Gauthier S.et al Pharmacotherapy of Alzheimer's disease: is there a need to redefine treatment success? Int J Geriatr Psychiatry 200116653–666. [DOI] [PubMed] [Google Scholar]
- 50.Rothwell P M. Responsiveness of outcome measures in randomised controlled trials in neurology. J Neurol Neurosurg Psychiatry 200068274–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DeLeon M J, Golomb J, George A E.et al The radiologic prediction of Alzheimer's disease: the atrophic hippocampal formation. Am J Neuroradiol 199314897–906. [PMC free article] [PubMed] [Google Scholar]
- 52.Visser P J, Verhey F R J, Hofman P A M.et al Medial temporal lobe atrophy predicts Alzheimer's disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry 200272491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montori V M, Guyatt G H. Intention‐to‐treat principle. CMAJ 20011651339–1341. [PMC free article] [PubMed] [Google Scholar]
- 54.Whitehouse P J, Kittner B, Roessner M.et al Clinical trial designs for demonstrating disease course‐altering effects in dementia. Alzheimer Dis Assoc Disord 199812281–294. [DOI] [PubMed] [Google Scholar]
- 55.Geda Y E, Petersen R C. Clinical trials in mild cognitive impairment. In: Gauthier S, Cummings JL, eds. Alzheimer's disease and related disorders annual. London, UK: Martin Dunitz, 200169–83.
- 56.Petersen R C, Thomas R G, Grundman M.et al Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005352(23)2379–2388. [DOI] [PubMed] [Google Scholar]
- 57.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease. Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 58.Salloway S, Ferris S, Kluger A.et al Efficacy of donepezil in mild cognitive impairment: a randomised placebo‐controlled trial. Neurology 200463651–657. [DOI] [PubMed] [Google Scholar]
- 59.Whitehead A, Perdomo C, Pratt R D.et al Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer's disease: a meta‐analysis of individual patient data from randomized controlled trials. Int J Geriatr Psychiatry 200419624–633. [DOI] [PubMed] [Google Scholar]
- 60.Farlow M R, He Y, Tekin S.et al Impact of APOE in mild cognitive impairment. Neurology 2004631898–1901. [DOI] [PubMed] [Google Scholar]
- 61. Johnson & Johnson Pharmaceutical Research and Development, L.L.C. Synopsis. GAL‐INT‐11. http://www.clinicalstudyresults.org/documents/company‐study_96_1.pdf
- 62. Johnson & Johnson Pharmaceutical Research & Development, L.L.C. Synopsis. GAL‐INT‐18. http://www.clinicalstudyresults.org/documents/company‐study_96_2.pdf
- 63.Thal L J, Ferris S H, Kirby L.et al A randomized, double‐blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology 200530(6)1204–1215. [DOI] [PubMed] [Google Scholar]
- 64.Visser P J, Scheltens P, Verhej F R J. Do MCI criteria in drug trials accurately identify subjects with predementia Alzheimer's disease? J Neurol Neurosurg Psychiatry 2005761348–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubinsztein D C, Easton D. Apolipoprotein E genetic variation and Alzheimer's disease. Dement Geriatr Cogn Disord 199910199–209. [DOI] [PubMed] [Google Scholar]
- 66.Mohs R C. The Alzheimer's disease assessment scale. Int Psychogeriatr 19968195–203. [DOI] [PubMed] [Google Scholar]
- 67.Graham D P, Cully J A, Snow A L.et al The Alzheimer's disease assessment scale‐cognitive subscale. Normative data for older adult controls. Alzheimer Dis Assoc Disord 200418236–240. [PubMed] [Google Scholar]
- 68.Knopman D, Gracon S. Observations on the short‐term “natural history” of probable Alzheimer's disease in a controlled trial. Neurology 199444260–265. [DOI] [PubMed] [Google Scholar]
- 69.Schneider L S, Olin J T, Lyness S A.et al Eligibility of Alzheimer's disease clinic patients for clinical trials. J Am Geriatr Soc 199745923–928. [DOI] [PubMed] [Google Scholar]
- 70.Gearing M, Mirra S S, Hedreen J C.et al The consortium to establish a registry for Alzheimer's disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer's disease. Neurology 199545461–466. [DOI] [PubMed] [Google Scholar]
- 71.Visser P J, Verhey F R, Scheltens P.et al Diagnostic accuracy of the Preclinical AD Scale (PAS) in cognitively mildly impaired subjects. J Neurol 2002249312–319. [DOI] [PubMed] [Google Scholar]
- 72.Dubois B. “Prodromal Alzheimer's disease”: a more useful concept than mild cognitive impairment? Curr Opin Neurol 200013367–369. [DOI] [PubMed] [Google Scholar]
- 73.Petersen R C, Smith G E, Ivnik R J. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory‐impaired individuals. JAMA 19952731274–1278. [PubMed] [Google Scholar]
- 74.Farrer L A, Cuppies L A, Haines J L.et al Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA 19972781349–1356. [PubMed] [Google Scholar]
- 75.Raber J, Huang Y, Ashford J W. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging 200425621–650. [DOI] [PubMed] [Google Scholar]
- 76.Caselli R J, Reiman E M, Osborne D.et al Longitudinal changes in cognition and behaviour in asymptomatic carriers of the APOE e4 allele. Neurology 2004621990–1995. [DOI] [PubMed] [Google Scholar]
- 77.DeKosky S T, Ikonomovic M D, Styren S D.et al Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 200251(2)145–155. [DOI] [PubMed] [Google Scholar]
- 78.Blacker D. Mild cognitive impairment – no benefit from vitamin E, little from donepezil. N Engl J Med 2005352(23)2439–2441. [DOI] [PubMed] [Google Scholar]
- 79.Poirier J, Delisle M‐C, Quirion R.et al Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer's disease. Proc Natl Acad Sci U S A 19959212260–12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aerssens J, Raeymaekers P, Lilienfeld S.et al APOE genotype: no influence on galantamine treatment efficacy nor on rate of decline in Alzheimer's disease. Dement Geriatr Cogn Disord 20011269–77. [DOI] [PubMed] [Google Scholar]
- 81.Rigaud A‐S, Traykov L, Latour F.et al Presence or absence of at least one E4 allele and gender are not predictive for the response to donepezil treatment in Alzheimer's disease. Pharmacogenetics 200212415–420. [DOI] [PubMed] [Google Scholar]
- 82.Rockwood K. Size of treatment effect on cognition of cholinesterase inhibition in Alzheimer's disease. J Neurol Neurosurg Psychiatry 200475677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fay S, Rockwood K. Do standard anti‐dementia drug trial tests capture symptoms that are important to patients? Neurobiol Aging 200425(Suppl 2)S325 [Google Scholar]