Abstract
Background
Many bacterial meningitis patients experience neurological or neuropsychological sequelae, predominantly deficits in short‐term memory, learning, and attention. Neuropsychological symptoms after viral meningitis are observed less frequently. Sleep disturbance has been reported after both viral and bacterial meningitis.
Objectives
To examine systematically the frequency and extent of sleep disturbance in meningitis patients.
Methods
Eighty six viral or bacterial meningitis (onset of acute disease at least 1 year previously) patients were examined using two standardised questionnaires (Schlaffragebogen B and the Pittsburgh Sleep Quality Index, PSQI) in conjunction with a standardised neurological examination, and compared to a control group of 42 healthy age‐matched volunteers.
Results
Patients after both viral and bacterial meningitis described their sleep as reduced in quality and less restful than that of healthy control subjects; both patient groups had a pathological mean PSQI total score. Impaired sleep scores after meningitis were not correlated to either the Glasgow Coma Scale or the Glasgow Outcome Scale. Moreover, no relationship between residual neurological dysfunction or depressivity and sleep quality was observed.
Conclusions
Impaired sleep is a long‐term consequence of meningitis. Additional, so far undetermined, factors other than the severity of concomitant neurological deficits are responsible for the development of this sequela.
Keywords: bacterial meningitis, Pittsburgh Sleep Quality Index, Schlaffragebogen B, sequelae, sleep impairment, viral meningitis
Insomnia is of clinical relevance due to both the direct burden of sleep disturbance and possible effects on quality of life of the individual patient. Insomnia is a risk factor for the later development of depression1 and other mental and physical illnesses,2 and people with insomnia have higher rates of health care utilisation.2 Insomnia also affects the workforce and is associated with an increased risk of accidents.3 It is therefore important to identify those at risk for insomnia. Although complaints of disturbed sleep are common4,5 in the general population, the frequency of severe insomnia (diagnosed according to DSM‐IV criteria) is low and affects only 4% of the German population.6
Neurological and neuropsychological sequelae frequently occur after bacterial meningitis (BM) and have been studied extensively.7,8 Neurological long‐term consequences following viral meningitis (VM) without encephalitis are rare but have been reported in some studies.9,10,11 Sleep disturbance following BM has been observed after Listeria rhombencephalitis12 and pneumococcal meningitis with brain stem lesions.13 VM induced a fatigue syndrome in two thirds of patients re‐examined 3 month after the acute stage of the disease, although after 1 year most patients had recovered.14 In animal models, exposure to bacterial cell wall components (for example, peptidoglycans, teichoic acid, endotoxin, and muramyl peptides) induced alterations in sleep patterns.15,16,17,18 In human volunteers, injection of endotoxin increased serum concentrations of TNF‐α, IL‐6, and IL‐1 receptor antagonist, increased EEG arousals in stage II sleep, and decreased arousals in slow wave sleep. In contrast to studies in rats15,19 and rabbits,17 endotoxin did not alter rapid eye movement (REM) sleep in humans, the duration of non‐REM sleep, or nocturnal wakefulness.20
The aim of this questionnaire study was to determine the potential impact of meningitis on persistent sleep disturbance in patients with a history of BM or VM.
Methods
This study was approved by the Ethics Committee of the University Hospital Goettingen, and prior informed consent was obtained from each participating patient or control group member.
Patients
Patients who had been treated in the University Hospital Goettingen for either definite VM or BM were contacted by post. Patients were excluded if they were younger than 16 years or had viral meningoencephalitis, addictive disorders, or any neurological or psychiatric disease not resulting directly from meningitis. The interval between treatment and contact was at least 1 year (to ensure complete rehabilitation) and not more than 12 years.
The diagnosis of BM was based on definite microbiological results (CSF culture, CSF microscopy, Gram stain) or on at least two clinical and laboratory results characteristic for BM (CSF pleocytosis ⩾1000/µl, CSF lactate ⩾3.0 mmol/l, serum CRP ⩾100 mg/dl).
As the search for causative agents in the VM group was focused on treatable viruses, namely Herpes viridae, the causative virus was identified in only 16 patients. To ensure that only those with VM were studied, patients taking antibiotics were excluded.
In the letter of contact, patients were asked to complete several questionnaires and attend the hospital for a neurological re‐examination including assessment according to the Glasgow Outcome Scale (GOS). Formal informed consent was obtained for the 86 patients who took part in the study (38 patients with BM and 48 with VM). For each patient, the Glasgow Coma Scale (GCS) score and neurological status (no neurological deficits, neurological deficits caused by meningitis, and neurological deficits caused by conditions other than meningitis) at original admission were obtained from the files.
Control group
A control group of 42 volunteers was recruited from relatives and friends of members of the study group. The following diseases or conditions were considered as exclusion criteria:
Subjective sleep disturbance
Diseases of the airways or lungs
Heart failure
History of myocardial infarction
History of neurological disorders of any origin
History of psychiatric disease including addiction to any substance
Shift work
The presence of children ⩽4 years old in the same household
Questionnaires
The Pittsburgh Sleep Quality Index (PSQI)5,21 and the Schlaffragebogen B (sleep questionnaire B; Sf‐B)22 were administered. Both questionnaires refer to the previous 2 weeks.
The PSQI consists of 19 questions, 18 of which build the following seven subscores:
Subjective quality of sleep
Sleep latency
Sleep duration
Sleep efficiency
Sleep disturbance
Use of sleeping medications
Daytime fatigue
The sum of these seven subscores yields the PSQI total score. Each subscore has a score of 0 to 3, giving a total PSQI score of 0 to 21. A total PSQI score exceeding 5 indicates disturbed sleep.21 Sf‐B comprises 28 self rating questions, giving rise to five scales: global sleep quality, feeling refreshed after sleep, feeling well‐balanced in the evening, mental exhaustion in the evening, and psychosomatic symptoms during sleep. Each scale is weighed and inversely transformed, where 1 is assigned to severe and 5 to no symptoms.
To rule out depression as a confounding factor, Beck's Depression Inventory (BDI)23 was completed by all meningitis patients.
Statistics
Whenever possible, data from questionnaires were presented as mean±standard deviation (SD). Because of the ordinal character of the scales, results were compared with ANOVA on ranks, and p values were adjusted for repeated testing with Dunn's correction. Patients' questionnaire results were compared to those of an age matched group of healthy volunteers.
For correlation analysis, Spearman's rank correlation coefficient was used. Differences in frequency distribution were examined by Fisher's exact test. Statistical significant was set at p<0.05.
Results
Study population
The VM patients did not differ from the BM patients as regards age or gender distribution (mean±SD: 44.1±15.8 v 41.3±11.8 years, NS; female/maleVM: 20/28, female/maleBM: 17/21, p = 0.57). Also, the age and gender distribution of both groups were not different from those of the control group (41.3±15.9 years; p = 0.23, female/malecontrol: 18/24; p = 0.90).
The BM group contained 10 patients who had had Streptococcus pneumoniae meningitis, 11 who had had Neisseria meningitidis meningitis, and 8 who were infected with other bacteria (Staphylococcus aureus, Listeria monocytogenes, or Streptococcus spp). In nine patients, the causative bacterium could not be identified by microscopy, culture, or agglutination tests, so the diagnosis of BM was based on clinical and laboratory information as described above (table 1).
Table 1 Microbiological findings in the patient groups.
| n | % | |
|---|---|---|
| Causative bacterium | ||
| S pneumoniae | 10 | 26.3 |
| N meningitidis | 11 | 28.9 |
| S aureus | 1 | 2.6 |
| Streptococci | 4 | 10.5 |
| L monocytogenes | 3 | 7.9 |
| Unknown bacteria | 9 | 23.7 |
| Total | 38 | 100 |
| Causative virus | ||
| Varicella‐zoster virus | 7 | 14.6 |
| Mumps virus | 3 | 6.3 |
| Epstein‐Barr virus | 3 | 6.3 |
| Cytomegalovirus | 1 | 2.1 |
| Enterovirus* | 2 | 4.2 |
| Unknown virus | 32 | 66.7 |
| Total | 48 | 100 |
*Enterovirus polymerase chain reaction was not routinely performed.
Clinical data
At hospital admission the GCS score of BM patients was worse than that of VM patients (13.5±2.6BMv 14.2±2.3VM; p<0.001). None of the VM patients displayed a lowered GOS score (4.8±0.4BMv 5.0±0.0VM; p = 0.0002). The frequency of neurological deficits at presentation (NBM = 24/38, 78% v NVM = 18/48, 38%) and at re‐evaluation (NBM = 24/38, 63% v NVM = 10/48, 21%) was significantly higher in the BM group than in the VM group (p = 0.0002).
When asked directly about sleep disturbance at re‐evaluation, 13 of 48 (27.1%) VM and 12 of 38 (31.6%) BM patients said they suffered from sleep impairment (NS). However, of those complaining of sleep disorders, more VM (7/13, 54%) than BM patients (3/12, 25%) felt that this symptom was definitely caused by the meningitis (non‐significant difference because of the small group size).
Sleep latency did not differ between the groups (22:53±42 minBM; 23:04±55 minVMv 23:05±55 mincontrol; p = 0.84).
The mean sleep duration of the VM patients was slightly longer than in the BM group but the difference failed to reach statistical significance when compared directly (7.9±1.4 hVM, 7.7±1.5 hBMv 7.9±0.9 hcontrol; p = 0.24). However, after transformation of the raw values of sleep duration into the respective PSQI subscale, the results indicated significantly longer sleep duration for the VM group than for the BM group.
Patients who had had S pneumoniae meningitis were compared to those who had had N meningitidis meningitis. Significant differences could only be detected for the GOS (which was lower for the former group, as expected).
Questionnaires
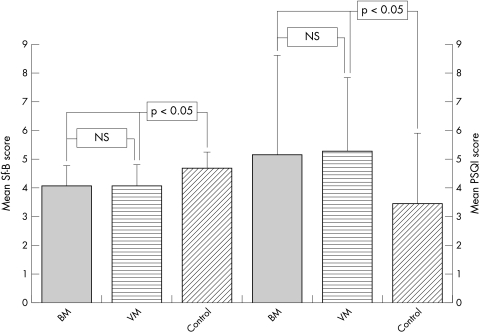
Compared to the healthy controls, the Sf‐B questionnaire identified differences in both the VM and BM groups as regards global sleep quality (fig 1), feeling of refreshment after sleep, and mental stability in the evening, but not mental exhaustion in the evening. The scales for psychosomatic symptoms during sleep indicated more pychosomatic symptoms in the VM group than in the BM group. However, psychosomatic symptoms during sleep in the BM and VM groups did not differ significantly from the control group.
Figure 1 Mean Sf‐B sleep quality scale score (left) and PSQI total score (right) for both BM and VM patients and the control group of healthy adults (mean±SD; *p<0.05).
The PSQI total score (fig 1) and subjective quality of sleep for both the VM and BM groups were significantly different from the control group, demonstrating the perception of impaired sleep in patients. The scales for sleep duration and sleep disturbance were higher (that is, more impaired) for VM patients (but not for BM patients) compared to the control group. Patient groups were comparable to the control group for the variables sleep latency, sleep efficiency, use of sleeping medications, and the extent of daytime fatigue (table 2).
Table 2 Descriptive and comparative statistics of sleep questionnaires.
| BM | VM | Control | ANOVA, p value | |
|---|---|---|---|---|
| PSQI | ||||
| PSQI total score | 5.18±3.46* | 5.29±2.56* | 3.48±2.44 | <0.01 |
| Subjective quality of sleep | 1.05±0.65* | 1.09±0.70* | 0.55±0.63 | <0.01 |
| Sleep duration scale | 0.55±0.86*† | 0.80±0.93† | 0.33±0.57 | 0.04 |
| Sleep disturbance | 1.18±0.66 | 1.17±0.63* | 0.90±0.43 | 0.04 |
| Sf‐B | ||||
| Global sleep quality | 4.10±0.69* | 4.08±0.74* | 4.71±0.55 | <0.01 |
| Feeling refreshed after sleep | 3.19±0.94* | 3.18±0.87* | 3.76±0.81 | 0.02 |
| Feeling well‐balanced in the evening | 3.04±0.63* | 2.96±0.79* | 3.98±0.63 | <0.01 |
Only significantly different subtests were integrated into the table. Values are mean±SD.
*Significantly different as compared to the control group; †significant difference between the patient groups.
A comparison of patients with S pneumoniae versus N meningitidis meningitis did not reveal important differences except for the Sf‐B scale mental exhaustion in the evening, which was 2.9±1.0 for patients who had had pneumococcal meningitis and 3.8±0.7 for those who had had meningococcal meningitis (p = 0.027).
According to the given cut‐off values for BDI (⩽11: “not depressive”, 12–19: “mild depression”, 20–26: “moderate depression”, ⩾26: “severe depression”) generally neither BM patients (5.1±7.2) nor VM patients (5.0±5.6) considered themselves as depressive. The VM and BM groups did not differ significantly from each other concerning their BDI depression scores (p = 0.92).
Correlations
The GCS scores at admission correlated significantly with the GOS scores at re‐evaluation (Spearman's rank correlation coefficient rs = 0.31; p = 0.004). Neither GCS nor any other clinical score (GOS, neurological symptoms at admission and at re‐evaluation) were substantially correlated with the results of the sleep questionnaires.
Discussion
Several neurological diseases are associated with an increased incidence of concomitant sleep disturbance. In addition to the known sleep related neurological disorders (that is, restless legs syndrome, narcolepsy, and fatal familial insomnia), other neurological diseases associated with sleep disturbance are Parkinson's disease,24,25 headache,26 multiple sclerosis,27,28 hereditary spinocerebellar ataxia,29 Huntington's disease,30 myasthenia gravis,31 and myotonia congenita.32,33
Decreased sleep quality has been frequently reported by survivors of meningitis in our outpatients clinic. Several clinical studies have dealt with the frequency and severity of neurological and neuropsychological sequelae after BM8,34,35 and VM.10,11 Yet, apart from a study by Hodgson et al who found that relatives report insomnia in survivors of meningococcal meningitis,36 and a follow‐up study with few participants,7 to the best of our knowledge, no data on the sleep quality of meningitis victims have been published.
Studies in which animals were exposed to bacterial cell wall components demonstrated marked alterations in sleep patterns. In humans, however, the effect of endotoxin on sleep patterns was less pronounced.20 Furthermore, there are no studies in either animals or humans on the long‐term effects on sleep of cerebral exposure to bacterial components.
Therefore, we examined the extent and frequency of persistent sleep disorders after meningitis. Sleep disturbance was significantly more frequent in survivors of meningitis than in a control population of healthy subjects. In 49 of 84 participating meningitis patients (58%), the mean PSQI score was ⩾5, demonstrating the severity of sleep disturbance.
To our knowledge, the morphological basis of sleep disturbance after meningitis has not yet been discovered.
Alterations in sleep patterns have been reported both in the acute stage of severe sepsis and after discharge from the intensive care unit. The circadian rhythm of melatonin secretion was abnormal in these patients, while critically ill patients without sepsis displayed preserved melatonin release.37
A possible reason for the sleep alterations induced by meningitis might be the preponderance of hyperexcitatory amino acids in acute inflammatory diseases of the brain.38 The relevance of excitatory amino acids for the clinical outcome of patients with BM has been demonstrated.39 In infectious diseases of the central nervous system, the kynurenine pathway is impaired, leading to an excess of quinolinic acid and glutamate.40 Both substances are able to induce neuronal cell death via the stimulation of NMDA receptors. Although the concentration of CSF kynurenic acid (an antagonist of the NMDA receptor41) is increased in BM, this increase does not outweigh the effect of increased NMDA receptor agonists.42 Simultaneously, the concentration of l‐tryptophan is decreased in meningitis.40l‐tryptophan is important for the synthesis of serotonin. The suppression of serotonin synthesis by the inhibition of tryptophan hydroxylase induces short lived total insomnia in the cat.43
Neuronal decay in brain regions that are important for the sleep‐wake rhythm might be another factor inducing sleep impairment in meningitis patients.
In this study, the decreased sleep quality could not be explained by environmental factors (for example, children or snoring partners) or the use of hypnotics, as items controlling (PSQI sleep duration and PSQI use of sleeping medications) for these parameters as well as for depressive mood disorders (BDI) did not differ from the control group.
From a clinical point of view, it is surprising that VM and BM patients were equally affected by sleep complaints, since the latter more often showed neurological symptoms and poor clinical outcome. An explanation for this could be that, as in BM, glutamate is released into the CSF in patients with VM.44
Neither the GCS scores nor the degree of neurological dysfunction on admission and at re‐evaluation were statistically linked to the extent of sleep dysfunction. Even when only BM patients were included into the statistical analysis, clinical scores did not correlate with the outcome of sleep questionnaires. At present, we are not able to explain this finding.
In conclusion, sleep disturbance is a frequent long‐term consequence of both VM and BM regardless of the severity of neurological symptoms. Subtle structural lesions in otherwise neurologically mute brain regions or simply the psychological stressor of a life threatening disease might be responsible for this persistent residual sequela.
Acknowledgements
We thank Mrs C Crozier for careful revision of the manuscript.
Abbreviations
BDI - Beck's Depression Inventory
BM - bacterial meningitis
GCS - Glasgow Coma Scale
GOS - Glasgow Outcome Scale
PSQI - Pittsburgh Sleep Quality Index
REM - rapid eye movement
SD - standard deviation
Sf‐B - Schlaffragebogen B questionnaire
VM - viral meningitis
Footnotes
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG Na 165/5‐1)
Competing interests: none declared
References
- 1.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord 200376255–259. [DOI] [PubMed] [Google Scholar]
- 2.Benca R. Consequences of insomnia and its therapies. J Clin Psychiatry 20016233–38. [PubMed] [Google Scholar]
- 3.Leger D, Guilleminault C, Bader G.et al Medical and socio‐professional impact of insomnia. Sleep 200225625–629. [PubMed] [Google Scholar]
- 4.Weyerer S, Dilling H. Prevalence and treatment of insomnia in the community: results from the upper Bavarian field study. Sleep 19912127–36. [PubMed] [Google Scholar]
- 5.Zeitlhofer J, Schmeiser‐Rieder A, Tribl G.et al Sleep and quality of life in the Austrian population. Acta Neurol Scand 2000102249–257. [DOI] [PubMed] [Google Scholar]
- 6.Hajak G. Epidemiology of severe insomnia and its consequences in Germany. Eur Arch Psychiatry Clin Neurosci 200125149–56. [DOI] [PubMed] [Google Scholar]
- 7.Merkelbach S, Sittinger H, Schweizer I.et al Cognitive outcome after bacterial meningitis. Acta Neurol Scand 2000102118–123. [DOI] [PubMed] [Google Scholar]
- 8.Grimwood K, Anderson V, Bond L.et al Adverse outcomes of bacterial meningitis in school‐age survivors. Pediatrics 199595646–656. [PubMed] [Google Scholar]
- 9.Hotopf M, Noah N, Wessely S. Chronic fatigue and minor psychiatric morbidity after viral meningitis: a controlled study. J Neurol Neurosurg Psychiatry 199660504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaiser R, Vollmer H, Schmidtke K.et al Verlauf und Prognose der FSME‐Enzephalitis. Nervenarzt 199768324–330. [DOI] [PubMed] [Google Scholar]
- 11.Günther G, Haglund M, Lindquist L.et al Tick‐borne encephalitis in Sweden in relation to aseptic meningo‐encephalitis of other etiology: a prospective study of clinical course and outcome. J Neurol 1997244230–238. [DOI] [PubMed] [Google Scholar]
- 12.Milhaud D, Bernardin G, Roger P.et al [Central apnea with consciousness impairment due to Listeria rhombencephalitis sequelae]. Rev Neurol (Paris) 1999155152–154. [PubMed] [Google Scholar]
- 13.Hasegawa T, Kohyama J, Kohji T.et al Impairment of respiratory rhythmogenesis and sequelae of bacterial meningitis. Pediatr Neurol 199512357–360. [DOI] [PubMed] [Google Scholar]
- 14.Lepow M, Coyne N, Thompson L. A clinical epidemiologic and laboratory investigation of aseptic meningitis during the four year period 1955–1958: II. The clinical disease and its sequelae. N Engl J Med 19622661188–1193. [DOI] [PubMed] [Google Scholar]
- 15.Masek K, Kadlecová O, Petrovický P. The effect of some bacterial products on temperature and sleep in rat. Z Immunitatsforsch Exp Klin Immunol 1975149273–282. [PubMed] [Google Scholar]
- 16.Pollmächer T, Mullington J, Korth C.et al Influence of host defense activation on sleep in humans. Adv Neuroimmunol 19955155–169. [DOI] [PubMed] [Google Scholar]
- 17.Johannsen L, Toth L, Rosenthal R.et al Somnogenic, pyrogenic, and hematologic effects of bacterial peptidoglycan. Am J Physiol 1990258R182–R186. [DOI] [PubMed] [Google Scholar]
- 18.Cady A, Riveau G, Chedid L.et al Interleukin‐1‐induced sleep and febrile responses differentially altered by a muramyl dipeptide derivative. Int J Immunopharmacol 198911887–893. [DOI] [PubMed] [Google Scholar]
- 19.Krueger J, Kubillus S, Shoham S.et al Enhancement of slow‐wave sleep by endotoxin and lipid A. Am J Physiol 1986251R591–R597. [DOI] [PubMed] [Google Scholar]
- 20.Haack M, Schuld A, Kraus T.et al Effects of sleep on endotoxin‐induced host responses in healthy men. Psychosom Med 200163568–578. [DOI] [PubMed] [Google Scholar]
- 21.Buysse D, Reynolds III C, Monk T.et al The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 198928193–213. [DOI] [PubMed] [Google Scholar]
- 22.Görtelmeyer R. On the development of a standardized sleep inventory for the assessment of sleep. In: Kubicki S, Herrmann WM, eds. Methods of sleep research. Stuttgart: Gustav Fischer, 198193–98.
- 23.Beck A, Ward C, Mendelson M.et al An inventory for measuring depression. Arch Gen Psychiatry 19614561–571. [DOI] [PubMed] [Google Scholar]
- 24.Tandberg E, Larsen J, Karlsen K. Excessive daytime sleepiness and sleep benefit in Parkinson's disease: a community‐based study. Mov Disord 199914922–927. [DOI] [PubMed] [Google Scholar]
- 25.Trenkwalder C, Wetter T, Tagaya H.et al Sleep disorders in Parkinson syndromes: the role of the dopaminergic system on motor disturbances and REM‐sleep. Electroencephalogr Clin Neurophysiol 199810750P [Google Scholar]
- 26.Bruni O, Galli F, Guidetti V. Sleep hygiene and migraine in children and adolescents. Cephalgia 19991957–59. [DOI] [PubMed] [Google Scholar]
- 27.Colosimo C, Millefiorini E, Grasso M.et al Fatigue in MS is associated with specific clinical features. Acta Neurol Scand 199592353–355. [DOI] [PubMed] [Google Scholar]
- 28.Ferrini‐Strambi L, Filippi M, Martinelli V.et al Nocturnal sleep study in multiple sclerosis: correlations with clinical and brain magnetic resonance imaging findings. J Neurol Sci 1994125194–197. [DOI] [PubMed] [Google Scholar]
- 29.Schöls L, Haan J, Riess O.et al Sleep disturbance in spinocerebellar ataxias. Neurology 1998511603–1607. [DOI] [PubMed] [Google Scholar]
- 30.Wiegand M, Möller A, Lauer C.et al Nocturnal sleep in Huntington's disease. J Neurol 1991238203–208. [DOI] [PubMed] [Google Scholar]
- 31.Guilleminault C, Philipe P, Robinson A. Sleep and neuromuscular disease: bilevel positive airway pressure by nasal mask as a treatment for sleep disordered breathing in patients with neuromuscular disease. J Neurol Neurosurg Psychiatry 199865225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Meche F, Bogaard J, van der Sluys J.et al Daytime sleep in myotonic dystrophy is not caused by sleep apnoe. J Neurol Neurosurg Psychiatry 199457626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begin P, Mathieu J, Almirall J.et al Relationship between chronic hypercapnia and inspiratory‐muscle weakness in myotonic dystrophy. Am J Resp Crit Care Med 199717492–498. [DOI] [PubMed] [Google Scholar]
- 34.Gomes I, Melo A, Lucena R.et al Prognosis of bacterial meningitis in children. Arq Neuropsiquiatr 199654407–411. [DOI] [PubMed] [Google Scholar]
- 35.Pikis A, Kavaliotis J, Tsikoulas J.et al Long‐term sequelae of pneumococcal meningitis in children. Clin Pediatr (Phila) 19963572–78. [DOI] [PubMed] [Google Scholar]
- 36.Hodgson A, Smith T, Gagneux S.et al Survival and sequelae of meningococcal meningitis in Ghana. Int J Epidemiol 2001301440–1446. [DOI] [PubMed] [Google Scholar]
- 37.Mundigler G, Delle‐Karth G, Koreny M.et al Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med 200230536–540. [DOI] [PubMed] [Google Scholar]
- 38.Spranger M, Krempien S, Schwab S.et al Excess glutamate in the cerebrospinal fluid in bacterial meningitis. J Neurol Sci 1996143126–131. [DOI] [PubMed] [Google Scholar]
- 39.Spranger M, Schwab S, Krempien S.et al Excess glutamate levels in the cerebrospinal fluid predict clinical outcome of bacterial meningitis. Arch Neurol 199653992–996. [DOI] [PubMed] [Google Scholar]
- 40.Heyes M P, Saito K, Crowley J S.et al Quinolinic acid and kynurenine pathway metabolism in inflammatory and non‐inflammatory neurological disease. Brain 19921151249–1273. [DOI] [PubMed] [Google Scholar]
- 41.Leib S, Kim Y, Ferriero D.et al Neuroprotective effect of excitatory amino acid antagonist kynurenic acid in experimental bacterial meningitis. J Infect Dis 1996173166–171. [DOI] [PubMed] [Google Scholar]
- 42.Medana I M, Day N P J, Salahifar‐Sabet H.et al Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J Infect Dis 2003188844–849. [DOI] [PubMed] [Google Scholar]
- 43.Denoyer M, Sallanon M, Kitahama K.et al Reversibility of para‐chlorophenylalanine‐induced insomnia by intrahypothalamic microinjection of L‐5‐hydroxytryptophan. Neuroscience 19892883–94. [DOI] [PubMed] [Google Scholar]
- 44.Shen E, Lai Y, Ho C.et al Excitatory and inhibitory amino acid levels in the cerebrospinal fluids of children with neurological disorders. Acta Paediatr Taiwan 19994065–69. [PubMed] [Google Scholar]