Abstract
This pilot study investigated whether 4 weeks of aerobic treadmill training in individuals with multiple sclerosis (MS) improved mobility and reduced fatigue. Individuals with MS were recruited to this prospective, randomised controlled trial. Individuals were assessed at baseline, week 7 and 12 with a 10 metre timed walk, a 2 minute walk, the Rivermead Mobility Index, and the Fatigue Severity Scale. After a pre‐assessment familiarisation session and a baseline assessment, individuals were randomly allocated to an initial intervention or delayed intervention group. Treadmill training consisted of 4 weeks of supervised aerobic exercise delivered weeks 3–6 in the immediate group and 8–11 in the delayed group. Of the initial 19 recruits, 16 individuals completed the study. There was a significant difference in walking endurance between the delayed and immediate groups at baseline (p<0.05). On reassessment in week 7, decreases in 10 metre walk time were found in both groups, which was significant in the immediate group (p<0.05). The 2 minute walk distance significantly increased in both groups (p<0.05). In the training group, reassessed at week 12 after training ceased, there was a return towards baseline scores. No significant changes in fatigue scores were found. This study showed that in individuals with MS, aerobic treadmill training is feasible and well tolerated. Walking speed and endurance increased following training with no increase in reported fatigue. Detraining occurred in the period following training. A larger randomised clinical trial is warranted.
Keywords: MS, treadmill training, speed, endurance, fatigue
Restricted mobility and fatigue are common problems for people with multiple sclerosis (MS),1 and 85% of individuals with MS report gait disturbance as their main complaint. Walking may be limited by fatigue and lack of safety in walking, which can lead to an interruption of optimum physical functioning1 that in turn can impact on quality of life.1 Limited evidence suggests that aerobic exercise may be beneficial to people with MS.2
Treadmill training is a ‘task orientated' method of gait training that is highly repetitive and promotes a specific practice of walking.3 After stroke, treadmill training may improve gait speed and endurance, normalise gait patterns with greater symmetry, and increase general wellbeing.4
To date, there is no published evidence of the efficacy of treadmill training in individuals with MS, although some studies that incorporate treadmill training into rehabilitation programmes report positive outcomes with no harmful effects.5
Fatigue is reported in 53–90% of people with MS.6 For many years such people limited their physical activity levels on the advice of physicians in an effort to help minimise the risk of exacerbations and symptoms of fatigue.7 However, contemporary opinion is changing; there is an increasing recognition that regular participation in aerobic exercise may benefit people with MS.2,7
This study investigates the effect of treadmill training, designed to increase aerobic fitness, on walking performance and levels of fatigue in people with MS.
METHODS
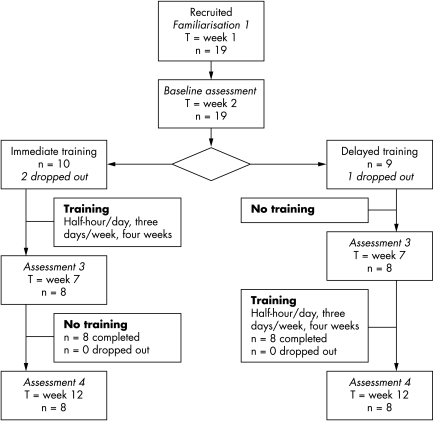
In this prospective single centre randomised crossover trial with blinded assessments (fig 1), subjects were randomly allocated to immediate or delayed training. Randomisation occurred in blocks of four using consecutively numbered sealed envelopes that contained computer generated random numbers. After pre‐assessment familiarisation in week 1, baseline assessment took place in week 2. Reassessments took place after 4 weeks. This design enabled the initial group to receive training while the delayed group acted as a control group. Individuals in the delayed group could then receive the exercise intervention, and the initial group could be followed up.
Figure 1 Flow diagram of study.
Participants were recruited through a consultant and physiotherapists at a specialist rehabilitation centre, through the local MS Society, from a local MS gymnasium group, and by word of mouth. Informed consent was obtained from all participants.
Individuals with a confirmed clinical diagnosis of MS and the ability to follow training instructions were included in the study. Additionally, individuals were required to walk 10 metres in <60 seconds without hands on support, using an aid if necessary, and to be able to walk on the treadmill with or without hands on support. Individuals were excluded if they had had a significant relapse within the past 8 weeks or were identified with any serious medical condition that might impair their ability to walk on a treadmill and participate in aerobic exercise.
Medical and therapy history was obtained, and screening for precautions and contraindications completed. Measurements of weight, height, and leg length, and the Guy's Neurological Disability Scale (GNDS),8 Rivermead Mobility Index (RMI)9 and Fatigue Severity Scale (FSS)10 were completed to assess level of disability, mobility, impairment, and fatigue. The 10 metre timed walk and the 2 minute walk were used to assess walking speed and endurance, respectively.9 All measurement tools have previously been used in MS populations and validity, reliability, and sensitivity described.8,9,10
Individuals were familiarised with the treadmill test and the procedure described by Holt et al11 was used to obtain preferred walking speed. To obtain steady state conditions, individuals walked for 3 minutes before heart rate was measured for the last minute.12
Intervention
Individuals received supervised treadmill training, three sessions each week, for 4 weeks. Walking duration was increased during the training period as tolerated, up to a maximum of 30 minutes with a maximum of three rest periods. Once maximum walking duration was attained, intensity was increased by increasing walking speed. Individuals were encouraged to train at an intensity of 55–85% of age predicted maximum heart rate (APMHR) (measured on a Polar Vantage 2000 heart rate monitor) according to American College of Sports Medicine guidelines.13 A fan and water were available to counter the effects of summer heat. Heart rate, speed, ratings of exertion using the CR10‐RPE14 scale, training time, and any comments were recorded.
Statistical analysis
Data were examined at first reassessment for between group differences. Data from the second reassessment were analysed for within group differences. Sample characteristics were summarised using descriptive statistics. Owing to the small sample size, non‐parametric tests were applied; for within group comparisons, Wilcoxon's signed ranks test, and for between group comparisons, the Mann‐Whitney U test, with significance set at p = 0.05. Data was analysed using SPSS software (version 11.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Nineteen individuals were recruited; one man and seven women (age range 30–65 years) in the immediate training group and two men and seven women (age range 30–65 years) in the delayed training group finished the study. None had used a treadmill recently or received other physiotherapy during the study period. All but two participants used a walking aid, with most using a walking cane. All individuals tolerated treadmill training. Results are shown in table 1.
Table 1 Outcome measures at baseline and re‐assessments.
| Week 1, baseline, mean (SD); IQR | Change in score,*, re‐assessment 1, mean (SD) | Week 12, re‐assessment 2, mean (SD); IQR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immediate group | Delayed group | Immediate group | Delayed group | Immediate group | Delayed group | |||||||
| 10‐metre timed walk (s) | 17.8 (5.4); 11.3–28.1 | 14.0 (5.5); 8.6–24.0 | −3.1 (2.5)†‡ | −0.6 (1.4) | 17.2 (6.2); 8.2–26.5 | 13.1 (6.5); 8.2–26.5 | ||||||
| 2‐minute walk (m) | 71.0 (22.8)‡; 44.6–105.3 | 99.5 (30.0); 49.8–139.7 | 10.8 (6.7)† | 5.8 (7.8)† | 74.5 (33.9); 46.3–141.5 | 106.8 (36.7); 56.5–156.1 | ||||||
| FSS | 30.6 (9.2); 15–44 | 32.1 (9.1); 15–42 | −4.5 (7.7) | −4.4 (7.8) | 26.1 (14.1); 7–44 | 26.9 (8.3); 13–41 | ||||||
| GNDS | 12.1 (3.8); 7–18 | 12.9 (4.9); 7–19 | 0.75 (1.8) | 0.13 (2.0) | 11.8 (5.9); 5–21 | 9.0 (4.1); 2–14 | ||||||
| Heart rate walking (beats/min) | 89.6 (13.3); 72–107.3 | 97.1 (11.4); 80.7–117.7 | 4.1 (8.6) | 4.3 (9.5) | 84.0 (12.0); 64–106.7 | 87.1 (12.7); 74.3–104.7 | ||||||
IQR, interquartile range; FSS, Fatigue Severity Scale; GNDS, Guy's Neurological Disability Scale. *Week 7 minus week 1; †Wilcoxon's signed ranks test p value<0.05; ‡Mann‐ Whitney U test p value<0.05.
At baseline, the immediate training group were slower on the 10 metre timed walk and covered significantly less distance on the 2 minute walk test than the delayed group. Statistical analysis showed that on reassessment 1, individuals in both groups improved their 2 minute walk times, and the trained group significantly improved their 10 metre walk time, compared with the untrained group (p<0.05). There was no change in fatigue levels. At week 12, after a 4 week rest period, walking performance returned towards baseline scores.
The immediate training group trained a mean (SD) total time of 323.8 (47.3) minutes, of which 55.1 (33.2)% was aerobic, with an average speed of 1.3 (1.2) mph and at 60% of their APMHR. The delayed group trained a total time of 295.6 (48.8) minutes, of which 61.9 (48.8)% was aerobic, with an average speed of 1 (0.97) mph and at 61% of their APMHR.
DISCUSSION
There was a significant increase in speed, a non‐significant increase in endurance, and a non‐significant downward trend in fatigue levels in the treated group compared with the untreated group at the crossover point. These findings accord with earlier investigations of the effect of outpatient programmes7 and aerobic cycling.15 Improved walking performance did not transfer into changes in overall disability, as measured using the GNDS. Future studies should further examine the effects of improved walking on community mobility as well disability.
In line with findings of Wiles et al,16 we found the training effects returned towards baseline scores within the follow up period. The rate of detraining observed in this clinical group suggests that longer interventions or an ongoing maintenance programme might be more effective than short interventions. Faster speeds12,17 and increased endurance16 are associated with increased functional mobility and independence, suggesting that a real benefit is possible.
The imbalance in walking endurance between the groups at baseline arose by chance and may have influenced our findings. The small sample number also raises the risk of missing significant findings. However, power calculations using MINITAB 14.1 showed that to identify a change of 3.1 seconds on the 10 metre timed walk with 80% power at a 0.05% significance level requires a sample size of only six. In other domains, a larger number would be needed.
We found a wide range in walking HR at baseline, in line with earlier studies18,19 but, in contrast with findings of Macko et al,20 there was no difference in walking HR after the initial study period between the training and non‐training group. This suggests that aerobic treadmill training did not increase fitness or improve movement economy. The small sample size, inherent variability of heart rate at lower exercise intensities, short duration of the intervention, and steady rise in environmental temperature during the study period may have contributed to these results. In the present study, on average the initial group spent 55% of time at aerobic levels and trained at 60% of their APMHR, which should have elicited a training response.17 Because of illness, unforeseen circumstances, and the heat in the last weeks of the study, one person completed only 11 training sessions and four were not able to complete their 12 training sessions within the 4 week period. This may have affected the results.
This study showed that treadmill training in MS individuals is feasible, well tolerated and increases gait speed and endurance, and importantly does not worsen symptoms of fatigue. A larger randomised clinical trial is warranted, with a larger sample size to determine whether broader aspects of activity limitation are helped. Further studies should also investigate whether longer intervention or a reduced intensity follow up period can avoid the loss of effect noted in this study.
ACKNOWLEDGEMENTS
We thank the individuals for their cooperation and the staff at the Oxford Centre of Enablement for their collaboration. We are thankful to the University of Maastricht and Oxford Brookes University for their contribution.
Abbreviations
APMHR - age predicted maximum heart rate
FSS - Fatigue Severity Scale
GNDS - Guy's Neurological Disability Scale
MS - multiple sclerosis
RMI - Rivermead Mobility Index
Footnotes
Competing interests: none
References
- 1.Freeman J A. Improving mobility and functional independence in persons with multiple sclerosis. J Neurol 2001248255–259. [DOI] [PubMed] [Google Scholar]
- 2.Mostert S, Kesselring J. Effect of a short‐term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult Scler 20028161–168. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd R, Carr J. Treadmill walking in neurorehabilitation. Neurorehabil Neural Repair 199913171–173. [Google Scholar]
- 4.Pohl M, Mehrholz J, Ritschel C.et al Speed‐dependent treadmill training in ambulatory hemiparetic stroke patients. Stroke 200233553–558. [DOI] [PubMed] [Google Scholar]
- 5.van Sint Annaland E, Lord S. Vigorous exercise for multiple sclerosis: a case report. N Z J Physiother 19992742–45. [Google Scholar]
- 6.Kesselring J, Thompson A J. Spasticity, ataxia and fatigue in multiple sclerosis. Baillieres Clin Neurol 19976429–445. [PubMed] [Google Scholar]
- 7.Solari A, Filippini G, Gasco P.et al Physical rehabilitation has a positive effect on disability in multiple sclerosis. Neurology 19995257–62. [DOI] [PubMed] [Google Scholar]
- 8.Rossier P, Wade D T. The Guy's Neurological Disability Scale in patients with multiple sclerosis: a clinical evaluation of its reliability and validity. Clin Rehabil 20021675. [DOI] [PubMed] [Google Scholar]
- 9.Rossier P, Wade D T. Validity and reliability comparinson of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil 2001829–13. [DOI] [PubMed] [Google Scholar]
- 10.Flachenecker P, Kümpfel T, Kallmann B.et al Fatigue in multiple sclerosis: a comparinson of different rating scales and correlation to clinical parameters. Mult Scler Clin Lab Res 20028523–526. [DOI] [PubMed] [Google Scholar]
- 11.Holt K G, Hamill J, Andres R O. Predicting the minimal energy costs of human walking. Med Sci Sports Exer 199023491–498. [PubMed] [Google Scholar]
- 12.Waters R L, Lunsford B R, Perry J.et al Energy‐speed relationship of walking: standard tables. J Orthop Res 19886215–222. [DOI] [PubMed] [Google Scholar]
- 13.Franklin B A, Whaley M H, Howley E T.ACSM's guidelines for exercise testing and prescription. Philadelphia: Lippincott Williams Wilkins, 2000
- 14.Neely G, Ljunggren G, Sylven C.et al Comparison between the Visual Analogue Scale (VAS) and the Category Ratio Scale (CR‐10) for the evaluation of leg exertion. Int J Sports Med 199213133–136. [DOI] [PubMed] [Google Scholar]
- 15.Petajan J H, Gappmaier E, White A T.et al Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol 199639432–441. [DOI] [PubMed] [Google Scholar]
- 16.Wiles C M, Newcombe R G, Fuller K J.et al Controlled randomised crossover trial of the effects of physiotherapy on mobility in chronic multiple sclerosis. J Neurol Neurosurg Psychiatry 200170174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waters R L, Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture 19999207–231. [DOI] [PubMed] [Google Scholar]
- 18.Chetta A, Rampello A, Marangio E.et al Cardiorespiratory response to walk in multiple sclerosis patients. Respir Med 200498522–529. [DOI] [PubMed] [Google Scholar]
- 19.Olgiati R, Jacquet J, di Prampero E. Energy cost of walking and exertional dyspnea in multiple sclerosis. Am Rev Respir Dis 19861341005–1010. [DOI] [PubMed] [Google Scholar]
- 20.Macko R F, Smith G V, Dobrovolny C L.et al Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil 200182879–884. [DOI] [PubMed] [Google Scholar]