Abstract
Background
Acquired diffuse paresis in an intensive care unit (ICU) can result from critical illness myopathy or polyneuropathy. Clinical examination and conventional neurophysiological techniques may not distinguish between these entities.
Objective
To assess the value of direct muscle stimulation (DMS) to differentiate myopathic from neuropathic process in critically ill patients with diffuse severe muscle weakness.
Methods
30 consecutive patients with ICU acquired diffuse motor weakness were studied. Responses of the right deltoid and tibialis anterior muscles to DMS and to motor nerve stimulation (MNS) were studied and compared with results of conventional nerve conduction studies and concentric needle electromyography (EMG). An original algorithm was used for differential diagnosis, taking into account first the amplitude of the responses to DMS, then the MNS to DMS amplitude ratio, and finally the amplitude of the sensory nerve action potentials recorded at the lower limbs.
Results
Evidence of neuropathy and myopathy was found in 57% and 83% of the patients, respectively. Pure or predominant myopathy was found in 19 patients. Other results were consistent with neuromyopathy (n = 5) and pure or predominant neuropathy (n = 2). Four patients had normal results with stimulation techniques, but spontaneous EMG activity and raised plasma creatine kinase suggesting necrotic myopathy.
Conclusions
A neurophysiological approach combining DMS and conventional techniques revealed myopathic processes in a majority of ICU patients. Reduced muscle fibre excitability may be a leading cause for this. The diagnosis of myopathy in ICU acquired paralysis can be established by a combination of DMS, needle EMG, and plasma creatine kinase.
Keywords: critical care, electromyography, myopathy, nerve conduction, neuropathy
Acquired neuromuscular disorders are particularly common during a stay in an intensive care unit (ICU). The main clinical features are difficulty in weaning from the ventilator and diffuse flaccid weakness, which can result in areflexic quadriplegia.1,2 Generalised motor deficits of this type have been attributed to various kinds of critical illness myopathy (CIM),3,4 first described in 1977,5 or to critical illness polyneuropathy (CIP),6,7 first described in 1983.8 A combination of both CIM and CIP has also been described, giving rise to the term “critical illness polyneuromyopathy”.9
Clinical examination is of little value in differentiating CIM from CIP in most critically ill patients.10 Conventional neurophysiological methods—that is, nerve conduction studies and needle electromyography (EMG)—may also fail to distinguish between CIM and CIP. Both these processes can be associated with amplitude reduction of compound muscle action potentials (CMAPs) to motor nerve stimulation (MNS) and with abnormal spontaneous EMG activity.11 Rich et al therefore introduced a technique of direct muscle stimulation (DMS) to improve the reliability of neurophysiological diagnosis in critically ill patients.12,13,14 Since these pioneering studies, DMS has rarely been applied in large series of patients with acquired muscle weakness during a stay in an ICU. The present study was designed to assess the value of DMS, coupled with MNS, to differentiate between CIM and CIP in a series of 30 consecutive patients with ICU acquired paresis, compared with conventional nerve conduction studies and needle EMG examination.
Methods
Patients
This study included 30 consecutive patients with ICU acquired diffuse paresis, nine women and 21 men, aged from 33 to 84 years (mean (SD), 63.1 (5.0)). All patients had been mechanically ventilated for more than seven days and were sedated. They all had severe weakness of all four limbs on awakening. The criterion for clinically significant weakness was an overall Medical Research Council (MRC) score of less than 48/60, obtained by assessment of three muscle groups in each of the upper and lower limbs.15 Patients were excluded if they had an identified pre‐existing neuromuscular disease present on ICU admission, a persistent neuromuscular transmission failure, a specific neuropathy such as Guillain‐Barré syndrome, or any central nervous system disease. Patients in whom the low motor score probably reflected an altered comprehension of orders or persisting alteration of consciousness were also excluded.
Neurophysiological recordings
Neurophysiological recordings were carried out at the bedside between two and 21 days after the patients had awakened and been found to have a paresis (mean, 13.7 (7.8) days), using a Keypoint EMG machine (Medtronic Functional Diagnostics, Skovlunde, Denmark). Before any further investigations, abductor digiti minimi muscle responses to 3 Hz repetitive stimulation of the ulnar nerve were recorded bilaterally to confirm the absence of neuromuscular transmission failure.
First, all patients underwent conventional nerve conduction studies, including bilateral examination of the median, ulnar, deep peroneal, and tibial motor nerves and of the median, ulnar, superficial peroneal, and sural sensory nerves. Surface electrodes were used for stimulation and recording, except for sensory nerve action potential (SNAP) recording, which was carried out using subdermal electrodes in case of oedema or when there was reduced amplitude with surface electrodes. Motor nerve conduction studies included the determination of CMAP amplitude and distal motor latency at both hands and feet to distal nerve stimulation, and the calculation of motor conduction velocity at the forearm or leg. Sensory nerve conduction studies included the determination of SNAP amplitude to distal nerve stimulation and the calculation of sensory conduction velocity at the wrist or ankle. Mean values of CMAP or SNAP amplitude (CMAPamp and SNAPamp) were calculated for upper and lower limbs.
Second, DMS was applied in the deltoid and tibialis anterior muscles of the right side. A monopolar needle electrode (length 37 mm; diameter 0.36 mm; stimulating surface 0.28 mm2) (No 53534T, Oxford Instruments–Medical, Woking, Surrey, UK) was inserted in the distal third part of the muscle, remote from the endplate zone.13,14 This electrode served as cathode, a subdermal needle electrode (length 12 mm) (No MF3.OE.1F35.12, Comepa, Saint‐Denis, France) being placed 10 mm lateral to serve as the anode.13,14 Then, using pulses of 0.1 ms in duration delivered at 0.5 Hz, the muscle was stimulated at various depths with gradually increasing strength (from 10 to 100 mA), until a palpable twitch was evoked.13,14 Guided by the twitch, a disposable concentric EMG needle (length 37 mm; diameter 0.46 mm; recording surface 0.07 mm2) (No N53156, Oxford Instruments–Medical) was inserted perpendicularly to skin surface, 15 mm proximal to the stimulating electrode, at increasing depths until a response of maximum amplitude was obtained.13,14 If no twitch could be elicited, the needle was also inserted 15 mm proximal to the stimulating cathode but oriented in various directions to ensure that responses were not missed.13,14 Bandpass filtering ranged from 20 Hz to 10 kHz.
The muscle fibre origin of the response was ascertained by careful observation of the latency of the response. The potentials which were indirectly evoked by nerve ending stimulation showed a shorter latency than those directly evoked by muscle fibre stimulation.16,17 Taking into account a mean interelectrode distance of 15 mm and muscle fibre conduction velocity values between 3.5 and 7.0 m/s,16,17 the range of latency for muscle fibre responses lay between 2.1 and 4.3 ms. Thus any response with latency shorter than 2 ms was suspected to be related to nerve ending stimulation and was discarded from analysis, after which the stimulating needle electrode was moved towards another site.
Thereafter, with the same recording electrode kept in place, the axillary or deep peroneal nerve was stimulated with surface electrodes (motor nerve stimulation, MNS). The axillary nerve was stimulated with a monopolar cathode at Erb's point and a cervical anode, while the deep peroneal nerve was given bipolar stimulation at the head of the fibula. Stimulation intensity was increased to obtain motor responses of maximum amplitude (supramaximal intensities) with a pulse duration of 0.5 ms. Finally, EMG activity was analysed at rest (spontaneous activity) and during contraction (interference pattern) using the same needle electrode in these muscles.
Peak to peak amplitudes and onset latencies of the responses evoked by DMS (DMSamp, DMSlat) or by MNS (MNSamp, MNSlat) were measured. The ratio MNSamp to DMSamp was calculated as previously described,14,17 and we also calculated the MNSlat to DMSlat difference (MNSlat−DMSlat).
Normative data were acquired in a series of 12 healthy subjects aged from 23 to 74 years (mean, 41.9 (14.5) years) for each neurophysiological variable used in the study. Normal limits were defined as the mean values obtained in the series of healthy subjects ±2 SD.
Discrimination between myopathy and neuropathy
First, we looked at DMS results. Criteria for myopathic process were reduced DMSamp, increased MNSamp/DMSamp ratio, or prolonged DMSlat. The only specific criterion for motor neuropathy was a reduced MNSamp/DMSamp ratio. Second, we looked at SNAPamp values: normal SNAPamp in the lower limbs was considered to be a sign of the absence of polyneuropathy; in contrast, decreased SNAPamp in the lower limbs was considered to be evidence of polyneuropathy. Finally, reduced MNSamp or CMAPamp, or both, and prolonged MNSlat were interpreted in the light of the previous observations.
Clinical variables
The clinical data collected included the following: drugs (in particular the use of corticosteroids or neuromuscular blocking agents); the presence of sepsis and the total number of antibiotics used during the ICU stay; the occurrence of multiple organ failure (respiratory, circulatory, renal, liver, haematological, hepatic, and neurological) according to ODIN (organ dysfunction and/or infection) score definitions,18 with particular reference to respiratory or renal failure; the presence of diabetes mellitus requiring insulin; creatine kinase (CK) concentrations in the serum (maximum value before neurophysiological examination); and clinical motor status on the day of discharge from the ICU. Unfortunately, it was impossible to collect data after ICU discharge for long term follow up assessment.
Statistical analyses
Statistical analyses were carried out using Fisher's exact test or the Mann–Whitney U test, a value of p<0.05 being considered significant.
Results
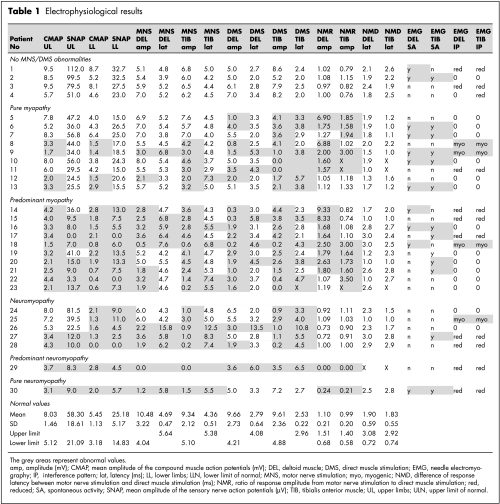
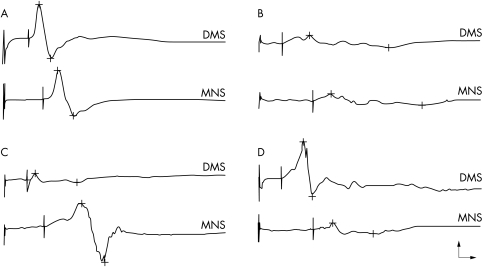
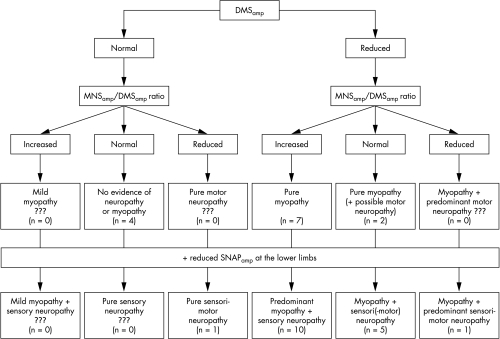
Electrophysiological results are given in table 1, including normative data. Examples of responses to DMS and MNS are illustrated in fig 1. The algorithm to discriminate between myopathy and neuropathy is summarised in fig 2.
Figure 1 Examples of responses elicited by direct muscle stimulation (DMS) and motor nerve stimulation (MNS) in the same recording site. (A) Normal pattern, with equal and normal response amplitude to both DMS and MNS. (B) Neuromyopathic pattern, with equal but severely reduced response amplitude to both DMS and MNS. (C) Predominant myopathic pattern, with response amplitude preferentially reduced to DMS. (D) Predominant neuropathic pattern, with response amplitude preferentially reduced to MNS. Calibration: 2 ms (horizontal arrow), 2 mV (vertical arrow).
Figure 2 Diagnostic algorithm for differentiating myopathy from neuropathy. In each case, the number of patients corresponding to this series is given in brackets. DMSamp, response amplitude to direct muscle stimulation; MNSamp/DMSamp ratio, ratio of response amplitude from motor nerve stimulation to direct muscle stimulation; SNAPamp, mean amplitude of the sensory nerve action potentials.
Discrimination between myopathy and neuropathy
Five patients (17%) had fully normal DMSamp values, showing no evidence of myopathy. Four of these (patients 1 to 4) did not show any sign of neuropathy on MNS/DMS and nerve conduction studies. The last patient (No 30) had a reduced MNSamp/DMSamp ratio, together with reduced SNAPamp in the lower limbs, suggesting sensorimotor neuropathy without myopathy.
Reduced DMSamp was found in the remainder (25 patients), indicating a large majority of myopathic processes (83.3%) in the present series. Among these 25 patients, nine (Nos 5 to 13) had a normal SNAPamp, suggesting the absence of concomitant neuropathy. This conclusion was reinforced in five of these nine patients who also had a normal CMAPamp. The other four patients, with normal SNAPamp but reduced CMAPamp, could have had motor neuropathy but they had a normal or increased MNSamp/DMSamp ratio, suggesting that the CMAPamp reduction was related to the myopathic process. In 16 patients (Nos 14 to 29), myopathy was associated with neuropathy—at the least a sensory polyneuropathy—as shown by a reduced SNAPamp. In 10 of these patients (Nos 14 to 23), the MNSamp/DMSamp ratio was increased, providing evidence of a predominant myopathy. In contrast, one patient (No 29) with a reduced MNSamp/DMSamp ratio suffered from predominant neuropathy.
We assumed that the severity of the myopathic process could be estimated by the absolute DMSamp values. Taking into account DMSamp values less than 50% of the lower limit of normal, a severe myopathy was observed in six patients for deltoid recording, in seven patients for tibialis anterior recording, and in six patients for both recordings.
We did not observe prolonged DMSlat or MNSlat without amplitude reduction and the MNSlat−DMSlat difference was normal in all cases, indicating that the latency parameter did not contribute additional diagnostic information compared with the amplitude parameter.
Electromyography
Spontaneous EMG activity—that is, fibrillation potentials—was found in 16 of 30 patients (53%), including two (Nos 1 and 2) with normal results using stimulation techniques. Fibrillation tended to be associated with a predominantly myopathic involvement: fibrillation potentials were found in 13 of 26 muscles (50%) with an increased MNSamp/DMSamp ratio v 11 of 34 muscles (32%) with a normal or reduced MNSamp/DMSamp ratio (Fisher's exact test, p = 0.19).
With respect to the interference pattern, 15 of 30 patients (50%) failed to produce any active muscle contraction, whereas MUP recruitment was severely but non‐specifically reduced in 11 patients (37%). The interference pattern contributed to the diagnosis in only four patients (13%) who had myogenic MUP changes—that is, small (<250 μV), brief (<8 ms), polyphasic (>3 phases) MUPs with early or increased spatial recruitment.
Correlation with clinical data
Clinical features are listed in table 2. In most cases, weakness was predominantly proximal, and deep tendon reflexes were all abolished or frankly diminished. It must be emphasised that almost all patients suffered from sepsis and respiratory failure. Steroid treatment consisted of a seven day course of 200 mg hydrocortisone to reduce the risk of death in patients with septic shock, except in three who received higher doses for other indications (pemphigus, immune thrombocytopenic purpura, and advanced prostate cancer). Neuromuscular blocking agents were used for two to four days (pancuronium 4 mg/hour or atracurium 1200 mg/day).
Table 2 Clinical results.
| Patient | Age (y) | Sex | dEMG | NMBA | CS | Seps | ATB | MOF | Resp F | Renal F | Diab M | CK | Recovery* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No MNS/DMS abnormalities | |||||||||||||
| 1 | 38 | M | 17 | Yes | 3 | Yes | 400 | Partial (D32) | |||||
| 2 | 35 | F | 20 | Yes | Yes | Yes | 3 | Yes | 6580 | Partial (D43) | |||
| 3 | 32 | M | 21 | Yes | 3 | Yes | 800 | None (D43) | |||||
| 4 | 81 | M | 4 | Yes | 3 | 2805 | None (D19) | ||||||
| Pure myopathy | |||||||||||||
| 5 | 69 | M | 21 | Yes | 6 | Yes | Yes | 17 | None (D43) | ||||
| 6 | 50 | M | 21 | Yes | 2 | Yes | Yes | 278 | Yes (D60) | ||||
| 7 | 66 | M | 21 | Yes | Yes | 5 | Yes | Yes | 192 | None (D41) | |||
| 8 | 65 | F | 3 | Yes | 43 | Yes (D40) | |||||||
| 9 | 71 | M | 2 | Yes | 700 | Partial (D10) | |||||||
| 10 | 58 | M | 14 | Yes | 4 | Yes | Yes | Yes | Yes | 103 | Yes (D55) | ||
| 11 | 67 | M | 21 | Yes | 2 | Yes | Yes | Yes | 17 | None (D35) | |||
| 12 | 69 | F | 2 | Yes | Yes | 4 | Yes | Yes | 17 | None (D24) | |||
| 13 | 46 | F | 2 | Yes | Yes | Yes | Yes | 12 000 | Partial (D25) | ||||
| Predominant myopathy | |||||||||||||
| 14 | 76 | M | 16 | Yes | Yes | Yes | 4 | Yes | Yes | Yes | 34 | Yes (D85) | |
| 15 | 57 | F | 4 | Yes | Yes | Yes | 94 | None (D123) | |||||
| 16 | 49 | M | 21 | Yes | Yes | Yes | 98 | None (D100) | |||||
| 17 | 79 | M | 10 | Yes | Yes | 3 | Yes | Yes | 35 | None (D88) | |||
| 18 | 71 | M | 21 | Yes | Yes | 3 | Yes | Yes | Yes | Yes | 240 | None (D48) | |
| 19 | 69 | M | 21 | Yes | 6 | Yes | 196 | None (D38) | |||||
| 20 | 69 | M | 21 | Yes | Yes | 5 | Yes | 164 | Yes (D90) | ||||
| 21 | 46 | F | 14 | Yes | Yes | Yes | 5 | Yes | Yes | Yes | 137 | Yes (D40) | |
| 22 | 67 | F | 21 | Yes | 6 | Yes | Yes | 38 | None (D40) | ||||
| 23 | 63 | M | 5 | Yes | Yes | 4 | Yes | 24 | Yes (D35) | ||||
| Neuromyopathy | |||||||||||||
| 24 | 72 | M | 21 | Yes | 3 | Yes | 17 | None (D63) | |||||
| 25 | 57 | F | 3 | Yes | Yes | 3 | Yes | 355 | None (D36) | ||||
| 26 | 32 | M | 21 | Yes | Yes | 5 | Yes | 800 | None (D64) | ||||
| 27 | 70 | M | 11 | Yes | Yes | Yes | 3 | Yes | Yes | 5990 | Yes (D48) | ||
| 28 | 82 | F | 2 | Yes | 182 | None (D15) | |||||||
| Predominant neuropathy | |||||||||||||
| 29 | 79 | M | 14 | Yes | Yes | 5 | Yes | Yes | Yes | 35 | None (D65) | ||
| Pure neuropathy | |||||||||||||
| 30 | 46 | M | 15 | Yes | Yes | 3 | Yes | Yes | 674 | None (D29) | |||
*Clinical motor status at Henri Mondor Hospital ICU discharge (D, day of final examination after ICU admission).
ATB, antibiotics; CK, creatine kinase plasma level (U/l); CS, corticosteroids; DEMG, day of neurophysiological examination after paresis observation; Diab M, insulin requiring diabetes mellitus; ICU, intensive care unit; MOF, multiple organ failure; NMBA, neuromuscular block agents; Renal F, renal failure; Resp F, respiratory failure; Seps, sepsis; y, years.
Correlation analyses between electrophysiological findings and clinical data failed to reach significance, but some trends could be seen. First, CK level tended to be increased in patients with spontaneous EMG activity compared with those without spontaneous activity (mean CK, 1055 v 375 U/l, Mann–Whitney U test, p = 0.07). Four of the six patients with the highest CK levels (⩾800 U/l) had been exposed to neuromuscular blocking agents. Second, steroid intake tended to be associated with neuropathic involvement (steroids were given in 12 of 17 patients (75%) with reduced SNAPamp but in four of 13 patients (31%) with normal SNAPamp (Fisher's exact test, p = 0.06).
Correlation analyses with clinical outcome were biased because follow up data after ICU discharge were missing. Nevertheless, we found that a better motor status at ICU discharge was significantly associated with the presence of spontaneous EMG activity (10 of 16 patients (63%) with spontaneous activity recovered partially or completely v two of 14 patients (14%) without spontaneous activity (Fisher's exact test, p = 0.01), and tended to correlate with the absence of neuropathy (seven of 13 patients (54%) with normal SNAPamp recovered before ICU discharge v five of 17 patients (29%) with reduced SNAPamp (Fisher's exact test, p = 0.26) and a high plasma CK (mean CK, 2204 U/l in patients who recovered before ICU discharge v 367 U/l in patients who did not recover (Mann–Whitney U test, p = 0.22).
Discussion
Using an original neurophysiological algorithm based on DMS results, we confirmed the predominance of a myopathic process (83% of the patients) as the origin of generalised weakness acquired in ICU.3,4 A brief historical overview of the DMS technique is required.
DMS technique
DMS was introduced in order to calculate conduction velocity along human muscle fibres.19 In curarised patients, Troni et al showed that muscle fibres could be stimulated by short duration electrical shocks delivered intramuscularly.16 Single fibre,16 then subdermal monopolar,20 and finally concentric EMG needles21 were used to record muscle fibre responses to DMS.
Rich et al applied this technique in the ICU environment and reported complete muscle fibre inexcitability in three patients with ICU acquired quadriplegia.13 In a second series of 14 weak ICU patients, they introduced the MNSamp/DMSamp ratio to differentiate neuropathy (ratio lower than 0.5) from myopathy (ratio higher than 0.5) and found myopathic process in 12 of 14 patients.14
To our knowledge, the DMS technique was applied in only two further studies carried out in an ICU. First, Trojaborg et al found a “myopathic” MNSamp/DMSamp ratio (above 0.5) in 20 critically ill patients, with reduced DMSamp values compared with controls (0.6 mV v 8.0 mV).17 Later, Bednarik et al22 studied 26 patients with ICU acquired diffuse paresis and discriminated between, first, a neuropathic profile with an MNSamp/DMSamp ratio below 0.5 (six patients); second, a myopathic profile with an MNSamp/DMSamp ratio above 0.5 and an absolute DMSamp below 2 mV (11 patients); and third, a normal profile with an MNSamp/DMSamp ratio above 0.5 and an absolute DMSamp above 2 mV (nine patients). In the present study, we included additional criteria for analysis, leading to an original diagnostic algorithm.
Original algorithm to discriminate between myopathy and neuropathy
In contrast to the previous studies, we analysed first the DMSamp values, then the MNSamp/DMSamp ratios, and we compared these data to SNAPamp values. We also differentiated normal from increased MNSamp/DMSamp ratios. In case of reduced muscle fibre excitability, MNS can recruit more muscle fibres than DMS, leading to an increased MNSamp/DMSamp ratio following stimulation of unequal muscle fibre populations.17
By applying our algorithm, we observed pure or predominant myopathy in 19 patients, neuromyopathy in five patients, and pure or predominant neuropathy in two patients. Four patients had normal DMS/MNS results, but high CK levels, and spontaneous EMG activity was found in two of these, suggesting necrotic myopathy. Thus needle EMG can be fruitfully added to the DMS technique to reveal myopathic involvement, which appeared to be the leading pathological process underlying the paresis in the present series.
A predominance of myopathies
In recent reports, myopathic changes in critically ill patients have been found more often than was recognised previously.23,24 Early reports had attributed the predominant motor involvement of the “pure motor syndrome”25 mostly to motor CIP on the basis of non‐specific electrophysiological abnormalities. These abnormalities—that is, CMAPamp reduction with normal SNAPamp and diffuse spontaneous EMG activity—were interpreted as features of motor axonopathy, neglecting the possibility of a primary muscle fibre disorder.26,27,28,29,30 As this pattern can be also considered suggestive of CIM,31,32 a controversial issue is whether or not there is a pure motor form of CIP.33 The present result did not provide any further evidence for the existence of pure motor CIP because we did not observe a reduced MNSamp/DMSamp ratio with normal DMSamp and SNAPamp. A pure motor demyelinating neuropathy with distal block was also unlikely, because no motor conduction slowing was observed, favouring demyelination. It must be emphasised that a reduced CMAPamp cannot differentiate muscle fibre inexcitability from neuropathy, and that SNAP abnormalities indicate only sensory neuropathy, but cannot be conclusive evidence of a neuropathic origin in any type of muscle weakness.11
Thus the present results were in favour of a myopathic process in most cases. The majority of investigators suggest that muscle disuse in critically ill patients—caused by prolonged exposure to neuromuscular blocking agents, immobilisation, or denervation—renders muscle more susceptible to steroids or toxins.3,34,35 However, in this study as in others36,37 some patients with myopathy had not been exposed to steroids or neuromuscular blocking agents. Muscle fibre inexcitability may be a leading cause of ICU acquired paralysis, but probably reflects various pathological processes.
Rich et al did a major study to explain the pathophysiological mechanisms of ICU acquired muscle inexcitability in the steroid denervated rat model. This model showed muscle fibre inexcitability to intracellular stimulation and reduced voltage gated sodium currents,38 associated with an upregulation of embryonic Nav1.5 sodium channel expression compensating for the loss of the adult Nav1.4 isoform.39 More recently, Rich et al highlighted the importance of increased fast inactivation of muscle sodium channels and the shift in the voltage dependence of channel inactivation to more negative potentials.40,41,42 Thus CIM with reduced muscle fibre excitability may be considered an acquired muscle channelopathy, triggered by still undetermined mechanisms. In addition, CIM might include alterations in the excitation–contraction coupling process, as blood serum fractions from patients with CIM were found to affect the release of calcium from the sarcoplasmic reticulum as well as the excitability of muscle fibre membrane.43
Muscle biopsies were not done in our patients. Not having histological correlates is a significant limitation of the interpretation of the results in our study, as in previous studies, except for the inconclusive data obtained in five patients in the series published by Bednarik et al.22 Reduced excitability might be observed in structurally intact muscle fibres on muscle biopsy,14,24,33 while the DMS technique may fail to reveal acute necrotising myopathy. It has been suggested that reduced muscle fibre excitability is common to all non‐necrotic forms of myopathy and is related to increased catabolic processes or to thick filament loss.14,44 It is worth differentiating these forms of ICU acquired myopathy from necrotising myopathy, which has specific diagnostic criteria (including high plasma CK and spontaneous EMG activity) and could be associated with a better clinical outcome.
Conclusions
This study suggests that myopathic involvement is more common than has been assumed in ICU acquired paresis. The DMS technique can be advocated for testing muscle fibre excitability and for establishing the correct diagnosis. By combining the DMS technique, needle EMG (spontaneous activity), and CK measurement, almost all myopathies may be uncovered. In contrast, neuropathic involvement cannot be determined by reduced CMAPamp, while reduced SNAPamp is inadequate to determine whether motor nerve lesions contribute to diffuse motor weakness. We feel that the use of the DMS technique may allow the mechanisms of ICU acquired paresis to be better understood, but whether this refinement would have significant impact on treatment and prognosis remains to be determined on large clinical series.45,46 Further studies are also awaited to determine what governs the individual responses to the ICU environment that generate either myopathic or neuropathic process.
Acknowledgements
We thank Isabelle Ménard‐Lefaucheur and Sylvie Wendling for their valuable technical assistance.
Abbreviations
CIM - critical illness myopathy
CIP - critical illness polyneuropathy
CK - creatine kinase
CMAP - compound muscle action potential
DMS - direct muscle stimulation
DMSamp - response amplitude to direct muscle stimulation
DMSlat - latency of direct muscle stimulation
ICU - intensive care unit
MNS - motor nerve stimulation
MNSamp/DMSamp ratio - ratio of response amplitude from motor nerve stimulation to direct muscle stimulation
MNSlat - latency of motor nerve stimulation
SNAP - sensory nerve action potential
SNAPamp - mean amplitude of the sensory nerve action potentials
Footnotes
Competing interests: none declared
References
- 1.Bolton C F. Neuromuscular conditions in the intensive care unit. Intensive Care Med 199622841–843. [DOI] [PubMed] [Google Scholar]
- 2.DeJonghe B, Cook D, Sharshar T.et al Acquired neuromuscular disorders in critically ill patients: a systematic review. Intensive Care Med 1998241242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lacomis D, Giuliani M J, Van Cott A.et al Acute myopathy of intensive care: clinical, electromyographic, and pathological aspects. Ann Neurol 199640645–654. [DOI] [PubMed] [Google Scholar]
- 4.Latronico N, Fenzi F, Recupero D.et al Critical illness myopathy and neuropathy. Lancet 19963471579–1582. [DOI] [PubMed] [Google Scholar]
- 5.McFarlane L A, Rosenthal F D. Severe myopathy after status asthmaticus. Lancet 1977ii615. [DOI] [PubMed]
- 6.Bolton C F, Gilbert J J, Hahn A F.et al Polyneuropathy in critically ill patients. J Neurol Neurosurg Psychiatry 1984471223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zochodne D W, Bolton C F, Wells G A.et al Critical illness polyneuropathy. A complication of sepsis and multiple organ failure. Brain 1987110819–841. [DOI] [PubMed] [Google Scholar]
- 8.Bolton C F, Brown J D, Sibbald W J. The electrophysiological investigation of respiratory paralysis in critically ill patients. Neurology 198333186 [Google Scholar]
- 9.Op de Coul A A, Verheul G A, Leyten A C.et al Critical illness polyneuromyopathy after artificial respiration. Clin Neurol Neurosurg 19919327–33. [DOI] [PubMed] [Google Scholar]
- 10.Gutmann L, Gutmann L. Critical illness neuropathy and myopathy. Arch Neurol 199956527–528. [DOI] [PubMed] [Google Scholar]
- 11.Breuer A C. Critical illness polyneuropathy. An outdated concept. Muscle Nerve 199922422–424. [DOI] [PubMed] [Google Scholar]
- 12.Rich M M, Raps E C, Bird S J. Distinction between acute myopathy syndrome and critical illness polyneuropathy. Mayo Clin Proc 199570198–200. [DOI] [PubMed] [Google Scholar]
- 13.Rich M M, Teener J W, Raps E C.et al Muscle is electrically inexcitable in acute quadriplegic myopathy. Neurology 199646731–736. [DOI] [PubMed] [Google Scholar]
- 14.Rich M M, Bird S J, Raps E C.et al Direct muscle stimulation in acute quadriplegic myopathy. Muscle Nerve 199720665–673. [DOI] [PubMed] [Google Scholar]
- 15.Kleyweg R, van der Méché F, Schmitz P. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain‐Barré syndrome. Muscle Nerve 1991141103–1109. [DOI] [PubMed] [Google Scholar]
- 16.Troni W, Cantello R, Rainero I. Conduction velocity along human muscle fibers in situ. Neurology 1983331453–1459. [DOI] [PubMed] [Google Scholar]
- 17.Trojaborg W, Weimer L H, Hays A P. Electrophysiologic studies in critical illness associated weakness: myopathy or neuropathy – a reappraisal. Clin Neurophysiol 20011121586–1593. [DOI] [PubMed] [Google Scholar]
- 18.Fagon J Y, Chastre J, Novara A.et al Characterization of intensive care unit patients using a model based on the presence or absence of organ dysfunctions and/or infection: the ODIN model. Intensive Care Med 199319137–144. [DOI] [PubMed] [Google Scholar]
- 19.Buchthal F, Guld C, Rosenfalck P. Propagation velocity in electrically activated muscle fibers in man. Acta Physiol Scand 19553475–89. [DOI] [PubMed] [Google Scholar]
- 20.Chino N, Noda Y, Oda N. Conduction study in human muscle fibers in situ – a useful technique for diagnosing myopathies. Electroencephalogr Clin Neurophysiol 198458513–516. [DOI] [PubMed] [Google Scholar]
- 21.Zwarts M J. Evaluation of the estimation of muscle fiber conduction velocity. Surface versus needle method. Electroencephalogr Clin Neurophysiol 198973544–548. [DOI] [PubMed] [Google Scholar]
- 22.Bednarik J, Lukas Z, Vondracek P. Critical illness polyneuromyopathy: the electrophysiological components of a complex entity. Intensive Care Med 2003291505–1514. [DOI] [PubMed] [Google Scholar]
- 23.Lacomis D. Critical illness myopathy. Curr Rheumatol Rep 20024403–408. [DOI] [PubMed] [Google Scholar]
- 24.Latronico N. Neuromuscular alterations in the critically ill patient: critical illness myopathy, critical illness neuropathy, or both? Intensive Care Med 2003291411–1413. [DOI] [PubMed] [Google Scholar]
- 25.Coakley J H, Nagendran K, Yarwood G D.et al Patterns of neurophysiological abnormality in prolonged critical illness. Intensive Care Med 199824801–807. [DOI] [PubMed] [Google Scholar]
- 26.Bolton C F, Lavery D A, Brown J D.et al Critical illness polyneuropathy: electrophysiological studies and differentiation from Guillain‐Barré syndrome. J Neurol Neurosurg Psychiatry 198649563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hund E, Genzwürker H, Böhrer H.et al Predominant involvement of motor fibres in patients with critical illness polyneuropathy. Br J Anaesth 199778274–278. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz J, Planck J, Briegel J.et al Single‐fiber electromyography, nerve conduction studies, and conventional electromyography in patients with critical‐illness polyneuropathy: evidence for a lesion of terminal motor axons. Muscle Nerve 199720696–701. [DOI] [PubMed] [Google Scholar]
- 29.Road J, Mackie G, Jiang T X.et al Reversible paralysis with status asthmaticus, steroids, and pancuronium: clinical electrophysiological correlates. Muscle Nerve 1997201587–1590. [DOI] [PubMed] [Google Scholar]
- 30.Geller T J, Kaiboriboon K, Fenton G A.et al Vecuronium‐associated axonal motor neuropathy: a variant of critical illness polyneuropathy? Neuromuscul Disord 200111579–582. [DOI] [PubMed] [Google Scholar]
- 31.Bird S J, Mackin G A, Schotland D L.et al Acute myopathic quadriplegia: a unique syndrome associated with vecuronium and steroid treatment. Muscle Nerve 1992151208 [Google Scholar]
- 32.Zochodne D W, Ramsay D A, Saly V.et al Acute necrotizing myopathy of intensive care: electrophysiologic studies. Muscle Nerve 199417285–292. [DOI] [PubMed] [Google Scholar]
- 33.Lacomis D, Zochodne D W, Bird S J. Critical illness myopathy. Muscle Nerve 2000231785–1788. [DOI] [PubMed] [Google Scholar]
- 34.Danon M J, Carpenter S. Myopathy with thick filament (myosin) loss following prolonged paralysis with vecuronium during steroid treatment. Muscle Nerve 1991141131–1139. [DOI] [PubMed] [Google Scholar]
- 35.DuBois D C, Almon R R. A possible role for glucocorticoids in denervation atrophy. Muscle Nerve 19814370–373. [DOI] [PubMed] [Google Scholar]
- 36.Höke A, Rewcastle N B, Zochodne D W. Acute quadriplegic myopathy unrelated to steroids or paralyzing agents: quantitative EMG studies. Can J Neurol Sci 199926325–329. [DOI] [PubMed] [Google Scholar]
- 37.Deconinck N, Van Parijs V, Beckers‐Bleukx G.et al Critical illness myopathy unrelated to corticosteroids or neuromuscular blocking agents. Neuromuscul Disord 19988186–192. [DOI] [PubMed] [Google Scholar]
- 38.Rich M M, Pinter M J, Kraner S D.et al Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Ann Neurol 199843171–179. [DOI] [PubMed] [Google Scholar]
- 39.Rich M M, Kraner S D, Barchi R L. Altered gene expression in steroid‐treated denervated muscle. Neurobiol Dis 19996515–522. [DOI] [PubMed] [Google Scholar]
- 40.Rich M M, Pinter M J. Sodium channel inactivation in an animal model of acute quadriplegic myopathy. Ann Neurol 20015026–33. [DOI] [PubMed] [Google Scholar]
- 41.Rich M M, Pinter M J. Crucial role of sodium channel fast inactivation in muscle fibre inexcitability in a rat model of critical illness myopathy. J Physiol (Lond) 2003547555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Filatov G N, Rich M M. Hyperpolarized shifts in the voltage dependence of fast inactivation of Nav1.4 and Nav1.5 in a rat model of critical illness myopathy. J Physiol (Lond) 2004559813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friedrich O, Hund E, Weber C.et al Critical illness myopathy serum fractions affect membrane excitability and intracellular calcium release in mammalian skeletal muscle. J Neurol 200425153–65. [DOI] [PubMed] [Google Scholar]
- 44.Ruff R L. Why do ICU patients become paralyzed? Ann Neurol 199843154–155. [DOI] [PubMed] [Google Scholar]
- 45.Leijten F, Harinck‐de‐Weerd J, Poortvliet D.et al The role of polyneuropathy in motor convalescence after prolonged mechanical ventilation. JAMA 1995275442–443. [PubMed] [Google Scholar]
- 46.De Jonghe B, Sharshar T, Lefaucheur J P.et al Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 20022882859–2867. [DOI] [PubMed] [Google Scholar]