Abstract
Objective
To describe a large pedigree with Charcot–Marie–Tooth disease type 1A (CMT1A) duplication in which severe pelvic and thigh musculature weakness occurred in two patients, detected by analysing the leg muscle atrophy pattern on magnetic resonance imaging (MRI).
Methods
The pedigree comprised 18 patients, aged between 15 and 85 (median 46) years, who were serially evaluated for up to three decades. All 18 patients and 13 non‐affected at‐risk people underwent electrophysiological examination. An MRI study of lower limb musculature was carried out in four patients. Three patients underwent sural‐nerve biopsy. Genetic testing was carried out in 17 patients and in all 13 at‐risk normal people.
Results
Fourteen patients were asymptomatic or slightly disabled. The two oldest patients, aged 84 and 80, showed a moderate phenotype. Two other patients, aged 70 and 53, showed late‐onset and gradually progressive peroneal paresis extending up to the thigh and pelvic musculature, resulting in waddling gait. MRI scans of all three patients with a mild phenotype showed subtle and subclinical fatty infiltration of calf anterolateral muscle compartments, with thigh muscle involvement in one patient, and extensive atrophy of intrinsic foot muscles. In the youngest patient with proximal leg weakness, the MRI scan showed massive fatty atrophy of all the calf muscles, posteromedial thigh muscle compartments, and internal and external hip rotator muscles. Sural‐nerve biopsy specimens showed hypertrophic neuropathy with no superimposed inflammation. Good correlation was seen between electrophysiological and genetic testing.
Conclusions
Late in the clinical course, a small proportion of patients with CMT1A develop severe proximal leg weakness, and long‐term follow‐up is essential for its detection. MRI scans may show subclinical involvement of the thigh musculature.
Charcot–Marie–Tooth disease type 1A (CMT1A) is an autosomal dominant demyelinating polyneuropathy, usually associated with a large DNA duplication on the short arm of chromosome 17.1,2,3 The hallmark of the disease is a peroneal muscular atrophy syndrome of variable severity, and a marked and diffuse slowing of nerve conduction velocity.4,5,6,7,8 Symptoms are seen during the first decade of life in >60% of patients.5 The clinical course is quiescent in adults,9 although a considerable age‐dependent increase in either mean weakness score5,6 or neuropathic deficit10 has been reported in cross‐sectional studies; furthermore, functional disability increases with disease duration.11 The predominant clinical signs are distally accentuated muscle weakness and wasting in the lower limbs usually associated with a loss of tendon reflexes and foot deformity.5,12 As the disease advances, more proximal muscles may become weak,13 although clinically perceptible paresis of the proximal limb muscles is extremely rare in CMT1A.14
Here, we describe a longitudinal study conducted over a period of three decades on a large pedigree comprising 18 examined patients with CMT1A duplication. Two patients showed severe pelvic and thigh musculature weakness. We analysed the magnetic resonance imaging (MRI) pattern of proximal lower limb muscle atrophy in one of them.
Methods
Pedigree MO
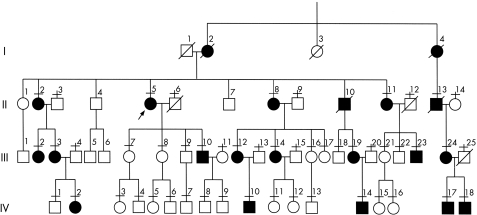
This is pedigree A in the reference by Combarros et al.15 Figure 1 shows the updated pedigree, which comprises 18 patients examined over three generations. By 1983, the relationship of patient III‐24 had not been established with certainty and, therefore, the corresponding branch of the pedigree does not appear in the referred pedigree A15; this question was clarified in 1983, when we examined the patient's affected father (II‐13), who provided genealogical information of his affected mother. Members of the pedigree were included in our previous papers on CMT‐16,15,16 and CMT1A.3,8,17,18,19,20
Figure 1 Pedigree of the family. I–IV represent four generations. Horizontal bars under the numbers indicate examined patients. The proband patient (II‐5, arrow) is the same proband patient as in pedigree A reported by Combarros et al15 in 1983. Squares are males and circles are females. Filled symbols are affected subjects and empty symbols are unaffected subjects.
Clinical assessment
Patients were clinically evaluated by recording a detailed history, as reported previously.6,8,15 Muscle power was evaluated manually, using the Medical Research Council (MRC) scale. To determine physical disability, we administered, either prospectively or retrospectively, the Functional Disability Scale (FDS)11 and the CMT Neuropathy Score (CMTNS).21 The hospital ethics committee approved the study.
Electrophysiological study
Motor conduction velocities (MCVs) of the median, ulnar, peroneal and tibial nerves were measured using standard methods, and the compound muscle action potential (CMAP) was recorded from the abductor pollicis brevis, abductor digiti minimi, extensor digitorum brevis and abductor hallucis muscles. The brachial plexus was stimulated at the Erb's point and the radial nerve at above the elbow, with recordings from the biceps brachii and extensor digitorum communis muscles. Femoral nerve stimulation was performed just distal to the inguinal ligament, with recording from the vastus medialis muscle. CMAP amplitude was measured from baseline to negative peak. Distal motor latency (DML) and the terminal latency index (TLI) were determined. Sensory conduction velocities (SCVs) of the median and ulnar nerves were assessed by stimulating digits III and V with ring electrodes, and recording the sensory nerve action potential (SNAP) at the wrist. Antidromic radial and sural‐nerve SNAPs were recorded at the wrist and ankle after 14 cm of proximal percutaneous stimulation; the peak‐to‐peak amplitude of the SNAP was measured.
An electromyogram (EMG) was recorded using concentric needle electrodes. We analysed the duration and morphology of the motor unit potentials (MUPs), the presence of spontaneous activity, and the EMG pattern at the maximum voluntary effort.22
MRI study
MRI was carried out using a 1.5‐T clinical scanner (Signa General Electric Medical Systems, Milwaaukee, Wisconsin, USA). The examinations were carried out with patients in a supine position. In scans of all four patients who underwent MRI we studied the thighs and legs, and in one patient we also evaluated the foot musculature in the same session. Leg muscle explorations were carried out with a commercially available phase array multicoil (GE Medical Systems, Slough, UK), using transverse (field of view 24–32 cm, slice thickness 10 mm and slice gap 0.5–1 mm) and coronal planes (field of view 38–40 mm, slice thickness 4–5 mm and slice gap 0.5–1 mm), with the following protocol: T1‐weighted fast spin echo sequence (repetition time/echo time (TR/TE) 400–540/9–11) and fat‐suppressed proton density–T2‐weighted fast spin echo sequence (TR/TE 3000–3500/50–70) in both planes. The thigh musculature was explored with the same sequences and planes, adapting the field of view, slice thickness and gap (44–48, 10 mm and 2 mm, respectively). We also obtained T1‐weighted spin echo using a chemical sift fat suppression sequence (TR/TE 500/9) before and after injection of a paramagnetic contrast agent (GdDPTA‐BMA (GE Healthcare, Chalfont St Giles, UK), 0.2 ml/kg) in the axial plane. Feet were studied with the same T1‐weighted spin echo sequences in coronal and axial planes using the manufacturer's standard head coil (GE Medical Systems).
Nerve biopsy
Whole sural‐nerve biopsy was carried out at the level of lateral malleolus under local anaesthesia in patients II‐5, II‐11 and II‐13. Histological study was carried out as reported previously.15
DNA analysis
With informed consent, all examined patients (except for patient II‐13, whose DNA sample was not available) and 13 at‐risk unaffected relatives (fig 1) were analysed for the presence of the 17p11.2 duplication, as reported previously.3,23
Results
Clinical findings
We present detailed clinical data of patient III‐24 and her father (II‐13) and sons (IV‐17 and IV‐18), and an overview of the remaining 14 patients examined (fig 1).
Patient III‐24
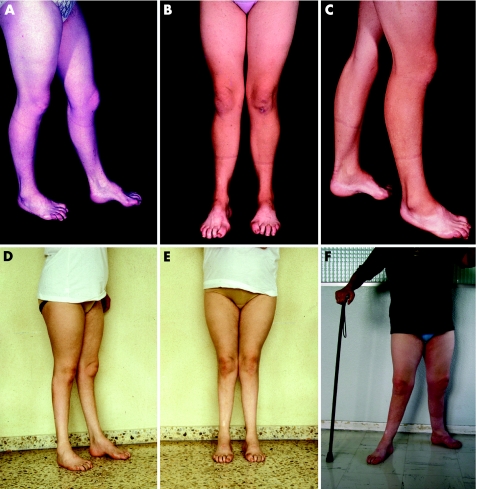
In 1976, the then 23‐year‐old woman was first attended as a secondary case; she was an illegitimate daughter of an affected member of family MO. She was asymptomatic at that time; during detailed history taking she referred to progressive foot deformities and gait clumsiness from adolescence, which did not prevent her from working as a farmer. Examination showed marked pes cavus and clawing of toes (fig 2A). No evidence of leg muscle atrophy was noted. Muscle power of the leg muscles, and particularly of the peronei, was preserved (MRC grade 5/5). She was unable to walk on her heels, but tiptoe walking was normal. Generalised areflexia was seen. Auricular nerves were visibly enlarged. Mild‐to‐moderate stocking hypoaesthesia was noted, which extended up to the ankles, predominating for propioception. Retrospectively, the FDS score was 1. Clinical and electrophysiological examination of her mother (II‐14) was normal. Re‐examination at age 31 showed incipient peroneal muscular atrophy (fig 2B,C). She had two sons born in 1982 and 1983; both were found to be affected on serial examinations (described later). Pregnancies did not worsen her gait difficulties.
Figure 2 Serial photographs of patient III‐24 taken between 1976 and 2005. (A) This picture, taken in 1976 (at age 23), shows pes cavus and toe clawing, but preservation of leg musculature. (B,C) Eight years later, incipient bilateral peroneal muscular atrophy is seen. (D,E) At age 38, marked and bilateral leg muscle atrophy is evident. (F) At age 53, wide‐based stance is observed, which is possible only with support; lower limb amyotrophy and foot deformities are now masked by weight gain.
At age 34, she gradually developed progressive foot and leg weakness, making gait increasingly more difficult; neither hand clumsiness nor sensory symptoms were noticed. Examination four years later showed marked leg muscle atrophy (fig 2D,E) with bilateral steppage; FDS score shifted from 1 to 3. Muscle strength of the flexoextensor ankle and toe muscles was MRC grade 2/5 for the plantar extensors and 3/5 for plantar flexors. Muscle strength of the knee and hip flexoextensor muscles was preserved. Mild hand weakness, pain, and vibration stocking and glove hypoaesthesia extending up to the knee and wrist were observed. She denied any drug misuse or alcoholism. Routine laboratory studies, including examination of cerebrospinal fluid, were normal.
Despite an initial improvement in her walking difficulties with foot orthotics, in the last decade, she has continued developing gradually progressive deterioration of gait; her walk was becoming ungainly, obliging her to continuously use a cane. Sensory positive symptoms, neuropathic pain and stepwise deterioration with acute or subacute relapses of gait semeiology were not observed. Despite disease progression, she is still able to do housework unaided. In October 2005, examination disclosed positive Gowers' manoeuvre in getting up from a chair. Walking was possible only with support and a combined bilateral steppage and waddling. Stance was wide based and also possible only with support (fig 2F). The FDS score shifted from 3 to 4. Marked atrophy of the foot, leg and posterior thigh muscles was noted. Muscle strength of the flexoextensor foot muscles was 0/5. Muscle strength of the remaining lower limb muscles was as follows:
a. Knee extensors, 4/5; flexors, 2/5
b. Thigh adductors, 4/5; abductors, 2/5
c. Internal hip rotators (mainly the gluteus medius muscle), 2/5; external rotators, 4/5
d. Hip flexors, 4/5; extensors (mainly the gluteus maximus muscles), 4/5.
A more advanced hand muscle atrophy was seen with incipient clawing, with intrinsic hand musculature strength being 3/5. Muscle power of the arm, forearm, and neck and face was preserved. Fasciculations were not observed. Repeated routine laboratory investigations including creatine kinase levels were normal. Investigation of serum tumour markers, antiganglioside antibodies and paraproteins was negative. CMTNS was 23, indicative of severe disease.
Patient II‐13
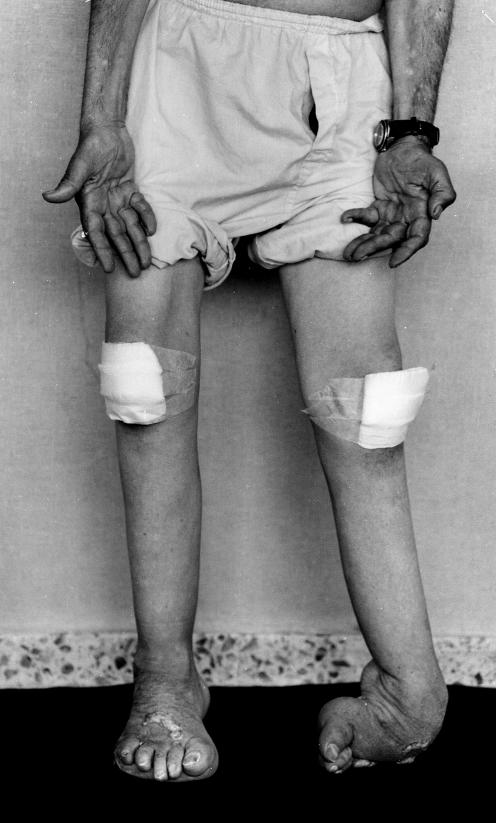
In 1983, the then 70‐year‐old herdsman referred to progressive foot deformity since infancy, although he had never consulted a doctor. At 40 years of age, he developed leg weakness with increasing gait difficulties, which obliged him to walk with a cane. At 65 years of age, he used to do some of his farmyard work by walking on his knees. Examination showed extensive psoriasis, bilateral pes cavus with left club‐foot deformity, marked leg amyotrophy and, to a lesser degree, atrophy of the thigh and hand musculature (fig 3). Walking was possible only with support and with bilateral steppage, and some waddling component was seen. Muscle power was 0/5 for the foot extensors and 2/5 for foot flexors. Muscle strength of the knee extensors and flexors, thigh adductors and abductors, hip internal and external rotators, hip flexors and extensors, and intrinsic hand muscles was graded 3/5 to 4/5. Generalised areflexia, and profound stocking (to knee) and glove (to third distal forearm) hypoaesthesia associated with exteroceptive and propioceptive sensations were seen; no foot ulcers were observed. The peripheral nerves were thickened. The FDS score was 5 and CMNTS was 25. Routine laboratory investigations were normal.
Figure 3 Photograph of patient II‐13 showing severe leg muscle atrophy and foot deformity with left club‐foot and clawing of toes. Hand and thigh muscle atrophy is also seen to a lesser degree. Psoriasis is evident on the dorsum of the feet; the knees are bandaged owing to combined lesions caused by psoriasis and the patient's habit of walking on his knees.
Patients IV‐17 and IV‐18
These patients are sons of patient III‐24. They have been evaluated yearly since infancy. They are asymptomatic. In October 2005, at 24 and 23 years of age, examination showed similar signs, including pes cavus, atrophy of the extensor digitorum brevis muscles, clawing of toes, flattening of transverse arcus plantaris, shortening of tendo Achillis, difficulty in walking on heels, generalised areflexia and thickened peripheral nerves. Neither muscle atrophy nor weakness of the peroneal musculature was noted. Sensory examination was normal. In both patients, the FDS scores were 0. CMTNS was 4 in patient IV‐18 and 3 in IV‐17.
Other members of the pedigree
Clinical data of the remaining 14 patients examined (fig 1) have been previously reported;6,8,15,18,19,20 so we briefly mentioned semeiology according to updated examinations carried out in the past 3 years. They have at most a moderate disability.
Ages of examined patients from the second generation ranged from 72 (II‐11) to 84 years (II‐2). Despite concomitant diseases (diabetes mellitus in II‐2 and II‐5, and brain infarct with left hemiparesis in II‐11), all four affected sisters of this generation remain ambulant, although the two eldest require support with a cane. FDS scores are 4 for II‐2 and II‐5, 2 for II‐11 and 0 for II‐8. Peroneal weakness (MRC score 3/5) and hand clawing were observed in patients II‐2 and II‐5. Patient II‐8, aged 73 years, is asymptomatic, and her examination showed pes cavus, difficulty in walking on her heels, generalised areflexia, nerve thickening and slight stocking hypoaesthesia, but absence of peroneal amyotrophy. Mild hand postural tremor (Roussy–Lewy) was noted only in patients II‐2 and II‐5. None of the four patients had proximal upper or lower limb weakness.
Examined patients of the third generation are aged 34 (III‐23) to 50 years (III‐2), and those of the fourth generation 15 (IV‐2) to 18 years (IV‐14). These patients are asymptomatic, except for frequent references to ill‐fitting shoes or foot deformities on specific questioning. Essential disease signs are almost identical to those in patients IV‐17 and IV‐18. Patient III‐12, aged 45 years, showed no signs of disease.15,24 The FDS scores of these 10 patients were 0 or 1. CMTNS scores could be determined in cases IV‐2, IV‐10 and IV‐14, and ranged between 4 and 6, corresponding to mild disease.
Electrophysiological findings
We will focus on electrophysiological studies in patient III‐24 and her father (II‐13) and sons (IV‐17 and IV‐18). Table 1 summarises the results of nerve conduction studies. A marked increase in DML and a slowing of MCV that ranged between 15 and 23 m/s (fig 4A) were observed. The slowing in SCV paralleled that in MCV. Except for CMAP of the median nerve in patient IV‐18 and that of the ulnar nerve in patient IV‐17, CMAP and SNAP were either attenuated or unobtainable; partial conduction block was not observed. Where applicable (patients III‐24, IV‐17 and IV‐18), TLI was normal.
Table 1 Nerve conduction findings at the last electrophysiological examination (in 1983 for patient II‐13 and in 2005 for the remaining patients).
| Patient | Motor | Sensory | ||||
|---|---|---|---|---|---|---|
| Nerve | DML (ms) | MCV (m/s) | CMAP (mV) | SCV (m/s) | SNAP (μV) | |
| II‐13 | Left brachial plexus | 15.1* | NA | 1* | ||
| Left radial | 8.7 | NA | 2 | |||
| Left median | A | A | A | A | A | |
| Left peroneal | A | A | A | |||
| III‐24 | Right median | 9.9 | 17.6 | 0.1 | A | A |
| Right radial | A | A | ||||
| Right peroneal | A | A | A | |||
| 10.1† | NA | 0.1† | ||||
| Left femoral | 11.6 | NA | 0.1 | |||
| Right femoral | 8.4 | NA | 0.1 | |||
| IV‐17 | Right median | 10.1 | 20.7 | 3.1 | A | A |
| Right ulnar | 4.6 | 19.2 | 6.3 | 27.8 | 0.9 | |
| Right peroneal | 9.1 | 16.9 | 1.1 | |||
| Right tibial | 12.2 | 15.8 | 2.3 | |||
| Right sural | A | A | ||||
| IV‐18 | Right median | 8.8 | 22.7 | 7.8 | A | A |
| Right ulnar | 7.8 | 20.3 | 4.8 | 19.9 | 0.3 | |
| Right peroneal | 12.5 | 17.5 | 0.3 | |||
| Right tibial | 10.2 | 20.4 | 0.1 | |||
| Right sural | A | A | ||||
A, absent; CMAP, compound muscle action potential; DML, distal motor latency; MCV, motor conduction velocity; NA, not applicable; SCV, sensory conduction velocity; SNAP, sensory nerve action potential.
*To biceps muscles.
†To tibialis anterior muscle.
Patient III‐24 underwent three previous electrophysiology examinations in 1977, 1990 and 1999. The recordings were destroyed when one of our hospital buildings collapsed in 1999. According to clinical record data, we can merely state that the peroneal nerve has been inexcitable at the ankle since 1977, and that the slowing of MCVs of the median nerve has remained stable.
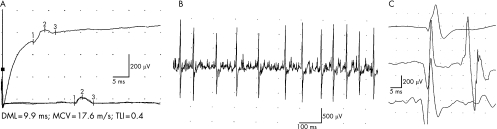
Figure 4 Electrophysiological study in patient III‐24. (A) Median nerve motor conduction study (upper trace, wrist stimulation; lower superimposed traces, elbow stimulation) showing prolonged distal motor latency (DML), low forearm motor conduction velocity (MCV), normal terminal latency index (TLI) and compound muscle action potential (CMAP) attenuation with preserved morphology. Changes in CMAP duration, amplitude or morphology on distal and proximal stimulation are absent. (B) Poor recruitment during full effort from the gluteus medius muscle. (C) Samples of long duration and high‐amplitude motor unit potentials from the gluteus medius muscle.
In patient III‐24, EMG from the gluteus medius, rectus femoris and right abductor pollicis brevis showed a similar pattern of chronic de‐reinnervation (fig 4B,C). The tibialis anterior muscle was severely denervated. No spontaneous potentials were present. In patient II‐13, EMG of the abductor digiti minimi and extensor digitorum brevis muscles showed complete denervation.
MRI findings
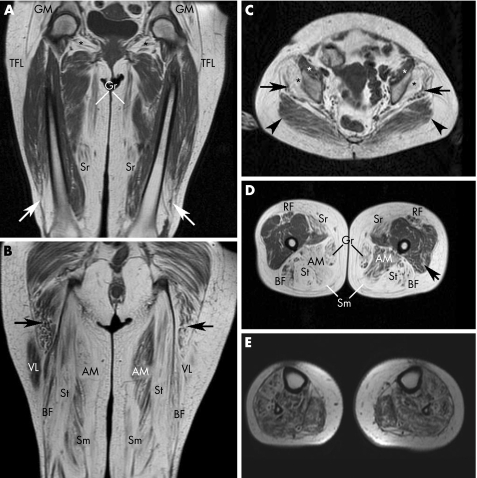
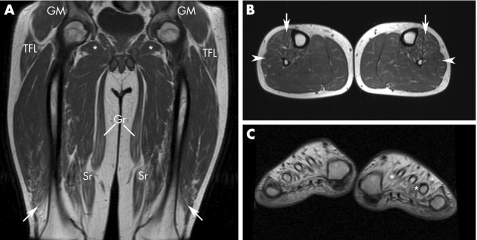
Patient III‐24, both her affected sons (IV‐17 and IV‐18) and patient II‐8, who is the oldest patient of the pedigree with no coexistent neurological comorbidity, underwent MRI. Figure 5 shows the MRI findings in patient III‐24. Marked fatty atrophy was seen in all four leg muscle compartments, the posteromedial thigh muscle compartments, gluteus medius and minimus, obturator externus and internus, tensor fasciae latae and, to a lesser degree, the gluteus maximus, vastus lateralis and medialis, sartorius, and rectus femoris. In the remaining three patients, there was subtle fatty infiltration of the anterolateral calf muscle compartments, with just minimal thigh involvement in patient IV‐17 (fig 6A,B); conversely, this patient showed severe fatty atrophy of the intrinsic foot muscles mainly associated with the extensor digitorum brevis, interossei, lumbricals and abductor hallucis (fig 6C). A slight oedema and gadolinium enhancement were observed in patients III‐24 and IV‐17, mainly affecting the tibialis anterior, tibialis posterior, peroneus and vastus lateralis muscles (data not shown).
Figure 5 T1‐weighted magnetic resonance images of the pelvis, thighs and legs of patient III‐24. (A) Anterior coronal thigh image through femoral heads, showing marked and symmetric hypersignal, indicative of fatty atrophy, of the gluteus medius and minimus (GM), tensor fasciae latae (TFL), obturator externus (*), gracilis (Gr) and sartorius (Sr) muscles. Distal fatty atrophy of both the vastus lateralis muscles (arrows) is evident. (B) Posterior coronal thigh image through ischiatic tuberosities showing severe and symmetric fatty atrophy of the posterior femoral muscles (BF, biceps femoris; Sm, semimembranosus; and St, semitendinosus). Fatty infiltration of the lower portion of the gluteus maximus muscles (arrows) and asymmetric involvement of the adductor magnus (AM) and vastus lateralis (VL) muscles are seen. (C) Pelvic axial image through the acetabular roof showing severe fatty atrophy of the gluteus medius (arrows) and gluteus minimus (black asterisks) muscles. The preservation at this level of the gluteus maximus (arrowheads) and iliopsoas (white asterisks) muscles can be seen. (D) Middle third axial thigh image showing extensive fatty atrophy of the posterior and medial muscle compartments (AM, adductor magnus; BF, biceps femoris; Gr, garcilis; Sm, semimembranosus; and St, semitendinosus) and sartorius (Sr) and rectus femoris (RF) muscles. There is slight fatty infiltration of the left vastus lateralis muscle (arrow); note also asymmetry of adductor magnus (AM) and rectus femoris (RF) muscle involvement. (E) Middle third axial calf image showing extensive fatty infiltration of all four muscle compartments.
Figure 6 T1‐weighted magnetic resonance images (MRIs) of the thighs, legs and feet of patient IV‐17. (A) Anterior coronal thigh image through femoral heads, showing subtle fatty infiltration of the gracilis (Gr), sartorius (Sr) and distal vastus lateralis (arrows) muscles. For comparative purposes with MRI findings in his mother, preservation of the gluteus medius and minimus (GM), and tensor fasciae latae (TFL) and obturator externus (*) muscles can be seen. (B) Middle‐third axial calf muscle image, showing subtle infiltration of the anterior (arrows) and, to a lesser degree, the lateral (arrowheads) muscle compartments. (C) Short‐axis image of the feet through the first metatarsal heads, showing massive fatty atrophy of the interosseus, lumbrical and abductor hallucis muscles, which are hardly recognisable (* indicate residual profiles of the first and third left interossei).
Nerve biopsy findings
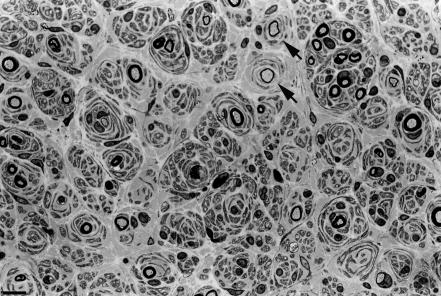
Sural‐nerve biopsy in patients II‐5, II‐11 and II‐13 showed similar findings, including severe depletion of large myelinated fibres, hypertrophic changes of onion bulb type and absence of inflammatory infiltrates, subperineurial oedema or active demyelination (fig 7).
Figure 7 Semithin transverse section of the sural nerve of patient II‐13, showing severe depletion of myelinated fibres and multiple onion bulbs, some of them surrounding remyelinated fibres (arrows). Bar indicates 6.25 μm.
Molecular findings
Molecular analysis of all 17 examined patients showed heterozygotic duplication of the chromosomal region 17p11.2–p12, whereas all 13 screened normal at‐risk people with normal clinico‐electrophysiological examination showed normal molecular findings.
Discussion
Our work describes a long‐term longitudinal study in a pedigree with CMT1A duplication, comprising 18 patients examined over three generations. At the end of the study, the ages of patients ranged between 15 and 84 years. After three decades of observation, we found that in 14 patients the clinical hallmark was a subtle phenotype, whereas four adult patients, aged between 53 and 84 years, showed a most severe phenotype. These four adult progressive patients represent 22% of the series, a fact that supports the notion that functional disability in CMT1A may increase with disease duration.5,6,10,11
Patient III‐24 is a 53‐year‐old woman who was serially evaluated from the age of 23 years. Initially, she was asymptomatic and her examination showed a mild phenotype. Incipient and subclinical atrophy of the peroneal musculature was noted at age 31 years, but, as occurs in half the patients with early‐onset disease,25 she denied pregnancy‐related exacerbation of CMT during both her gestations at ages 29 and 30 years. At age 34 years and throughout the next 4 years, she developed gradually progressive and symmetric leg amyotrophy and weakness, obliging her to use foot orthotics. Known causes of acquired polyneuropathy were reasonably discarded. Several features argue against the possibility of coexistent chronic inflammatory demyelinating polyneuropathy (CIDP):
a. No evidence of stepwise deterioration
b. Absence of positive sensory symptoms or neuropathic pain
c. Stable nerve conduction over time, slowing in the CMT1A range, with normal TLI
d. Preservation of CMAP morphology even in the presence of severe attenuated potentials
e. Absence of motor conduction block
f. Normal cerebrospinal fluid with no oligoclonal bands.26,27,28,29
Furthermore, sural‐nerve biopsy in her father, who had a similar clinical course, showed neither inflammatory cell infiltrates nor active demyelination suggestive of superimposed CIDP.29
Both patient III‐24 and her father (II‐13) developed late‐onset symptomatic proximal lower limb weakness. An EMG of the gluteus medius and quadriceps femoris in patient III‐24 showed a pattern of poor recruitment at full effort, with MUP of increased amplitude and duration, and absence of spontaneous activity, which is suggestive of a chronic neurogenic process.22,30 By means of motor unit number estimation in patients with CMT1A, it has been demonstrated that the proximal upper limb muscles, which appear strong clinically, had evidence of chronic denervation or reinnervation, although to a lesser extent than the weak distal hand muscles.31 We therefore infer that the pelvic girdle weakness observed in our two patients, who represent 11% of the series, is accounted for by the extension of secondary axonal degeneration to include the thigh and pelvic muscles. In a recent series of 51 patients with CMT1A, 40% of the patients had apparently non‐symptomatic moderate weakness of the thigh and hip muscles, although none of them showed severe paresis as described here.32
MRI has an important role in the detection and characterisation of pathological conditions of the skeletal muscle that causes changes in the muscle signal intensity.33,34,35 We have reported that clinical–MRI patterns of the lower limb amyotrophy in patients with CMT1A vary with the evolution of semeiology.20 Selective involvement of the intrinsic foot muscles is the characteristic pattern of patients with minimal disease; afterwards this pattern usually combines variable involvement of the leg muscles. In keeping with these observations, we found here that in all three patients with mild disease signs, MRI showed subtle fatty infiltration of the anterior and lateral leg muscle compartments, and severe fatty atrophy of the intrinsic foot muscles in the only patient whose feet were investigated. This proximal‐to‐distal gradient of muscle changes concurs with the proposal of a length‐dependent degeneration as the mechanism of muscle denervation in patients with CMT1A.18,36,37 Previous MRI studies of the lower limb musculature in patients with CMT did not include the proximal muscles.20,38 We therefore obtained MRI scans of the thigh in coronal and axial planes in all four patients. In one patient, there was subclinical distal fatty infiltration of the vastus lateralis muscles, and of the gracilis and sartorius muscles with no proximal‐to‐distal gradient of muscle changes as reported for gastrocnemius.20,38 A good clinical–MRI correlation occurred in patient III‐24:
a. The completely paralysed flexoextensor foot muscles showed massive fatty atrophy on MRI of the legs.
b. Severe paretic proximal muscles, including the knee flexors, thigh abductors and hip rotators, also showed extensive fatty atrophy on the pelvic and thigh images.
c. Mild paretic muscles, such as the knee extensors and thigh adductors, showed less fatty atrophy, proximal‐to‐distal gradient again being identified for the gluteus maximus and vastus lateralis muscles.
A small proportion of affected muscles showed subtle oedema and contrast enhancement, suggesting that subacute muscle denervation may coexist with chronic denervation in the disease process.20 MRI studies can help us to identify distinct patterns of muscle involvement in genetically distinct entities.39,40 Given the small number of scanned patients in this study, the specificity of the pattern of distal–proximal lower limb musculature involvement described here would require further investigation in patients with CMT1A at different stages of the disease.
We conclude that late in the clinical course, a small proportion of patients with CMT1A develop severe proximal weakness in the legs and that long‐term follow‐up is essential for its detection.
Acknowledgements
We are grateful to Mar Ruiz and Rosario Repoila for technical assistance, to Marta de la Fuente for secretarial help and to John Hawkins for stylistic revision of the manuscript.
Abbreviations
CIDP - chronic inflammatory demyelinating polyneuropathy
CMAP - compound muscle action potential
CMT1A - Charcot–Marie–Tooth disease type 1A
CMTNS - CMT Neuropathy Score
DML - distal motor latency
EMG - electromyogram
FDS - Functional Disability Scale
MCV - motor conduction velocity
MRC - Medical Research Council
MRI - magnetic resonance imaging
MUP - motor unit potential
SCV - sensory conduction velocity
SNAP - sensory nerve action potential
TLI - terminal latency index
TR/TE - repetition time/echo time
Footnotes
This study was supported by the Centro de Investigación de Enfermedades Neurólogicas (CIEN), Nodo CO3/CO6, (ISCIII, Madrid, Spain), Instituto de Formación e Investigación Marqués de Valdecilla (IFIMAV, Santander, Spain) and FIS grant number PI050879.
Competing interests: None.
Patient consent was obtained to publish the figures in this paper.
References
- 1.Raeymaekers P, Timmerman V, Nelis E.et al Duplication in chromosome 17p11.2 in Charcot‐Marie‐Tooth neuropathy type Ia (CMT Ia). Neuromuscul Disord 1991193–97. [DOI] [PubMed] [Google Scholar]
- 2.Lupski J R, de Oca‐Luna R M, Slaugenhaupt S.et al DNA duplication associated with Charcot‐Marie‐Tooth disease type 1A. Cell 199166219–232. [DOI] [PubMed] [Google Scholar]
- 3.Hallam P J, Harding A E, Berciano J.et al Duplication of part of chromosome 17 is commonly associated with hereditary motor and sensory neuropathy type I (Charcot‐Marie‐Tooth disease type I). Ann Neurol 199231570–572. [DOI] [PubMed] [Google Scholar]
- 4.Dyck P J, Lambert E H. Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. I. Neurologic, genetic, and electrophysiologic findings in hereditary polyneuropathies. Arch Neurol 1968391032–1038. [DOI] [PubMed] [Google Scholar]
- 5.Harding A E, Thomas P K. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain 1980103259–280. [DOI] [PubMed] [Google Scholar]
- 6.Berciano J, Combarros O, Calleja J.et al The application of nerve conduction and clinical studies to genetic counseling in hereditary motor and sensory neuropathy type I. Muscle Nerve 198912302–306. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson G A. Penetrance of the hereditary motor and sensory neuropathy Ia mutation: assessment by nerve conduction studies. Neurology 199141547–552. [DOI] [PubMed] [Google Scholar]
- 8.García A, Combarros O, Calleja J.et al Charcot‐Marie‐Tooth disease type 1A with 17p duplication in early infancy and childhood. A longitudinal clinical and electrophysiological study. Neurology 1998501061–1067. [DOI] [PubMed] [Google Scholar]
- 9.Hoogendijk J, de Visser M, Bolhuis P A.et al Hereditary motor and sensory neuropathy type I: clinical and neurographical features of the 17p duplication subtype. Muscle Nerve 19941785–90. [DOI] [PubMed] [Google Scholar]
- 10.Dyck P J, Karnes J L, Lambert E H. Longitudinal study of neuropathic deficits and nerve conduction abnormalities in hereditary motor and sensory neuropathy type 1. Neurology 1989391302–1308. [DOI] [PubMed] [Google Scholar]
- 11.Birouk N, Gouider R, Le Guern E.et al Charcot‐Marie‐Tooth disease type 1A with 17p11.2 duplication. Clinical and electrophysiological phenotype study and factors influencing disease severity in 119 cases. Brain 1997120813–823. [DOI] [PubMed] [Google Scholar]
- 12.Lupski J R, Garcia C A, Parry G J.et al Charcot‐Marie‐Tooth polyneuropathy syndrome: clinical, electrophysiologic, and genetic aspects. In: Appel S, ed. Current neurology. Chicago: Mosby Yearbook, 19911–25.
- 13.Thomas P K, Marques W, Davis M B.et al The phenotypic manifestations of chromosome 17p11.2 duplication. Brain 1997120465–478. [DOI] [PubMed] [Google Scholar]
- 14.Hoogendijk J E, De Visser M. Hereditary motor and sensory neuropathy types I and II (Charcot‐Marie‐Tooth disease). In: de Jong JMBV, ed. Handbook of clinical neurology. Vol 16 (60). Amsterdam: Elsevier Science, 1991185–211.
- 15.Combarros O, Calleja J, Figols J.et al Dominantly inherited motor and sensory neuropathy type I. Genetic, clinical, electrophysiological and pathological features in four families. J Neurol Sci 198361181–191. [DOI] [PubMed] [Google Scholar]
- 16.Middleton‐Price H R, Harding A E, Berciano J.et al Absence of linkage of hereditary motor and sensory neuropathy type I to chromosome 1 markers. Genomics 19894192–197. [DOI] [PubMed] [Google Scholar]
- 17.Middleton‐Price H R, Harding A E, Monteiro C.et al Linkage of hereditary motor and sensory neuropathy type I to the pericentromeric region of chromosome 17. Am J Hum Genet 19904692–94. [PMC free article] [PubMed] [Google Scholar]
- 18.Berciano J, García A, Calleja J.et al Clinico‐electrophysiological correlation of extensor digitorum brevis muscle atrophy in children with Charcot‐Marie‐Tooth disease 1A duplication. Neuromuscul Disord 200010419–424. [DOI] [PubMed] [Google Scholar]
- 19.Berciano J, García A, Combarros O. Initial semeiology in children with Charcot‐Marie‐Tooth disease. Muscle Nerve 20032734–39. [DOI] [PubMed] [Google Scholar]
- 20.Gallardo E, García A, Combarros O.et al Charcot‐Marie‐Tooth disease type 1A duplication: spectrum of clinical and magnetic resonance imaging features in leg and foot muscles. Brain 2006129426–437. [DOI] [PubMed] [Google Scholar]
- 21.Shy M E, Blake J, Krajewski K.et al Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology 2005641209–1214. [DOI] [PubMed] [Google Scholar]
- 22.Buchthal F.An introduction to electromyography. Copenhagen: Scandinavian University Books, 1957
- 23.Infante J, García A, Combarros O.et al Diagnostic strategy for familial and sporadic cases of neuropathy associated with 17p11.2 deletion. Muscle Nerve 2001241149–1155. [DOI] [PubMed] [Google Scholar]
- 24.Berciano J, Calleja J, Combarros O. Charcot‐Marie‐Tooth disease. Neurology 1994441985–1986. [DOI] [PubMed] [Google Scholar]
- 25.Rudnik‐Schöneborn S, Röhrig D, Nicholson G.et al Pregnancy and delivery in Charcot‐Marie‐Tooth disease type 1. Neurology 1993432011–2016. [DOI] [PubMed] [Google Scholar]
- 26.Kaku D A, Parry G J, Malamut R.et al Nerve conduction studies in Charcot‐Marie‐Tooth polyneuropathy associated with a segmental duplication of chromosome 17. Neurology 1993431806–1808. [DOI] [PubMed] [Google Scholar]
- 27.Lewis R A, Sumner A J. The electrodiagnostic distinction between chronic familial and acquired demyelinative neuropathies. Neurology 198232592–596. [DOI] [PubMed] [Google Scholar]
- 28.Attarian S, Azulay J P, Boucraut J.et al Terminal latency index and modified F ratio in distinction of chronic demyelinating neuropathies. Clin Neurophysiol 2001112457–463. [DOI] [PubMed] [Google Scholar]
- 29.Ginsberg L, Malik O, Kenton A R.et al Coexistent hereditary and inflammatory neuropathy. Brain 2004127193–202. [DOI] [PubMed] [Google Scholar]
- 30.Daube J R. Needle examination in clinical electromyography. Muscle Nerve 199114685–700. [DOI] [PubMed] [Google Scholar]
- 31.Lewis R A, Li J, Fuerst D R.et al Motor unit number estimate of distal and proximal muscles in Charcot‐Marie‐Tooth disease. Muscle Nerve 200328161–167. [DOI] [PubMed] [Google Scholar]
- 32.Verhamme C, van Schaik I N, Koelman J H T M.et al Clinical disease severity and axonal dysfunction in hereditary motor and sensory neuropathy Ia. J Neurol 20042511491–1497. [DOI] [PubMed] [Google Scholar]
- 33.Fleckenstein J L, Watumull D, Conner K E.et al Denervated human skeletal muscle: MR imaging evaluation. Radiology 1993187213–218. [DOI] [PubMed] [Google Scholar]
- 34.May D A, Disler D G, Jones E A.et al Abnormal signal intensity in skeletal muscle at MR imaging: patterns, pearls, and pitfalls. RadoGraphics 200020S295–S313. [DOI] [PubMed] [Google Scholar]
- 35.Farber J M, Buckwalter K A. MR imaging in nonneoplastic muscle disorders of the lower extremity. Radiol Clin N Am 2002401013–1031. [DOI] [PubMed] [Google Scholar]
- 36.Gabreëls‐Festen A A, Joosten E M, Gabreëls F J.et al Early morphological features in dominantly inherited demyelinating motor and sensory neuropathy (HMSN) type 1. J Neurol Sci 199210745–54. [DOI] [PubMed] [Google Scholar]
- 37.Krajewski K M, Lewis R A, Fuerst D R.et al Neuronal dysfunction and axonal degeneration in Charcot‐Marie‐Tooth disease type 1A. Brain 20001231516–1527. [DOI] [PubMed] [Google Scholar]
- 38.Stilwell G, Kilcoyne R F, Sherman J L. Patterns of muscle atrophy in the lower limbs in patients with Charcot‐Marie‐Tooth disease as measured by magnetic resonance imaging. J Foot Ankle Surg 199534583–586. [DOI] [PubMed] [Google Scholar]
- 39.Mercuri E, Jungbluth H, Muntoni F. Muscle imaging in clinical practice: diagnostic value of muscle magnetic resonance imaging in inherited neuromuscular disorders. Curr Opin Neurol 200518526–537. [DOI] [PubMed] [Google Scholar]
- 40.Chan W P, Liu G C. MR imaging of primary skeletal muscle diseases in children. Am J Roentgenol 2002179989–997. [DOI] [PubMed] [Google Scholar]