Abstract
Background
Adult opsoclonus‐myoclonus (OM), a disorder of eye movements accompanied by myoclonus affecting the trunk, limbs, or head, is commonly associated with an underlying malignancy or precipitated by viral infection.
Methods
We present the first two reports of post‐streptococcal OM associated with antibodies against a 56 kDa protein. Two young girls presented with opsoclonus and myoclonus following a febrile illness and pharyngitis. Protein purification techniques were employed. Amino acid sequences of human neuroleukin (NLK) and streptococcal proteins were compared using the protein‐protein BLAST application.
Results
The antigen was identified as NLK (glucose‐6‐phosphate isomerase, GPI). GPI is present on the cell surface of streptococcus making the protein a candidate target for molecular mimicry.
Conclusions
We have identified NLK as an antigenic target in two patients with post‐streptococcal OM. The pathogenicity of the antibodies is uncertain. The potential role of anti‐neuroleukin antibodies in the pathogenesis of OM is discussed. We propose that OM may represent a further syndrome in the growing spectrum of post‐streptococcal neurological disorders. The role of streptococcus in OM and the frequency with which anti‐NLK responses occur in both post‐infectious and paraneoplastic OM should be investigated further.
Keywords: antibodies, opsoclonus‐myoclonus, post‐infectious, streptococcus
Opsoclonus‐myoclonus (OM) is characterised by chaotic saccadic, large amplitude multidirectional eye movements usually associated with myoclonus affecting the trunk, limbs, and head. Adult OM may be the presenting feature of an occult malignancy, most frequently breast or small cell lung cancer.1,2 OM may also occur in the absence of an identified malignancy. In most cases, the syndrome is post‐infectious or idiopathic. OM following infection with Epstein‐Barr virus, coxsackie virus B2, or enterovirus has been reported.3,4,5,6 Post‐infectious OM is thought to be the result of an immune mediated pathology although the evidence for this is limited. Post mortem investigations are often unremarkable. The lack of pathological findings, such as inflammatory infiltration and neuronal loss, correlates with the frequent resolution of non‐paraneoplastic OM. With no intervention post‐infectious OM is often self limiting, but resolution may be hastened by the use of immunomodulatory therapies.7
Although anti‐Ri and anti‐Hu are associated with paraneoplastic OM, no such antibody association exists with post‐infectious OM. Attempts to define an antibody response using serum from paraneoplastic and idiopathic patients suggest a heterogeneous immune response occurs in which antibodies are directed against various antigens within the nervous system.8 More recently, Blaes et al have suggested that antibodies against an extra‐cellular antigen on cerebellar granule cells can be detected in cases of OM.9
We report the first documented cases of post‐streptococcal OM associated with an antibody response against a 56 kDa protein, identify the antigen, and demonstrate its presence on the surface of neuronal cells.
Methods
Case reports
Patient 1
One week after a febrile illness and pharyngitis, a previously well 10 year old girl presented with chaotic, multi‐directional eye movements. The opsoclonus progressed rapidly over the next few days and was complicated by myoclonus and ataxia. In addition, the patient became profoundly insomniac and suffered a change in personality. Her speech became pressured, disinhibited, and inappropriate, and she experienced auditory hallucinations. Brain MRI, EEG, and echocardiogram were normal. CSF examination revealed 85 lymphocytes/mm3, CSF protein 0.48 g/dl, and normal CSF glucose/lactate. CSF Gram stain was negative. The patient was started on acyclovir and ceftriaxone pending CSF PCR for herpes simplex, varicella, and enterovirus, all of which were negative. No organisms were cultured from the CSF. Extensive serology for mycoplasma, influenza, chlamydia, adenovirus, Epstein‐Barr virus, and measles virus were all negative or normal. Anti‐streptolysin‐O titre (ASOT) was elevated (400 IU/ml, normal <200 IU/ml). Throat culture was negative. Biochemistry including copper metabolism, and liver and thyroid function tests was normal. Urinary vanillylmandelic acid (VMA) and homovanillic acid (HVA) were negative. An ultrasound of the abdomen and metaiodobenzylguanidine (MIBG) scanning were normal. The patient was treated with ACTH 40 U/day for 3 days and then with oral prednisolone 2 mg/kg for 2 weeks. In addition, she was given penicillin 500 mg bd for 2 weeks. Within 1 week, her sleep pattern and movement disorder had significantly improved, although her mood became labile. A convalescent ASOT performed 6 weeks after the first ASOT was <200 IU/ml. The prednisolone dose was tapered over 6 weeks, during which time her opsoclonus and movement disorder steadily improved leaving only a residual intention tremor. One year after her illness the patient had no neurological signs, although she remained hyperactive, a finding not reported prior to the onset of the neurological disease.
Patient 2
A 16 year old girl presented with a neurological disorder 1 week after a febrile illness characterised by pharyngitis and rash. The neurological dysfunction was initially characterised by gait disturbance followed by generalised myoclonus. In addition, her eye movements demonstrated jerky pursuit and reduced pupillary response to accommodation. Brain MRI, EEG, ECG, and echocardiogram were normal. CSF was acellular with CSF protein 0.5 g/dl and normal CSF glucose/lactate. CSF PCR for herpes simplex, varicella, and enterovirus were negative. Serology for mycoplasma, chlamydia, Epstein‐Barr virus, HIV, Lyme disease, and measles virus were all negative or normal. ASOT was elevated (800 IU/ml, normal <200 IU/ml), although throat culture was negative. Biochemistry including copper metabolism, urine toxicology, liver and thyroid function tests, autoimmune profile, and immunoglobulins was normal. Urinary VMA and HVA were negative and an ultrasound of the abdomen was normal. The patient was treated with oral prednisolone and 2 g/kg intravenous immunoglobulin over 24 h. Her illness was resistant to the initial treatment and progressed over the next month with the development of frank opsoclonus. In addition, her illness became complicated by the development of psychiatric symptoms, particularly anxiety and low mood. A repeat ASOT 6 weeks after the first ASOT had fallen to 235 IU/ml. The patient remained on 2 mg/kg of prednisolone for 2 months. The dose was tapered over a further 2 months. A repeat MRI at 6 months remained normal. Her OM had completely resolved by 9 months, although 2 years later she required rehabilitative care for her impaired motor function resulting from her prolonged admission.
Serum and CSF samples
Autoantigen identification in CNS disorders has ethical approval from the hospital research and development committee. Serum and CSF were taken from both patients (with ethical approval and consent) and stored at −80°C. CSF and serum pairs were obtained from 30 paediatric controls (mean age 11.7 years) and serum only from a further 24 controls (mean age 11.1 years). Clinical details are shown in table 1.
Table 1 Clinical details of control serum and CSF (n = 54).
| CSF and serum pairs (n = 30) | Serum only (n = 24) |
|---|---|
| Inflammatory conditions (n = 16) | Post‐streptococcal disorders (n = 8) |
| ADEM (n = 8) | Sydenham's chorea (n = 4) |
| Multiple sclerosis (n = 4) | Glomerulonephritis (n = 4) |
| Rasmussen's encephalitis (n = 2) | |
| Cerebral lupus (n = 2) | |
| Non‐inflammatory neurological | Healthy controls (n = 16) |
| conditions (n = 14) |
ADEM, acute disseminated encephalomyelitis.
Preparation of tissues
Human brain tissue snap frozen within 12 h of death was provided by Queen Square Brain Bank. Wistar rat brains were isolated and snap frozen immediately after death. Brain tissue was homogenised in a Teflon homogeniser with tissue protein extraction reagent (1 ml/g brain tissue; T‐per, Pierce, Rockford, IL) and mammalian protease inhibitors (50 μl/g brain tissue; Sigma, St Louis, MO). The homogenate was centrifuged at 10 000 g for 12 min and the supernatant was aliquoted and stored at −80°C until use.
Polyacrylamide gel electrophoresis and immunoblotting
Polyacrylamide gel electrophoresis (PAGE) was performed using Invitrogen 4–12% Bis‐Tris gels and NuPAGE MES buffer (Invitrogen, Carlsbad, CA). The rat and human brain tissues were diluted 1:16 (v/v) with double distilled water, and heated at 65°C for 15 min with 25% LDS sample buffer (Invitrogen) and 0.05 M dithiothreitol. Gels were run at 200 V, 110 mA/gel for 39 min. Silver staining was performed using Amersham Biosciences silver stain kit (Amersham Biosciences, Little Chalfont, UK).
Following PAGE, proteins were electroblotted for 2 h onto nitrocellulose in 5% transfer buffer (Invitrogen), 20% methanol, and 75% distilled water. Nitrocellulose was then blocked for 2 h in 2% milk. Serum from patients and controls was diluted 1:300, CSF was diluted 1:50. The blots were incubated overnight and then washed with 10 changes of 0.9% saline containing 0.1% Tween. Rabbit anti‐human IgG HRP (Dako, Glostrup, Denmark) diluted 1:1000 was then incubated with the blots for 2 h at room temperature. The blots were washed as before and then developed colourimetrically.
Ammonium sulphate fractionation
Ammonium sulphate fractionation was performed using 300 μl of rat brain supernatant and 50 μl Tris‐HCl (pH 9.0) diluted to 10 ml with water. Ammonium sulphate was added to give the following saturations: 0–20%, 20–40%, 40–60%, 60–80%, and 80–100%. At each stage the solution was stirred for 1 h and proteins precipitated by centrifugation at 10 000 g for 20 min. The precipitate was stored for later use and the supernatant used for further ammonium sulphate fractionation. Precipitated proteins were re‐suspended, subjected to PAGE, and immunoblotted. The 56 kDa antigen was detected using serum from patient 1.
Ion exchange chromatography (IEX)
IEX was performed using the ÄKTA fluid phase liquid chromatography system and the anionic exchange HiTrap Mono Q FF (5 ml) column (Amersham Biosciences). Proteins from the 40–60% ammonium sulphate fraction were exchanged into IEX binding buffer (20 mM Tris‐HCl, pH 8.35) and injected onto the column. Proteins were eluted over a gradient of increasing ionic strength by injection of elution buffer (20 mM Tris‐HCl, 1.5 M NaCl, pH 8.35) 0–100% over 50 ml. Fractions were collected using a Frac‐900 fraction collector and those corresponding to peaks on the chromatogram selected, subjected to PAGE, and immunoblotted. The 56 kDa antigen was detected using serum from patient 1. Further IEX was conducted using proteins from the initial IEX separation and a lower pH binding buffer (20 mM Tris‐HCl, pH 8.0). Proteins were subjected to gradient elution as described using 20 mM Tris‐HCl, 1.5 M NaCl, pH 8.0. Fractions were collected and analysed as above. Silver stained proteins were subjected to in gel trypsinolysis and identified using a Q‐TOF hybrid quadrupole/orthogonal acceleration time of flight spectrometer.
Production of recombinant neuroleukin (rNLK)
cDNA was synthesised by RT‐PCR amplification of human cerebellar mRNA using gene specific primers to amplify the entire open reading frame of neuroleukin (NLK) (GenBank accession no. K03515). The following sense and anti‐sense primers were used: sense primer, 5′‐CCGGAATTCATGGCCGCTCTCTCACCC‐3′ (EcoRI site underlined); anti‐sense primer, 5′‐GCCCAAGCTTATTGGACTCTGGCCTCG‐3′ (HindIII site underlined). Amplified cDNA was gel purified and ligated into the pRSETB bacterial expression vector (Invitrogen) and used to transform Escherichia coli BL21 (DE3) pLysS (Invitrogen) in preparation for expression of the recombinant protein. Cells were grown at 37°C to OD 0.4–0.6 and protein production induced by addition of 1 mM IPTG. After 3 h, the cells were harvested by centrifugation, re‐suspended in His‐binding buffer (8 M urea, 20 mM Tris‐HCl, 0.5 M NaCl, 20 mM imidazole, 1 mM 2‐mercaptoethanol) containing bacterial protease inhibitors (Sigma), and subjected to four 10 s rounds of sonication on ice. Recombinant protein was purified using a 1 ml HiTrap chelating column (Amersham). His‐tagged proteins were eluted from the column using His‐elution buffer (8 M urea, 20 mM Tris‐HCl, 0.5 M NaCl, 0.5 M imidazole, 1 mM 2‐mercaptoethanol), aliquoted, and stored at −20°C.
Immunoblotting rNLK
A 30 μg sample of purified rNLK was subjected to PAGE, immunoblotted, and probed with serum or CSF. Rabbit anti‐NLK antibodies were raised in New Zealand rabbits (CovalAb, Cambridge, UK) as described10 and used as a positive control (secondary antibodies: rabbit anti‐human IgG HRP and swine anti‐rabbit IgG HRP diluted 1:5000).
Immunocytochemistry
Live neurones were cultured from Sprague‐Dawley rat foetuses at 17 days' gestation. On day 10 of culture, cells were rinsed in Tris‐buffered saline (TBS) and fixed by addition of ice cold methanol for 10 min. The methanol was removed, the cells rinsed in blocking buffer (TBS, 1% BSA, 10% serum (corresponding to the species of the secondary antibody)) for 2 h at room temperature. Blocking solution was removed and rabbit anti‐NLK (diluted 1:1000), patient's serum (diluted 1:70), or anti‐Hu positive serum (diluted 1:70) applied and incubated overnight at 4°C. Cells were washed twice in TBS and incubated with the appropriate secondary antibody for 1 h at room temperature. The cells were washed and stained with 0.01% 4,6‐diamidino‐2‐phenylindole HCl (DAPI) for 10 min prior to mounting on glass cover slip slides using 10 μl of citiflour. Serum from healthy controls and patients with ADEM was used as controls. Primary and secondary antibodies were omitted to provide negative controls. Cells were visualised by confocal microscopy using a Zeiss 510 laser scanning confocal microscope.
Determination of sequence homology
A comparison of the amino acid sequences of human NLK and streptococcal proteins was conducted using the protein‐protein BLAST application (http://www.ncbi.nih.gov/BLAST). The default parameters were used for comparison.
Results
Protein purification and identification
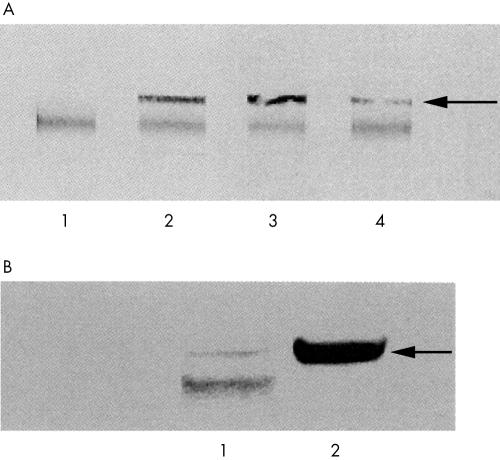
Antibodies in the serum and CSF of the two patients reacted with a 56 kDa protein present in immunoblots of human brain homogenate (fig 1A) which was also present in the 40–60% ammonium sulphate fraction of rat brain (fig 1B). Fractionated rat brain was used in subsequent protein purification and separation.
Figure 1 (A) Antibodies against a 56 kDa protein in serum and CSF of OM patients. Lane 1: control serum; lane 2: serum from patient 1; lanes 3 and 4, serum and CSF from patient 2, respectively. Arrow: 56 kDa antigen. The common band represents human IgG within the tissue preparation. (B) Comparison of human and rat brain. Serum from patient 1 detects the 56 kDa antigen in both human brain (lane 1) and the 40–60% ammonium sulphate fraction of the rat brain homogenate (lane 2) (arrow). The lower band (lane 1) represents human IgG in the preparation of the tissue homogenate.
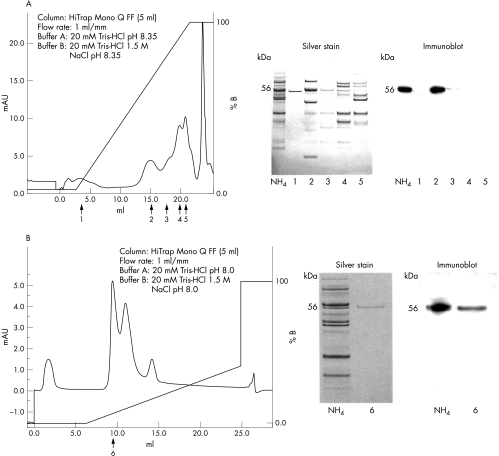
IEX chromatography was conducted over two stages. During the initial stage, the 56 kDa candidate autoantigen eluted when elution buffer equalled 55% of the elution gradient. During the second stage, the 56 kDa candidate autoantigen eluted after 10% of the elution gradient (fig 2). Q‐TOF mass spectral analysis identified the purified protein as NLK.
Figure 2 Two rounds of IEX were used to purify the 56 kDa antigen. (A) Partially purified proteins from the 40–60% ammonium sulphate fraction were separated. Peaks 1–5 were selected, separated by PAGE, and the gel stained or immunoblotted and probed with serum from patient 1. Immunoblotting showed that the antigen was contained within peak 2. (B) The proteins from peak 2 were subjected to further IEX under different conditions. A number of peaks were selected and the antigen again detected by immunoblotting. The antigen was contained in peak 6. A silver stain showed a single protein at 56 kDa, the molecular weight of the antigen. No other proteins were observed in the gel. The band was digested and subjected to mass spectrometry (NH4 = pre‐IEX 40–60% ammonium sulphate fraction of rat brain homogenate).
rNLK: patients and controls
Antibodies in the serum and CSF of both patients showed strong reactivity with rNLK. Five of 54 (9%) controls showed reactivity with rNLK (cerebral lupus, n = 1; normal, n = 3; encephalitis, n = 1). The blot using CSF from patient 2 is shown (fig 3). Only one of 30 (3%) CSF controls was positive. This patient had cerebral lupus with cognitive and gait disturbance.
Figure 3 Antibodies against rNLK were detected in the CSF. Lane 1: rabbit anti‐NLK (positive control); lane 2: CSF from patent 2; lanes 3–7: control CSF.
Immunohistochemistry
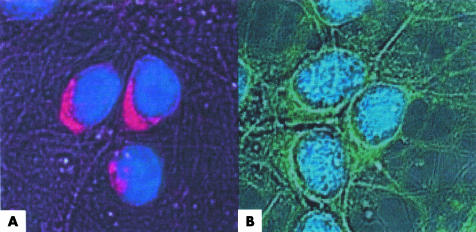
Permeablisation steps were omitted during the preparation of rat neurones in an attempt to determine whether NLK existed on the plasma membrane. No cytoplasmic or nuclear staining was observed in neurones stained with the rabbit anti‐NLK antibody. Bright staining was seen around the perimeter of the cell (fig 4). Serum from both patients produced a similar staining pattern to that produced by the positive control. No nuclear staining was produced when anti‐Hu positive serum was used suggesting that the integrity of the plasma membrane had been maintained during the preparation of the slides. Staining was not observed in the control preparations.
Figure 4 Rat neurones were incubated with (A) rabbit anti‐NLK antibody or (B) serum from patient 1. Staining can clearly be seen localised to the plasma membrane. Staining of the cytoplasm did not occur as evidenced by the separation of the nucleus (blue) from the stained membrane.
Comparison of human NLK and streptococcal glucose‐6‐phosphate isomerase (GPI)
There is significant sequence homology between human NLK and streptococcal GPI. A BLAST comparison of the amino acid sequences of human NLK with Streptococcus pyogenes GPI revealed 89 exact amino acid matches (23% of total sequence), with 147 close matches (38%) (expected chance homology was less than 1×10−6).
Discussion
OM can be subclassified as a paraneoplastic, post‐infectious or idiopathic neurological disease. It is probable that many cases of idiopathic OM are post‐infectious, but definitive identification of the precipitating pathogen is not always possible.
We present the first documented cases of post‐streptococcal OM. Both patients experienced a prodromal upper respiratory tract illness with elevated ASOT. The patients were much older than the typical patient with paediatric paraneoplastic OM (6–36 months) and much younger than the typical adult patient with paraneoplastic OM (40–77 years).5 Neither had evidence for a precipitating tumour and both experienced complete resolution of their OM.
Using a proteomic approach we were able to characterise NLK as an antigen recognised by antibodies in both the CSF and serum of both patients. NLK is an example of a moonlighting protein with both intracellular and extracellular functions and has thus been given a number of different names. Intracellular NLK exists as GPI which catalyses the interconversion of glucose 6‐phosphate and fructose 6‐phosphate in the glycolytic pathway. The extracellular protein has various functions including the regulation of cell migration during tumour invasion and metastasis (autocrine motility factor, AMF) and cell maturation (maturation factor, MF). The name NLK was assigned because of the neurotrophic effects observed when the protein was incubated with cultures of spinal and sensory neurones.11
Interference with the function of NLK has been implicated in AIDS associated neuropathology12 and mutation in the NLK gene has been connected with myopathy and mental retardation.13 Antibodies against NLK have been implicated in amylotrophic lateral sclerosis.14
Investigation suggests NLK is secreted by a non‐classical pathway15 and that the extracellular effects of NLK are mediated though its interaction with the cell surface glycoprotein gp78. It is also possible that a form of NLK is associated with the plasma membrane. This is supported by the fact that NLK has been detected with synaptosomal membranes16 and that other glycolytic enzymes have been shown to be associated with the neuronal plasma membrane.17 We clearly demonstrate that rabbit anti‐NLK and patients' serum detect a protein associated with the plasma membrane. It is possible that NLK represents the antigen recently detected on the surface of cerebellar granule cells using serum from 10/14 patients with OM.9 The patients described in that series were predominantly paraneoplastic. However, given the role of NLK in tumour metastasis and its identification and occurrence in human pathogens (see below), it is possible that antibodies to this protein represent the final common pathway of the post‐infectious and paraneoplastic pathological processes.
The extracellular nature of the antigen suggests that the antibodies may be able to exert an effect. This would be consistent with the absence of inflammatory changes in post mortern specimens. Clearly, further work such as in vitro investigation, the passive transfer of antibodies to animals, and immunisation will be required to determine whether the antibodies are indeed pathogenic.
The significant sequence homology between human NLK and streptococcal GPI raises the possibility that the protein represents a molecular mimic. The process of molecular mimicry may explain the frequent resolution of post‐infectious OM since successful eradication of the pathogen by the immune system would remove the antigenic stimulus for antibody production, thus resulting in a self limiting illness.
Recently, others have speculated on the role of antibodies that cross‐react with glycolytic enzymes on the surface of group A streptococcus and neuronal tissue.18 In addition, there is increasing evidence for the role of streptococcal infection in a growing spectrum of movement disorders, many of which have been associated with an anti‐neuronal antibody response.19 OM may represent another of these disorders, although further studies will be required.
In conclusion, we have identified NLK as an antigenic target in two patients with post‐streptococcal OM. The pathogenicity of the antibodies is uncertain. Despite this, the accessibility of the antigen, its role in the normal nervous system, and the presence of anti‐NLK antibodies in the CSF make NLK an interesting target. Furthermore, an antibody mediated disease would account for the pathological and clinical findings. Further investigation involving cohorts of patients with post‐infectious, paraneoplastic, and idiopathic OM will be required. The role of streptococcus in OM and the frequency with which anti‐NLK responses occur in both post‐infectious and paraneoplastic OM will need to be investigated to elucidate the significance of the pathogen and antibodies in the disease.
Electronic‐database information
The protein‐protein BLAST application can be found at www.ncbi.nih.gov/BLAST.
Copyright © 2006 BMJ Publishing Group
Abbreviations
ASOT - anti‐streptolysin‐O titre
GPI - glucose‐6‐phosphate isomerase
HVA - homovanillic acid
IEX - ion exchange chromatography
NLK - neuroleukin
OM - opsoclonus‐myoclonus
PAGE - polyacrylamide gel electrophoresis
rNLK - recombinant neuroleukin
TBS - Tris‐buffered saline
VMA - vanillylmandelic acid
Footnotes
Competing interests: none declared
Patient details are published with consent
The protein‐protein BLAST application can be found at www.ncbi.nih.gov/BLAST.
References
- 1.Luque F A, Furneaux H M, Ferziger R.et al Anti‐Ri: an antibody associated with paraneoplastic opsoclonus and breast cancer. Ann Neurol 199129241–251. [DOI] [PubMed] [Google Scholar]
- 2.Pittock S J, Lucchinetti C F, Lennon V A. Anti‐neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol 200353580–587. [DOI] [PubMed] [Google Scholar]
- 3.Delreux V, Kevers L, Callewaert A.et al Opsoclonus secondary to an Epstein‐Barr virus infection. Arch Neurol 198946480–481. [DOI] [PubMed] [Google Scholar]
- 4.Verma A, Brozman B. Opsoclonus‐myoclonus syndrome following Epstein‐Barr virus infection. Neurology 2002581131–1132. [DOI] [PubMed] [Google Scholar]
- 5.Digre K B. Opsolonus in adults. Arch Neurol 1986431165–1175. [DOI] [PubMed] [Google Scholar]
- 6.Imtiaz K E, Vora J P. Dancing eyes‐dancing feet. Lancet 1999354390. [DOI] [PubMed] [Google Scholar]
- 7.Bataller L, Graus F, Saiz A.et al Clinical outcome in adult onset idiopathic or paraneoplastic opsoclonus‐myoclonus. Brain 2001124437–443. [DOI] [PubMed] [Google Scholar]
- 8.Bataller L, Rosenfeld M R, Graus F.et al Autoantigen diversity in the opsoclonus‐myoclonus syndrome. Ann Neurol 200353347–353. [DOI] [PubMed] [Google Scholar]
- 9.Blaes F, Fühlhuber V, Korfei M.et al Surface‐binding autoantibodies to cerebellar neurons in opsoclonus syndrome. Ann Neurol 200558317. [DOI] [PubMed] [Google Scholar]
- 10.Niinaka Y, Paku S, Haga A.et al Expression and secretion of neuroleukin/phosphohexose isomerase/maturation factor as autocrine motility factor by tumor cells. Cancer Res 1998582667–2674. [PubMed] [Google Scholar]
- 11.Gurney M E, Heinrich S P, Lee M R.et al Molecular cloning and expression of neuroleukin, a neurotrophic factor for spinal and sensory neurons. Science 1986234566–574. [DOI] [PubMed] [Google Scholar]
- 12.Lee M R, Ho D D, Gurney M E. Functional interaction and partial homology between human immunodeficiency virus and neuroleukin. Science 19872371047–1051. [DOI] [PubMed] [Google Scholar]
- 13.Kugler W, Breme K, Laspe P.et al Molecular basis of neurological dysfunction coupled with haemolytic anaemia in human glucose‐6‐phosphate isomerase (GPI) deficiency. Hum Genet 1998103450–454. [DOI] [PubMed] [Google Scholar]
- 14.urney M E, Belton A C, Cashman N.et al Inhibition of terminal axonal sprouting by serum from patients with amyotrophic lateral sclerosis. N Engl J Med 1984311933–939. [DOI] [PubMed] [Google Scholar]
- 15.Haga A, Niinaka Y, Raz A. Phosphohexose isomerase/autocrine motility factor/neuroleukin/maturation factor is a multifunctional phosphoprotein. Biochim Biophys Acta 20001480235–244. [DOI] [PubMed] [Google Scholar]
- 16.Knull H R, Fillmore S J. Glycolytic enzyme levels in synaptosomes. Comp Biochem Physiol B 198581349–351. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima K, Hamanoue M, Takemoto N.et al Plasminogen binds specifically to a alpha‐enolase on rat neuronal plasma membrane. J Neurochem 1994632048–2057. [DOI] [PubMed] [Google Scholar]
- 18.Pancholi V. Multifunctional α‐enolase: its role in disease. Cell Mol Life Sci 200158902–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dale R C, Heyman I. Post‐streptococcal autoimmune psychiatric and movement disorders in children. Br J Psychiatry 2002181188–190. [DOI] [PubMed] [Google Scholar]