Abstract
Background
Granulysin, a recently defined cytolytic molecule, is expressed in cytotoxic T cells and natural killer cells in a similar way to perforin, which is reported to have a major role in the pathogenesis of polymyositis and inclusion‐body myositis (IBM).
Objective
To clarify the role of granulysin in polymyositis and IBM.
Methods
The expression of granulysin and perforin was examined by double staining with CD8, CD4 and CD56 in endomysial infiltrating cells and autoinvasive cells in muscle biopsy specimens of 17 patients with polymyositis (6 steroid resistant and 11 steroid responsive) and of 7 patients with IBM.
Results
Similar to perforin, granulysin was expressed in CD8, CD4 or CD56 cells in patients with polymyositis and IBM. The ratio of cells double positive for granulysin and CD8 to all CD8 cells at endomysial sites was notably higher in steroid‐resistant polymyositis than in steroid‐responsive polymyositis and IBM.
Conclusion
Granulysin expression in CD8 cells seems to be correlated with steroid resistance in polymyositis.
Cytotoxic T cells and natural killer cells are the primary effector cells in cell‐mediated immunity. A major pathway for them to destroy their targets is the release of cytolytic granules stored in their cytoplasm by exocytosis.1,2 Perforin, a pore‐forming protein,1 and granzymes are released towards the target cells in response to target recognition signals.2 Granulysin, a recently defined cytolytic molecule, is expressed in cytotoxic T cells and natural killer cells.3 Granulysin and perforin colocalise in cytolytic granules and generally work together.4,5
Autoimmune mechanisms have an important role in the pathogenesis of inflammatory myopathies. In polymyositis and inclusion‐body myositis (IBM), CD8 T cells and natural killer cells, together with macrophages, surround non‐necrotic muscle fibres expressing major histocompatibility complex (MHC) class I antigen.6 These infiltrating cells contain perforin and granzymes.7 Perforin, which is reported to have a crucial role in muscle fibre damage in polymyositis and IBM, makes a pore on the sarcolemmal surface and granzymes are released into the sarcoplasm.6,7,8 Perforin expression between polymyositis and IBM, however, did not differ markedly in this study, although their responses to immunotherapy are quite different.
This study aims to clarify differences in the expression of granulysin in infiltrating cells in polymyositis and IBM, and also to examine its role. We also consider the correlation between granulysin expression and steroid resistance, especially in polymyositis.
Materials and methods
Muscle specimens
We studied muscle specimens taken just before starting steroid treatment from 17 patients with polymyositis (6 were steroid resistant and 11 steroid responsive) and 7 with IBM. We defined steroid‐resistant polymyositis as a state needing the addition of immunosuppressants or the continuation of prednisolone with a dosage of >25 mg/day, because of insufficient improvement in muscle strength or in serum concentration of creatine kinase (>300 IU/l in men and >200 IU/l in women) 6 months after beginning the initial treatment with 60 mg/day prednisolone for 4–8 weeks and then a tapered dosage. Steroid‐responsive polymyositis was defined as a state showing good recovery, both in weakness and serum level of creatine kinase, with prednisolone of <20 mg/day or with no drugs 6 months after beginning the initial treatment.9 We also gave the initial prednisolone treatment to all patients with IBM; however, all were resistant to steroid.
The diagnosis of polymyositis was in accordance with the criteria given by Bohan and Peter.10 Two additional criteria were included: endomysial mononuclear cell infiltration surrounding and invading non‐necrotic fibres,11 and MHC class I antigen expression. The diagnosis of IBM relied on (1) surrounding and invasion of non‐necrotic fibres by mononuclear cells, (2) MHC class I antigen expression, (3) rimmed vacuoles, and (4) SMI‐31 positivity or tubulofilamentous inclusions of 15–18 nm.12,13
During a follow‐up period of at least 2 years, no patient with polymyositis showed prominent distal, finger or wrist flexor, or weakness from disproportionate quadriceps.
Immunohistochemistry
Muscle specimens were frozen in isopentane cooled in liquid nitrogen. Sections, 7‐μm thick, were fixed in 4% paraformaldehyde at 4°C for 15 min, then immersed in a blocking solution at room temperature for 30 min and incubated with primary antibodies at 4°C overnight. As a negative control, we used normal mouse immunoglobulin G (IgG) in place of primary antibodies. The next day, sections were incubated with a fluorescein isothiocyanate (FITC)‐labelled secondary antibody at 37°C for 1 h. We examined the sections by confocal laser scanning microscopy (Olympus, Tokyo, Japan). We also double stained granulysin with CD4, CD8 and CD56 with a Zenon one mouse IgG1 labelling kit (Molecular Probes, Invitrogen, Tokyo, Japan) with FITC‐labelled and Texas Red‐labelled secondary antibodies. We counted the positive cells for lymphocyte subsets, perforin and granulysin in endomysial sites, including autoinvasive cells containing at least 1000 muscle fibres. Significance between data was estimated by Mann–Whitney U test or Kruskal–Wallis rank test. We considered p<0.05 to be significant. With the Bonferroni‐adjusted Mann–Whitney U test, p<0.0167 (0.05/3) was considered to be significant.
Antibodies
The following monoclonal primary antibodies were used: anti‐CD4 (1:30; Dako, Tokyo, Japan), anti‐CD8 (1:30; Dako), anti‐CD56 (1:30; Dako), anti‐perforin (1:50; Novocastra, Tokyo, Japan) and anti‐granulysin RC8 (20 μg/ml; as published previously14).
Results
The mean (SD) number of CD4 cells per 1000 muscle fibres at endomysial sites was 275 (140) in all cases of polymyositis (both steroid resistant and steroid responsive) and 250 (120) in cases of IBM. The mean (SD) numbers of CD8 and CD56 cells were 355 (150) and 25 (13), respectively, in all cases of polymyositis and 330 (200) and 31 (16), respectively, in cases of IBM. We found no significant differences in any of these numbers between all cases of polymyositis and IBM.
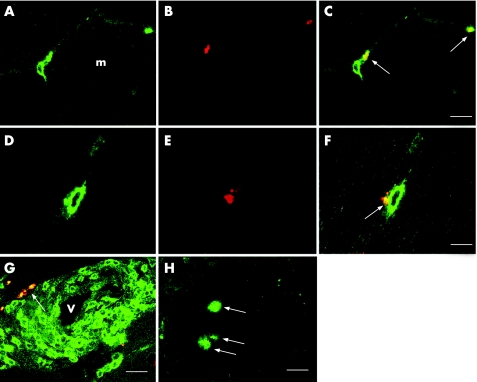
Granulysin was expressed in the cytoplasm of infiltrating cells in cases of polymyositis and IBM (fig 1); most infiltrating cells with granulysin were CD8 or CD4, but small numbers of CD56 were also seen. We detected many more infiltrating cells with granulysin at endomysial than at perivascular sites. These results were similar to those reported for perforin.7,8
Figure 1 Granulysin expression in infiltrating cells in biopsy specimens of patients with polymyositis. (A–C) Granulysin is located in the cytoplasm of CD8 cells infiltrating around muscle fibres (denoted by m; arrows). (A) CD8 (fluorescein isothiocyanate (FITC)); (B) RC8 (Texas Red); (C) merged. Bar = 10 μm. (D–F) In this longitudinal section, granulysin (arrow) seems to have been released from a CD8 cell into the non‐necrotic muscle fibre located at the left. (D) CD8 (FITC); (E) RC8 (Texas Red); (F) merged. Bar = 5 μm. (G) Granulysin is occasionally expressed in CD4 cells surrounding blood vessels (denoted by v; arrow); CD4 (FITC)+RC8 (Texas Red). Bar = 15 μm. (H) Granulysin is also expressed in autoinvasive cells (arrows); RC8 (FITC). Bar = 10 μm.
In all patients with polymyositis, the ratio of the number of double‐positive cells for granulysin and CD8 to that for all CD8 cells (G:CD8) was 0.37 (SD 0.16). The ratio of double‐positive cells for granulysin and CD4 to all CD4 cells (G:CD4) and that for granulysin and CD56 to all CD56 cells (G:CD56) was 0.30 (SD 0.13) and 0.39 (0.13), respectively; Kruskal–Wallis analysis showed no significant difference (p = 0.1628). Similarly, in patients with IBM, G:CD4, G:CD8 and G:CD56 ratios were 0.30 (SD 0.07), 0.22 (SD 0.11) and 0.35 (SD 0.14), respectively.
Next we compared these ratios between all patients with polymyositis and IBM by Mann–Whitney U test. Although these ratios showed no significant differences (p = 0.8039 in G:CD4, p = 0.0549 in G:CD8 and p = 0.4741 in G:CD56), G:CD8 tended to be higher in all patients with polymyositis than in those with IBM.
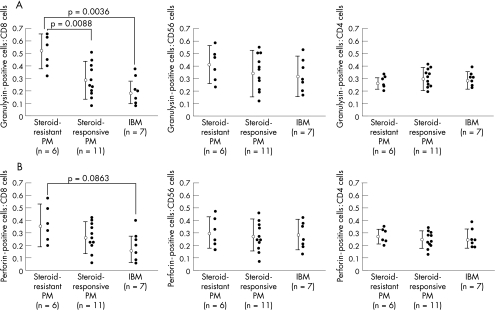
We then compared G:CD8 among the three groups—namely, steroid‐resistant polymyositis, steroid‐responsive polymyositis and IBM (0.51 (SD 0.14), 0.29 (SD 0.13) and 0.19 (SD 0.10), respectively; fig 2A). Kruskal–Wallis analysis indicated significance (p = 0.0017). In the comparison between each of two groups, G:CD8 showed a significant difference between steroid‐resistant polymyositis and steroid‐responsive polymyositis (p = 0.0088) and between steroid‐resistant polymyositis and IBM (p = 0.0036; Bonferroni‐adjusted Mann–Whitney U test).
Figure 2 Ratios of double‐positive cells for (A) granulysin and (B) perforin, and CD8, CD56 or CD4 to all CD8, CD56 or CD4 cells, respectively, in endomysial infiltrating and autoinvasive cells in steroid‐resistant polymyositis (PM), steroid‐responsive polymyositis and inclusion‐body myositis (IBM).
In contrast, although the ratio of the number of CD8 cells with perforin to that of all CD8 cells tended to be higher in steroid‐resistant polymyositis than in steroid‐responsive polymyositis and IBM (0.37 (SD 0.16), 0.27 (SD 0.12) and 0.17 (SD 0.10), respectively), Kruskal–Wallis analysis showed no significant difference (p = 0.1776; fig 2B).
Discussion
In this study, we showed that granulysin expression in CD8 cells, but not in CD4 or CD56 cells, tended to be positively correlated with steroid resistance in polymyositis. In polymyositis, cytotoxic CD8 cells have a major role in pathogenesis.15 Cytotoxic CD8 cells are clonally expanded, and recognise antigenic peptides bound to HLA class I molecules at the sarcolemma.15,16 Cytotoxic granules such as perforin and granzymes are reported to be released from cytotoxic T cells into the sarcoplasm to initiate muscle fibres into necrosis.7,8 Therefore, it seems reasonable to speculate that the upregulation of granulysin expression in CD8 cells, but not in CD4 and CD56 cells, leads to steroid resistance in polymyositis.
In this study, perforin expression in CD8 cells tended to be higher in steroid‐resistant polymyositis than in steroid‐responsive polymyositis, although there was no statistical significance. It has recently been reported that perforin expression in CD8 cytotoxic cells in polymyositis is preserved even in phases where there is no relapse, and that CD8 cytotoxic cells are reactivated quickly by interleukin‐2.17 Therefore, the higher expression of perforin in steroid‐resistant polymyositis may be a reaction to particular stimulations—that is, both granulysin and perforin are upregulated when CD8 cytotoxic cells are in an activated state. Granulysin is expressed in cytotoxic T cells and natural killer cells in humans, but not in rodents.2 Accordingly, it is speculated that granulysin may provide a backup membrane‐disrupting molecule to perforin in humans.2 Moreover, granulysin and perforin are considered to work cooperatively; however, in some cases they function independently. Granulysin, but not perforin, contributes to host defence in leprosy.18 Conversely, perforin, rather than granulysin, is used by natural killer cells in cryptococcal infection.19 Taken together, we believe that probably the extra granulysin upregulation under certain conditions—that is, apart from the basic upregulation of granulysin and perforin in CD8 cytotoxic cells in polymyositis—is related to steroid resistance.
Despite there being lower granulysin expression in patients with IBM than in those with steroid‐responsive polymyositis, patients with IBM were steroid resistant. In IBM muscle rimmed vacuole formation and the accumulation of many kinds of proteins in and around it are distinctive, even though clonal expansion of cytotoxic CD8 cells and HLA class I antigen at the sarcolemma takes place,20 similar to that in polymyositis. Therefore, steroid resistance in IBM seems to be based on factors other than granulysin, such as endoplasmic reticulum stress.21
In human renal transplantation, dense granulysin expression in tissues along with raised peripheral blood lymphocyte granulysin are markers for acute rejection and steroid resistance.22 Although there is the limitation that only a small number of muscles were examined in the present study, we postulate that granulysin has an important role in steroid resistance in polymyositis and that examination of serum granulysin expression may help understand the steroid response and prognosis in patients with polymyositis.
Abbreviations
FITC - fluorescein isothiocyanate
IBM - inclusion‐body myositis
Footnotes
Competing interests: None declared.
References
- 1.Kagi D, Ledermann B, Burki K.et al Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin‐deficient mice. Nature 199436931–37. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman J. The ABCs of granule‐mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol 20033361–370. [DOI] [PubMed] [Google Scholar]
- 3.Pena S V, Krensky A M. Granulysin, a new human cytolytic granule‐associated protein with possible involvement in cell‐mediated cytotoxicity. Semin Immunol 19979117–125. [DOI] [PubMed] [Google Scholar]
- 4.Dieli F, Troye‐Blomberg M, Ivanyi J.et al Granulysin‐dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis 20011841082–1085. [DOI] [PubMed] [Google Scholar]
- 5.Gamen S, Hanson D A, Kaspar A.et al Granulysin‐induced apoptosis. I. Involvement of at least two distinct pathways. J Immunol 1998611758–1764. [PubMed] [Google Scholar]
- 6.Dalakas M C, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003362971–982. [DOI] [PubMed] [Google Scholar]
- 7.Orimo S, Koga R, Goto K.et al Immunohistochemical analysis of perforin and granzyme A in inflammatory myopathies. Neuromuscul Disord 19944219–226. [DOI] [PubMed] [Google Scholar]
- 8.Goebels N, Michaelis D, Engelhardt M.et al Differential expression of perforin in muscle‐infiltrating T cells in polymyositis and dermatomyositis. J Clin Invest 1997972905–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas M W., III Ch16 Disease of nerve and muscle: polymyositis. In: Martin AS, ed. Manual of neurologic therapeutics. 6th edn. Philadelphia: Lippincott Williams & Wilkins, 1999411
- 10.Bohan A, Peter J B. Polymyositis and dermatomyositis. N Engl J Med 1975292344–347. [DOI] [PubMed] [Google Scholar]
- 11.van der Meulen M F G, Bronner I M, Hoogendijk J E.et al Polymyositis. An overdiagnosed entity. Neurology 200361316–321. [DOI] [PubMed] [Google Scholar]
- 12.Askanas V, Engel W K. Sporadic inclusion‐body myositis and hereditary inclusion‐body myopathies: current concepts of diagnosis and pathogenesis. Curr Opin Rheumatol 199810530–534. [DOI] [PubMed] [Google Scholar]
- 13.van der Meulen M F G, Hoogendijk J E, Moons K G M.et al Rimmed vacuoles and the added value of SMI‐31 staining in diagnosing sporadic inclusion body myositis. Neuromuscul Disord 200111447–451. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa K, Takamori Y, Suzuki K.et al Granulysin in human serum as a marker of cell‐mediated immunity. Eur J Immunol 2003331925–1933. [DOI] [PubMed] [Google Scholar]
- 15.Arahata K, Engel A G. Monoclonal antibody analysis of mononuclear cells in myopathies. V: identification and quantitation of T8+ cytotoxic and T8+ suppressor cells, Ann Neurol 198823493–499. [DOI] [PubMed] [Google Scholar]
- 16.Mantegazza R, Andreetta F, Bernasconi P.et al Analysis of T cell receptor repertoire of muscle‐infiltrating T lymphocytes in polymyositis. Restricted V alpha/beta rearrangements may indicate antigen‐driven selection. J Clin Invest 1993912880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benveniste O, Herson S, Salomon B.et al Long‐term persistence of clonally expanded T cells in patients with polymyositis. Ann Neurol 200456867–872. [DOI] [PubMed] [Google Scholar]
- 18.Ochoa M T, Stenger S, Sieling P A.et al T‐cell release of granulysin contributes to host defense in leprosy. Nat Med 20017174–179. [DOI] [PubMed] [Google Scholar]
- 19.Ma L L, Wang C L C, Neely G G.et al NK cells use perforin rather than granulysin for anticryptococcal activity. J Immunol 20041733357–3365. [DOI] [PubMed] [Google Scholar]
- 20.Figarella‐Branger D, Pellissier J F, Bianco N.et al Inflammatory and non‐inflammatory inclusion body myositis. Characterization of the mononuclear cells and expression of the immunoreactive class I major histocompatibility complex product. Acta Neuropathol (Berl) 199079528–536. [DOI] [PubMed] [Google Scholar]
- 21.Vattemi G, Engel W K, McFerrin J.et al Endoplasmic reticulum stress and unfolded response in inclusion body myositis muscle. Am J Pathol 20041641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarwal M M, Jani A, Chang S.et al Granulysin expression is a marker for acute rejection and steroid resistance in human renal transplantation. Hum Immunol 20016221–31. [DOI] [PubMed] [Google Scholar]