Abstract
Background
Neurocysticercosis is a major cause of epilepsy in developing countries and is endemic in Brazil. To test the hypothesis that the aetiological profile of patients with intractable epilepsy in Brazil includes neurocysticercosis, we conducted a cross sectional study investigating the aetiology of intractable epilepsy.
Methods
A total of 512 patients evaluated at the outpatient clinic for intractable epilepsy at the Ribeirão Preto School of Medicine were included in the survey. Medical intractability was determined on the basis of seizure incidence and severity, and response to appropriate epilepsy management. Neuroimaging included brain CT with non‐contrasted and contrasted phases and high resolution MRI. Patients were divided into neurocysticercosis and non‐neurocysticercosis groups according to previous diagnostic criteria.
Results
The most common epileptogenic lesions were mesial temporal sclerosis (MTS; 56.0%), malformations of cortical development (12.1%), and brain tumours (9.9%). Neuroimaging was normal in 8.7% of patients. Calcifications were found in 27% of patients and were significantly more common in patients with MTS than in those without MTS (p<0.001). Isolated neurocysticercosis was found in only eight patients (1.56%).
Conclusions
These data suggest that neurocysticercosis is an uncommon cause of intractable epilepsy, even in an endemic region such as Brazil, and that it may only represent a coexistent pathology. However, an analysis of our findings reveals that neurocysticercosis was more common in patients with MTS. This finding could suggest either that there is a cause‐effect relationship between MTS and neurocysticercosis, or that MTS and neurocysticercosis co‐vary with a missing variable, such as socio‐economic status.
Keywords: hippocampal sclerosis, initial precipitating injury, intractable epilepsy, neurocysticercosis, neuroimaging
Epilepsy is one of the most common neurological disorders, affecting 1% of the population in developed countries.1 In developing countries, epilepsy is even more frequent,2 and it is estimated that patients from these regions account for about three quarters of all epileptic patients.1 In fact, epidemiological studies indicate higher lifetime prevalence and incidence rates for epilepsy in the general population of Latin America than in countries in the northern hemisphere.3
Trauma and brain infection can cause epilepsy at any age, and may account for the higher incidence of epilepsy in developing countries. For example, a common cause in Latin America is neurocysticercosis, caused by tapeworm infection,4 and malaria and meningitis are common causes in Africa,5 while in India, neurocysticercosis and tuberculosis often lead to epilepsy.6
Neurocysticercosis is an important cause of seizures and other neurological problems in developing countries.7 Neurocysticercosis, although less common, is also becoming a health concern in developed countries, mostly due to the migration of infected persons. Neurocysticercosis was found in 10% of patients with seizures who were evaluated in an emergency department in Los Angeles, and in 6% of patients in New Mexico, qualifying neurocysticercosis as an important emerging infection.8
Epidemiological studies indicate that most patients receiving treatment for epilepsy in the community have long standing epilepsy, which is frequently medically intractable and associated with considerable social handicap.9 The main causes of intractable epilepsy are mesial temporal sclerosis (MTS), brain tumours, and malformations of cortical development (MCD).10 Although studies in endemic regions have indicated that cysticercosis is the leading cause of symptomatic epilepsy, no study has investigated the relationship between calcified cysticercotic lesions and intractable epilepsy.
It would be reasonable to suppose that the aetiological profile of intractable epilepsy in our country includes neurocysticercosis, which is endemic in the region; if this were indeed the case, the aetiological profile would differ from that in developed countries. To test this hypothesis, we carried out a cross sectional study investigating the aetiological profile of intractable epilepsy in a tertiary referral centre, paying special attention to the presence of calcifications and their relationships with various types of epilepsy and cerebral lesions.
Methods
Five hundred and twelve patients (273 women) evaluated at the outpatient clinic for intractable epilepsy at the Ribeirão Preto School of Medicine Hospital and aged >18 years were included in the survey. The study examined the presence or absence of neurocysticercosis in patients with intractable epilepsy. Thus, all patients with intractable epilepsy evaluated at our institution between January and June 2003 who fulfilled the inclusion criteria were included in the study. The outpatient clinic receives referrals of patients with intractable epilepsy from the general epilepsy clinic, general practitioners, hospitals, and specialists from several regions of Brazil. All patients had a comprehensive investigation including clinical and neurological examination, classification of seizure types and epilepsy syndromes, routine scalp EEG recordings, and CT and high resolution MRI studies.
Ascertainment of cases
For this study, medical intractability was determined by the epilepsy program staff on the basis of seizure incidence and severity, and response to appropriate epilepsy management. Intractability was defined as: (i) one or more seizures every 3 months during the last year of follow up or until successful seizure surgery, and (ii) failure of two or more first line antiepileptic drugs at maximum tolerated doses. We excluded patients with clinical or video‐EEG evidence of non‐epileptic seizures.
Of the 512 patients, 374 (73%) underwent long term video‐EEG with ictal and interictal EEG recordings and ictal and interictal SPECT. Written informed consent was obtained, and the study was approved by the local research ethics committee.
Clinical features
Clinical and demographic characteristics included sex, age of epilepsy onset, epilepsy duration, family history of seizures in first degree relatives, the presence of initial precipitating injury (IPI), and MRI findings. Patients submitted to epilepsy surgery had their resected tissue evaluated for neuropathological diagnosis.
Neuroimaging studies
Neuroimaging investigation included brain CT with non‐contrasted and contrasted phases (Somatom ARC and Somatom Emotion; Siemens, Erlangen, Germany) and high resolution MRI (Magneton Vision 1.5 T scanner; Siemens), with special protocols for epilepsy evaluation. CT and MRI scans for all patients were available for review. The neuroimaging findings were carefully reviewed specifically for the purpose of the study by neuroradiologists skilled in epilepsy and neurocysticercosis neuroimaging. The neuroradiologists looked for the presence of lesions commonly responsible for intractable epilepsy, such as MTS, MCD, brain tumours, vascular lesions, perinatal brain damage, and post‐traumatic and post‐stroke gliosis.
Patients were divided into neurocysticercosis and non‐neurocysticercosis groups, according to diagnostic criteria previously published by Del Brutto et al.11 Patients with definitive and probable diagnoses of cysticercosis were included in the neurocysticercosis group.
Definitive neurocysticercosis was diagnosed if the following were present: (i) an absolute criterion, such as histological demonstration of the parasite or cystic lesions showing the scolex on CT or MRI; (ii) two major criteria, such as lesions highly suggestive of neurocysticercosis on neuroimaging studies, spontaneously resolving small single enhancing lesions, or resolution of intracranial cystic lesions after therapy with albendazole or praziquantel; or (iii) one major and two minor criteria, such as lesions compatible with neurocysticercosis on neuroimaging studies, clinical manifestations suggestive of neurocysticercosis, and positive CSF ELISA for detection of anticysticercal antibodies, plus epidemiological evidence. According to the above criteria, the presence of solid, dense, supratentorial calcifications, 1–10 mm in diameter, in the absence of other illnesses should be considered to be highly suggestive of neurocysticercosis.11
Probable neurocysticercosis was diagnosed if the following were present: (i) one major and two minor criteria; (ii) one major criterion, one minor criterion, and epidemiological evidence, and (iii) three minor criteria and epidemiological evidence.11
Statistical analysis
We applied the Kolmogorov‐Smirnov test to define the distribution type of variables. If numerical variables presented a normal distribution, we used parametric tests such as Student's t test. When variables did not present a normal distribution, we used the non‐parametric Mann‐Whitney test. For categorical variables we applied Fisher's exact test.
Results
A total of 512 patients were included in this survey. Most (78.3%) came from the southeast of the country, but patients from all regions of Brazil were included. Mean (±SD) age at enrolment was 38.6±10.6 years (range 7–56 years) and mean (±SD) age at epilepsy onset was 11.6±7.2 years (range 1–36 years). Mean (±SD) epilepsy duration was 21.8±10.3 years (range 7–48 years). The median seizure frequency before surgery was six seizures per month (range 1–27). There were 271 women (52.9%) and 241 men (47.1%).
The most common epileptogenic lesion found was MTS in 56.0% of patients. MCD accounted for 12.1% of patients and brain tumours were found in 9.9%, gliosis in 6.0%, porencephaly in 4.8%, Rasmussen encephalitis and Sturge‐Weber disease in 0.8%, and vascular malformations in 0.3% of patients. Neuroimaging investigation was normal in 8.7% of patients. Neurocysticercosis was the unique neuroimaging feature in only eight patients (1.56%) (table 1). All patients diagnosed as having neurocysticercosis had at least one major criterion for neurocysticercosis (lesions highly suggestive of neurocysticercosis on neuroimaging studies), one minor criterion (epileptic seizures or a positive CSF ELISA), and epidemiological evidence. Cerebral calcifications also occurred in patients with Sturge‐Weber disease and cavernous angioma, but the calcifications were not typical of neurocysticercosis.
Table 1 Neuroimaging features of intractable epilepsy and the proportion of patients with calcified cysticercotic lesions.
| Neuroimaging | Number (%) | Number of patients with calcifications (%) |
|---|---|---|
| Hippocampal sclerosis | 281 (54.8) | 104 (37.0) |
| MCD | 62 (12.1) | 11 (17.74) |
| Brain tumours | 51 (9.9) | 8 (15.6) |
| Normal | 44 (8.56) | 0 (0.0) |
| Gliosis | 31 (6.0) | 5 (16.1) |
| Porencephaly | 25 (4.8) | 3 (12.0) |
| Isolated neurocysticercosis | 8 (1.56) | 8 (100.0) |
| Rasmussen encephalitis | 4 (0.8) | 0 (0.0) |
| Sturge‐Weber | 4 (0.8) | 0 (0.0) |
| Vascular malformations | 2 (0.3) | 0 (0.0) |
| Total | 512 (100.0) | 139 (27.14) |
MTS was unilateral in 82.4% of patients and bilateral in 17.6%. The most common MCD were focal cortical dysplasia (FCD) in 27 patients, polymicrogyria in 12, and subependymal or subcortical grey matter heterotopia in nine, or a combination of these malformations. In three patients the association of FCD and ipsilateral MTS was demonstrated by MRI. The most frequent brain tumour was dysembryoplastic neuroepithelial tumour in 16 patients (31.3%), but oligodendrogliomas, astrocitomas, and gangliogliomas were also observed. The concomitance of FCD and oligodendroglioma was pathologically confirmed in one patient with suspected FCD on MRI. Gliosis was found mostly in patients with perinatal insults, brain trauma, meningitis, or cerebral abscess.
The mean (±SD) age at enrolment was 39.4±9.7 years in the MTS group and 32.5±8.8 years in the non‐MTS group (p<0.01, two sample t test). The mean (±SD) age was 40.9±8.8 years in patients with calcified lesions and 36.1±10.1 years in those without calcification (p<0.01, two sample t test). Cerebral calcifications were more frequent in women (34.5%) than in men (25%) (p = 0.02, Fisher's exact test).
Clinical and preoperative investigations showed that calcification was involved in the process of epileptogenesis only in two patients with calcified cysticercotic lesions. In one patient, a gross calcification located in the right parietal lobe was associated with right hippocampal sclerosis. As the clinical semiology suggested parietal lobe seizures (dizziness and paresthesias on the left side) and the ictal EEG showed right temporal theta activity, the patient underwent invasive investigation with an 8×8 subdural grid in the right parietal lobe and 1×6 subdural strips in the right temporal lobe. The ictal onset zone was simultaneous in both epileptogenic lesions, in the vicinity of the parietal lobe and in the mesial contacts of the temporal strips.
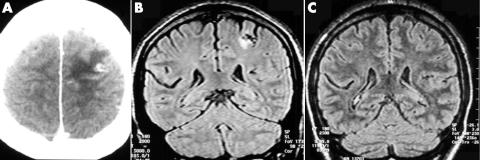
Epilepsy developed in another patient at 23 years of age with medically intractable clonic seizures in the right hand. A CT showed an active parenchymal cyst in the left posterior superior frontal gyrus, close to the motor hand area (fig 1), and a CSF ELISA was positive for anticysticercal antibodies. A subsequent MRI 1 year later revealed reduction of the cyst with gliosis, while the final MRI was absolutely normal, although CT confirmed the presence of calcification in the left posterior frontal lobe. Epileptic seizures persist despite the use of adequate antiepileptic drug regimens, including topiramate or lamotrigine.
Figure 1 (A) Brain CT shows an active parenchymal cyst in the left posterior superior frontal gyrus, close to the motor hand area. (B) A subsequent MRI 1 year later revealed reduction of the cyst with gliosis, and (C) MRI 1 year later is absolutely normal.
The relative frequency of calcified cysticercotic lesions in our sample was 27.14%. The frequency of calcifications was higher in patients with MTS (37.01%) than in those with other types of epileptogenic lesions (15.15%) (p<0.001, Fisher's exact test; OR 3.29, range 2.13–5.07) (table 2). There were no differences between groups with or without calcification regarding age of epilepsy onset, family history of epilepsy, presence of IPI, and epilepsy duration. However, women had significantly more calcifications than men in our sample (p = 0.03, Fisher's exact test).
Table 2 Frequency of patients with mesial temporal sclerosis (MTS) with neurocysticercosis, sex differences, and presence of initial precipitant injury (IPI).
| Variable | Neurocysticercosis | OR | 95% CI | p value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| MTS (n = 281) | 104 (37%) | 177 (63%) | 3.29 | 2.13 to 5.07 | <0.001 |
| No MTS (n = 231) | 35 (15%) | 196 (85%) | |||
| History of IPI | 32 (23%) | 100 (27%) | 0.81 | 0.51 to 1.28 | 0.45 |
| Men (n = 241) | 54 | 186 | 1.58 | 1.07 to 2.32 | 0.02 |
| Women (n = 271) | 85 | 187 | |||
| Age (mean) | 40.9 | 36.1 | – | – | <0.001 |
The 2 year post‐operative follow up revealed similar post‐surgical outcome: 72.3% of patients in the group without calcified cysticercotic lesions and 78.3% of patients in the group with calcifications had class I (Engel) epilepsy (p = 0.108, Fisher's exact test).
Discussion
In a cross sectional study of 512 adult patients with intractable epilepsy, we found calcified cysticercotic lesions in 27% of patients. Although the frequency of such calcifications is high, the result is not surprising, and is consistent with other reports in the literature showing that neurocysticercosis affects a great number of individuals in less developed countries, even in general population studies.4,12,13 In most endemic areas, more than 10% of the general population is seropositive, but this proportion can reach 25%.12,14 In addition, population based studies have shown that 10–18% of asymptomatic individuals have CT features suggestive of neurocysticercosis, mainly brain calcifications.12,14. Finally, an epilepsy population based study in eastern Honduras found that symptomatic epilepsy was primarily due to neurocysticercosis (37%).15
One central question that may rise from our study is whether the punctuate calcified lesions observed on CT were in fact due to neurocysticercosis. In addition to diagnostic criteria for neurocysticercosis,11 we based our diagnosis on the fact that, although lesions such as tuberculosis and fungal infections may present as single or multiple calcifications, when there is spontaneous resolution, the diagnosis of neurocysticercosis is almost certain.11,12,14 In fact, none of our patients had been treated with any antituberculous or antifungal drugs or had any systemic evidence of these infections. Finally, in five patients who had the calcification removed with the epileptogenic tissue, the neuropathological findings suggested neurocysticercosis. For this reason, our data indicate that such inactive calcified lesions found at CT are caused by cysticercosis. Serological tests, although available, were not used to diagnose neurocysticercosis because patients with parenchymal calcified lesions and single enhanced CT lesions are often serologically negative.
Apart from the high relative frequency of calcified cysticercotic lesions in this study, in the vast majority of patients, another lesion which could explain the presence of intractable seizures (such as MTS, brain tumours, or MCD) was found in 89.23% of patients with calcified cysticercotic lesions. Calcifications were the unique neuroimaging feature in only eight patients (1.56%), but a clear relationship between calcified cysticercotic lesions and intractable epilepsy was confirmed in only two patients (0.39%). In addition, the 2 year post‐operative outcome in the group without calcifications was similar to that in the group with calcifications, even though the calcification was not included in the resected tissue. This finding also confirms previous descriptions of successful surgical treatment of patients with well defined localisation related epilepsy, despite the existence of single or disseminated calcified cysticercotic lesions.16,17 In summary, although calcified cysticercotic lesions were present in 27% of patients, in most cases (98.56%) they can not be considered epileptogenic lesions.
MTS was the most frequent epileptogenic lesion (56%), followed by MCD (12.1%), brain tumours (9.9%), and normal MRI (8.56%). Temporal lobe lesions were found in 67% of cases. Such findings are similar to those reported in developed countries, except for the higher proportion of MTS in our sample.
These data suggest that neurocysticercosis is an uncommon cause of intractable epilepsy, even in an endemic region such as Brazil, and that in most patients neurocysticercosis may only represent a coexistent pathology. Indeed, Carpio and Hauser reported that seizure recurrence in patients with neurocysticercosis is low, and 85% of patients with a solitary cerebral cysticercus granuloma have a good seizure outcome following resolution of the lesion and early withdrawal of antiepileptic drugs.18
However, a more reflective analysis of our findings reveals that calcified cysticercotic lesions were twice as common in MTS than in non‐MTS patients. This finding could suggest a cause‐effect relationship between MTS and neurocysticercosis. There is some evidence supporting this hypothesis. First, various reports suggested that acute neurocysticercosis infection may cause MTS and late onset temporal lobe epilepsy.17,19,20 Second, in the present series, in some patients the calcification was very close to the hippocampus head in the resected tissue.
It is reasonable to suppose that some patients who presented to our emergency department 30 or 40 years ago with febrile or non‐febrile epileptic generalised seizures or status epilepticus may have had neurocysticercosis. In children and teenagers, an acute meningoencephalitic presentation of neurocysticercosis can occur and is mostly due to the inflammatory reaction of the host to parasite infection.17,21 In such a case, neurocysticercosis could result in an IPI, such as bacterial meningitis, febrile seizures, or brain injury. It has been reported that hippocampal sclerosis is likely an acquired pathology and most of the neuronal loss occurs during the IPI.22
Conversely, the presence of a relationship between calcified cysticercotic lesions and MTS does not mean that there is a causal relationship. A third variable or factor could be responsible for this association. This is sometimes referred to as the “missing variable problem”. In other words, a possible alternative explanation is that cysticercosis and hippocampal sclerosis could be associated with a third variable, such as low socio‐economic status. Individuals living in regions characterised by socio‐economic deprivation could be more exposed to risk factors generally associated with hippocampal sclerosis, such as meningitis, prolonged febrile seizures, or parasitic infections. Controlling for socio‐economic status would help investigate this hypothesis, but controlling retrospectively for socio‐economic status three or four decades ago would be very difficult due to missing data. A prospective cohort study would better establish a causal relationship between neurocysticercosis and MTS.
In conclusion, our results suggest that neurocysticercosis is an uncommon cause of intractable epilepsy as previously reported. Epilepsy surgery for patients with intractable seizures may be indicated despite the presence of calcified cysticercotic lesions if the epileptogenic zone can be identified during careful preoperative evaluation. In addition, an association between MTS and neurocysticercosis was found, suggesting that neurocysticercosis may be a risk factor for the development of hippocampal sclerosis or that both co‐vary with socio‐economic status.
Abbreviations
FCD - focal cortical dysplasia
IPI - initial precipitating injury
MCD - malformations of cortical development
MTS - mesial temporal sclerosis
Footnotes
This work was supported by FAPESP (CINAPCE Project no. 04/14004‐9) (Drs Velasco, Sakamoto, Alexandre Jr, Santos, and Leite), by PIBIC‐CNPq (Dr Zanello), by CNPq Proc. no. 300937/2003‐2 (Dr Takayanagui), and by FAPESP Project 02/03743‐0 (Dr Bianchin)
Competing interests: none declared
References
- 1.Sander J W. The epidemiology of epilepsy revisited. Curr Opin Neurol 200316165–170. [DOI] [PubMed] [Google Scholar]
- 2.Borges M A, Min L L, Guerreiro C A.et al Urban prevalence of epilepsy: populational study in Sao Jose do Rio Preto, a medium‐sized city in Brazil. Arq Neuropsiquiatr 200462199–204. [DOI] [PubMed] [Google Scholar]
- 3.Burneo J G, Tellez‐Zenteno J, Wiebe S. Understanding the burden of epilepsy in Latin America: a systematic review of its prevalence and incidence. Epilepsy Res 20056663–74. [DOI] [PubMed] [Google Scholar]
- 4.Del Brutto O H, Santibanez R, Idrovo L.et al Epilepsy and neurocysticercosis in Atahualpa: a door‐to‐door survey in rural coastal Ecuador. Epilepsia 200546583–587. [DOI] [PubMed] [Google Scholar]
- 5.Brewster D R, Kwiatkowski D, White N J. Neurological sequelae of cerebral malaria in children. Lancet 19903361039–1043. [DOI] [PubMed] [Google Scholar]
- 6.Rajshekhar V, Joshi D D, Doanh N Q.et al Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Trop 20038753–60. [DOI] [PubMed] [Google Scholar]
- 7.Nash T E, Del Brutto O H, Butman J A.et al Calcific neurocysticercosis and epileptogenesis. Neurology 2004621934–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallin M T, Kurtzke J F. Neurocysticercosis in the United States: review of an important emerging infection. Neurology 2004631559–1564. [DOI] [PubMed] [Google Scholar]
- 9.Hart Y M, Shorvon S D. The nature of epilepsy in the general population. I. Characteristics of patients receiving medication for epilepsy. Epilepsy Res 19952143–49. [DOI] [PubMed] [Google Scholar]
- 10.Leppik I E. Intractable epilepsy in adults. Adv Exp Med Biol 20024971–7. [DOI] [PubMed] [Google Scholar]
- 11.Del Brutto O H, Rajshekhar V, White A C., Jret al Proposed diagnostic criteria for neurocysticercosis. Neurology 200157177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medina M T, Rosas E, Rubio‐Donnadieu F.et al Neurocysticercosis as the main cause of late‐onset epilepsy in Mexico. Arch Intern Med 1990150325–327. [PubMed] [Google Scholar]
- 13.Pal D K, Carpio A, Sander J W. Neurocysticercosis and epilepsy in developing countries. J Neurol Neurosurg Psychiatry 200068137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia H H, Gonzalez A E, Evans C A.et al Taenia solium cysticercosis. Lancet 2003362547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medina M T, Duron R M, Martinez L.et al Prevalence, incidence, and etiology of epilepsies in rural Honduras: the Salama Study. Epilepsia 200546124–131. [DOI] [PubMed] [Google Scholar]
- 16.Leite J P, Terra‐Bustamante V C, Fernandes R M.et al Calcified neurocysticercotic lesions and postsurgery seizure control in temporal lobe epilepsy. Neurology 2000551485–1491. [DOI] [PubMed] [Google Scholar]
- 17.Wichert‐Ana L, Velasco T R, Terra‐Bustamante V C.et al Surgical treatment for mesial temporal lobe epilepsy in the presence of massive calcified neurocysticercosis. Arch Neurol 2004611117–1119. [DOI] [PubMed] [Google Scholar]
- 18.Carpio A, Hauser W A. Prognosis for seizure recurrence in patients with newly diagnosed neurocysticercosis. Neurology 2002591730–1734. [DOI] [PubMed] [Google Scholar]
- 19.Chung C K, Lee S K, Chi J G. Temporal lobe epilepsy caused by intrahippocampal calcified cysticercus: a case report. J Korean Med Sci 199813445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi E, Guerreiro C A, Cendes F. Late onset temporal lobe epilepsy with MRI evidence of mesial temporal sclerosis following acute neurocysticercosis: case report. Arq Neuropsiquiatr 200159255–258. [DOI] [PubMed] [Google Scholar]
- 21.Rangel R, Torres B, Del Bruto O.et al Cysticercotic encephalitis: a severe form in young females. Am J Trop Med Hyg 198736387–392. [DOI] [PubMed] [Google Scholar]
- 22.Mathern G W, Adelson P D, Cahan L D.et al Hippocampal neuron damage in human epilepsy: Meyer's hypothesis revisited. Prog Brain Res 2002135237–251. [DOI] [PubMed] [Google Scholar]