Abstract
Background
Attentional dysfunction is believed to be a prominent and distinguishing neuropsychological feature of dementia with Lewy bodies (DLB); yet, the specific nature of the attentional deficit and factors that can potentially influence attentional processing in DLB have not been fully defined.
Aims
To clarify the nature of the attentional deficit in early‐stage DLB relative to patients with early‐stage dementia of the Alzheimer's type (DAT) and elderly controls, and examine the effect of task complexity and type of cognitive load on attentional processing in DLB.
Methods
Attentional impairment and fluctuating attention were investigated in three groups of subjects—patients with clinical features of early probable DLB (n = 20), a group with early probable DAT (n = 19) and healthy elderly controls (n = 20)—using an experimental computerised reaction time paradigm.
Results
Patients with DLB showed greater attentional impairment and fluctuations in attention relative to patients with DAT and elderly controls. The attentional deficit was generalised in nature but increased in magnitude as greater demands were placed on attentional selectivity. Attentional deficits in DLB were most pronounced under task conditions that required more active recruitment of executive control and visuospatial cognitive processes.
Conclusions
Attentional deficits in DLB are widespread and encompass all aspects of attentional function. Deficits in higher cortical function influence the degree of attentional impairment and fluctuating attention, suggesting that attentional processing in DLB is mediated by interacting cortical and subcortical mechanisms. These findings serve to clarify the nature of the attentional deficit in DLB and have potentially important ramifications for our understanding of the neurocognitive underpinnings of fluctuations.
Dementia with Lewy bodies (DLB) is a neurodegenerative condition characterised by progressive, disabling cognitive impairment and one or more additional core clinical features.1 These include recurrent well‐formed visual hallucinations, fluctuations in cognition and spontaneous motor features of parkinsonism. Clinicopathological studies have indicated that many cases of DLB are often misdiagnosed as probable or possible dementia of the Alzheimer's type (DAT) during life,2,3 bringing the reliability and validity of the consensus diagnostic criteria into question. Studies that have examined the clinical utility of the consensus criteria have typically shown a pattern of high specificity but low to medium diagnostic sensitivity.4,5,6,7
A central criterion required for a diagnosis of DLB is cognitive decline. The specific pattern of cognitive change in DLB, and the features which distinguish it from the cognitive impairment of DAT have not been fully defined. Studies aimed at refining this criterion may, therefore, prove beneficial in improving diagnostic sensitivity. In a recent meta‐analytic review,8 only 21 controlled‐comparison studies of the cognitive performance of patients with DLB were identified, highlighting the paucity of research examining the neuropsychological profile associated with DLB.
Some studies suggest that impairments of attention are prominent in DLB, differentiating the condition from DAT,8,9,10 but others have not replicated this finding.11,12,13 A problem that characterises this literature is a reliance on simple tests of attentional capacity (eg, digit span). These measures require a low degree of mental effort, and depend on “the commitment of just one or a few diffuse attentional resources” (van Zomeren and Brouwer,14 p 39) and might not be appropriate for differentiating between dementia syndromes.
Attention is considered to be a neurocognitively complex and hierarchically organised process, which can be subdivided into several distinct functions, such as selective, focused and sustained attention.15,16 In the literature on DLB, only a handful of studies have used theoretically derived measures capable of distinguishing between the various components of attention.9,17,18,19 Sahgal et al,18 using a computerised test of visual attention, found that patients with DLB exhibit a “focal” or selective attentional impairment. Ayre et al17 suggested that patients with DLB have a prominent sustained attention deficit. By contrast, two recent studies9,10 have raised the possibility of a more global attentional breakdown. In addition to these uncertainties about the nature of the attentional impairment, differentiating cases of DLB from DAT using more sophisticated measures of attention has not been consistently achieved.18,19
Clinical and neuropsychological observations also suggest that patients with DLB are highly variable in their attentional performance.1,10,20,21 Greater intraindividual variability has been shown in patients with DLB relative to those with DAT.22,23 This finding, and particularly the observation of marked second‐to‐second variation in attentional performance, has sparked intense clinical interest and has led some researchers to suggest that patients with DLB can experience a continuously variable pattern of attentional performance.22 This is at odds with previous reports1,24 that fluctuations occur in the form of discreet individual episodes. It has also been proposed that this fluctuating attentional profile may represent a quantifiable measure of the gross fluctuations in cognition and global performance that have long been regarded as a cardinal clinical feature of DLB, but paradoxically have been so problematic to identify in a reliable manner.22,23
Research has shown that attentional function can vary for many reasons (for comprehensive reviews, see Ballard25 and Stuss et al26). In the literature on DLB, several of these factors have been investigated, including, severity of dementia, severity of Parkinson's disease, mood status and processing speed (ie, mean length of reaction time). After controlling for these variables, attentional variability remains markedly greater in patients with DLB.10,20,23 One potentially important variable that has not been dealt with is the effect of task demands. In other neurological conditions, task demands have been shown to influence the presence and degree of intraindividual variability.26,27,28 The degree of intraindividual variability observed seems to depend on which cognitive processes are tapped by the task used to assess variability,29 suggesting that higher cognitive functions may also contribute to the regulation of attention. Relatively greater impairments of executive and visuospatial function are now well documented in DLB, in addition to attentional deficits.8,9,30,31 To date, there has been no attempt to systematically investigate the potential influence of these higher cognitive deficits on the nature of the attentional impairment and degree of fluctuating attention in DLB.
Our aim was twofold: firstly, we sought to clarify the nature of the attentional impairment in early‐stage DLB relative to patients with early‐stage DAT and elderly controls. Secondly, we examined the effect of varying task demands, specifically task complexity and type of cognitive load, on attentional processing in DLB and DAT. We hypothesised that patients with DLB would show a generalised breakdown of attentional function, but that specific cognitive task demands would influence the degree of impairment and intraindividual variability observed. It was anticipated that patients with DLB would experience more pronounced deficits on attentional tasks that were framed within an executive or spatial context than would patients with DAT and elderly controls.
Methods
Participants
The sample comprised three groups of subjects—patients with clinical features of early probable DLB (n = 20), a group with early probable DAT (n = 19) and a group of healthy controls (n = 20). The patients with dementia were matched as groups on a range of demographic and dementia severity variables to ensure comparability. Severity of illness was determined by the Clinical Dementia Rating (CDR) Scale32 and Mini‐Mental Status Examination (MMSE).33 Controls were matched to the dementia groups on the basis of age, education and premorbid intellectual function, estimated by the National Adult Reading Test (NART).34
Clinical diagnoses were made according to the NINCDS–ADRDA criteria for DAT35 and the consensus criteria for DLB.1 Well‐recognised clinical rating scales—namely, the Unified Parkinson's Disease Rating Scale (UPDRS‐motor examination),36 Brief Psychiatric Rating Scale,37 and Hospital Anxiety and Depression Scale,38 were used to document the presence or absence of key psychiatric and neurological features, and patient selection was restricted to subjects in a mild‐to‐moderate stage of illness.
Patients were recruited over a 2½‐year period, principally from neurology, psychiatry, and movement disorder outpatient clinics and aged care inpatient units at Austin Health, a large metropolitan tertiary hospital in Melbourne, Australia. Regional memory clinics and consultant neurologists and psychiatrists in private practice were other key referral sources. This ensured a broad referral base and aimed to minimise the influence of ascertainment bias on the nature of presenting clinical symptoms.
All patients were examined and the diagnosis confirmed in the Brain Disorders Program neurobehaviour clinic at the hospital. Routine clinical investigations were conducted to exclude reversible causes of dementia. Patients were also excluded if formal neurological or psychiatric examination showed evidence of any other brain disorder, physical or mental illness sufficient to contribute considerably to the clinical picture, or if they showed signs of significant cerebrovascular disease evident as focal neurological signs or on brain imaging.
Patient selection was strictly consecutive and included all patients referred to the study who met the clinical criteria, were English speaking and agreed to participate. Patients remained on any drugs that had been prescribed by their treating doctor due to ethical considerations. The study was approved by the Austin Health Human Research Ethics Committee and informed consent was obtained according to hospital ethics committee guidelines.
Tasks and procedures
The experimental paradigm was modelled after a Visual Focused Attention Test developed by Eriksen and Eriksen39 and adapted by Sharma et al.40 A computerised reaction time paradigm was selected to measure attentional function, as this allowed the investigators to manipulate the specific demands of the attentional task, while maintaining equivalence across all other task parameters.
Participants were seated in front of a 35×35 cm2 colour monitor. They were trained to focus on a central fixation point and to press the appropriate response key with their preferred hand as soon as the target stimulus appeared. The target stimulus consisted of a 5×2.5 cm coloured rectangle, presented centrally, surrounded by 1.25×1.25 cm2 distractors. The distractors appeared around the central rectangle within ½ degree of visual angle at a viewing distance of approximately 1 m from the participant's head position.
There were eight different levels to the task, which varied in terms of complexity and type of cognitive load (box 1). Manipulations to task complexity were made to the first four levels by gradually increasing the level of selective attention required, while the final four task levels manipulated the type of cognitive processing required—namely, the executive control and spatial processing demands. During the simple reaction time task, targets appeared on screen at random intervals, lasting up to 2 s. The interstimulus interval was fixed at 2 s for all subsequent levels as attentional selectivity and cognitive load were manipulated. Target stimuli remained on screen for a fixed duration of 1.5 s or until a response was recorded.
Tasks were presented in a fixed order and the total task duration lasted approximately 35 min. Environmental and situational variables were controlled where possible. Feedback was provided when participants sought clarification of task instructions to avoid the confounding effect of deficits in recent memory function.
Measures
Reaction time measures of speed and intraindividual variability were calculated to measure the degree of attentional impairment and fluctuating attention, respectively. For each task, these measures included mean response time (in ms) and individual variability in length of reaction time (mean individual variance)41 for correct target trials, averaged across participants within each group.
Box 1: A description of the eight task levels that comprised the reaction time paradigm
Simple reaction time (36 trials): Each time the blue rectangle appeared in the centre of the screen, the subject was required to press the response key as quickly as possible.
Choice reaction time (108 trials): A randomised series of blue, red or yellow rectangles appeared on the screen, one at a time, and the subject was required to press the response key as quickly as possible only when the blue rectangle appeared.
Focused attention (108 trials): The blue, red or yellow rectangles appeared on the screen, as in the previous task, but were now surrounded by a varying number of green distractor squares (0, 2 or 5 distractors). Subjects were instructed to ignore the green squares and to respond just as they did on the previous task—that is, only when a blue rectangle appeared (regardless of the number of distractors).
Divided attention (108 trials): The previously irrelevant distractors became relevant, thus requiring the subject to attend to two sources of information simultaneously. Subjects were instructed to respond as quickly as possible only when a blue rectangle appeared surrounded by two green squares.
Executive control 1—Response organisation (60 trials): A randomised series of red or green rectangles appeared in the centre of the screen. Subjects were required to press the “up” arrow key if the rectangle was red or the “down” arrow key if the rectangle was green, necessitating a dual response and a degree of response organisation.
Executive control 2—Set shift and response inhibition (60 trials): Having mastered the previous task, the rule was reversed. Subjects were now required to press the “up” arrow if the rectangle was green and the “down” arrow if it was red, thus requiring a set shift and demanding an inhibitory response to a previously entrenched rule.
Spatial 1—Easy judgement (60 trials): Subjects were required to make a spatial judgement about the position of a single green distractor square in relation to a centrally positioned blue rectangle. They were instructed to respond only when the green square appeared above the blue rectangle.
Spatial 2—Difficult judgement (60 trials): In this second stage, the blue rectangle moved position around the screen (ie, it did not remain fixed in a central location). Subjects were again instructed to respond only when the green square appeared above the blue rectangle, but now had to take into consideration the relative positions of both stimuli, which changed each trial.
Impulsive responses (<100 ms) were excluded from the computation of mean response time and mean individual variance, as they were considered to be too fast for participants to have processed the stimulus before registering a response, thereby potentially confounding the data.
Statistical analysis
Data were screened for accuracy of data entry, missing values, numerical outliers and normality of distributions. A two‐way (group × task) mixed analysis of variance with repeated measures on the within‐subjects factor (task) was conducted to compare the three groups on the eight task levels for measures of speed and variability. The α level was set at 0.05 for all inferential tests. Post hoc comparisons were undertaken using Tukey's method to examine the specific pattern of group differences.
The influence of parkinsonism on response time and variability was examined using analysis of covariance and the UPDRS score as the covariate. The relationship between age and attentional performance was examined using Pearson's correlations. Pearson's correlations were also computed to examine the relationship between length of reaction time and intraindividual variability on each task level. The coefficient of variation was compared between the groups as another way of controlling for group differences in mean response time.
Finally, each task level was divided into three time blocks and a three‐way (group×task×time) mixed analysis of variance with repeated measures on the within‐subjects factor (task) was carried out to examine the potential confounding effect of declining sustained attention, particularly on the executive and spatial tasks, due to the fixed order in which the tasks were administered.
Results
Sample characteristics
Men were disproportionately represented in the DLB group (70%) relative to the DAT group (37%) and healthy controls (50%), although the group difference did not reach significance (χ22,59 = 4.38, p = 0.11; table 1). There was a significant age difference between the groups (F2,56 = 4.80, p = 0.01). On average the DAT group (mean = 80.37) was 4 years older than the DLB group (mean = 75.55), whereas the control group was not considerably different in age from either of the dementia groups (mean = 77.35). There were no group differences in years of education, Kruskal–Wallis (χ22,59 = 3.87, p = 0.14), or premorbid level of intellectual function based on the NART (F2,56 = 0.42, p = 0.66). The dementia groups obtained equivalent dementia severity ratings on the CDR and MMSE (table 1), consistent with recruitment procedures designed to select patients in a mild to moderate stage of dementia.
Table 1 Demographic characteristics of the sample.
| Group | |||
|---|---|---|---|
| Control | DAT | DLB | |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| n | 20 | 19 | 20 |
| Sex (M:F) | 10:10 | 7:12 | 14:6 |
| Age (years)*† | 77.35 (3.81) | 80.37 (3.73) | 75.55 (6.55) |
| Education (years) | 9.25 (2.43) | 9.21 (2.51) | 11.15 (4.22) |
| NART | 108.25 (9.48) | 105.74 (10.52) | 109.21 (15.59) |
| CDR***ठ| 0.00 (0.00) | 1.16 (0.37) | 1.30 (0.47) |
| MMSE***ठ| 29.00 (1.29) | 23.21 (3.21) | 23.05 (3.33) |
CDR, Clinical Dementia Rating; DAT, dementia of Alzheimer's type; DLB, dementia with Lewy bodies; F, female; M, male; MMSE, Mini‐Mental State Examination; NART, National Adult Reading Test.
*p<0.05; ***p<0.001.
†Significant difference between DAT and DLB groups.
‡Significant difference between control and DAT groups.
§Significant difference between control and DLB groups.
Attentional impairment
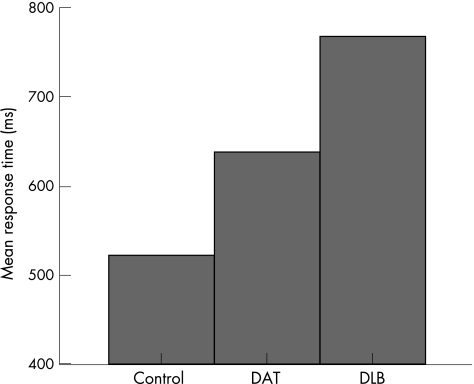
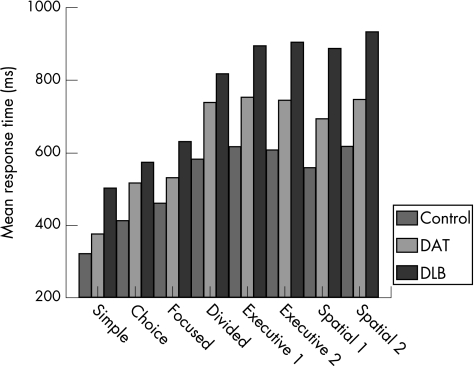
There was a significant group difference in mean response time (F2,56 = 40.21, p<0.001). Among the polynomial contrasts, there was a significant linear effect (p<0.001), with the control group recording the fastest response times and the DLB group the slowest (fig 1). There was also a main effect of task (F7,392 = 184.21, p<0.001), characterised by a graded increase in mean response time across task levels. This pattern was evident in all groups (fig 2). The presence of a significant interaction between diagnostic group and task level (F14,392 = 4.29, p<0.001), indicated that group differences in mean response time were influenced by the nature of the task.
Figure 1 Mean response time for each group, collapsed across all task levels. DAT, dementia of the Alzheimer's type; DLB, dementia with Lewy bodies.
Figure 2 Group differences in mean response time as a function of task. DAT, dementia of the Alzheimer's type; DLB, dementia with Lewy bodies.
Univariate analyses showed that group differences on each level of the task were significant at the 0.1% level (p<0.001). The DLB group was impaired relative to controls on all task levels. The DAT group also showed greater attentional impairment than controls on all tasks, except for simple reaction time. Significant differences between the dementia groups were identified. The patients with DLB were slower than their DAT counterparts on all task levels, except choice reaction time. The magnitude of the difference between the dementia groups was greatest on the executive control and spatial processing tasks (fig 2).
Attentional variability
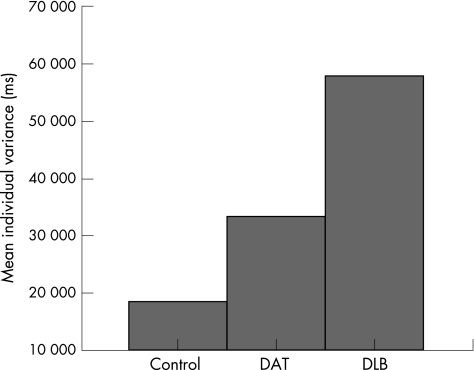
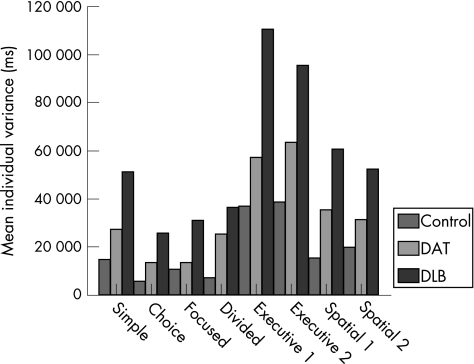
There was a significant group difference in mean individual variance (F2,56 = 46.93, p<0.001). The polynomial contrasts showed a significant linear effect (p<0.001), with the DLB group demonstrating the most intraindividual variability and the control group showing the least (fig 3). There was also a main effect of task (F7,392 = 49.23, p<0.001). Figure 4 presents the differing degrees of intraindividual variability associated with each level of the task. Group differences were influenced by task demands, as evidenced by the significant interaction between diagnostic group and task level (F14,392 = 3.96, p<0.001).
Figure 3 Mean individual variance for each group, collapsed across all task levels. DAT, dementia of the Alzheimer's type; DLB, dementia with Lewy bodies.
Figure 4 Group differences in mean individual variance as a function of task. DAT, dementia of the Alzheimer's type; DLB, dementia with Lewy bodies.
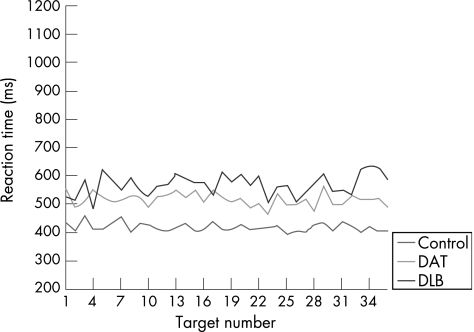
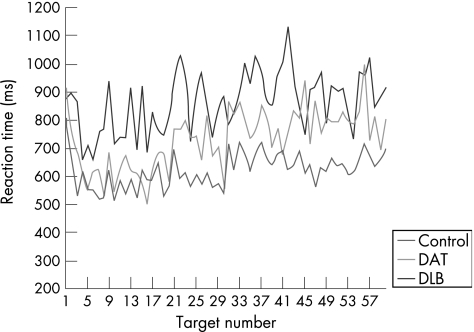
Univariate analyses showed that group differences on each level of the task were significant at the 0.1% level (p<0.001). The intraindividual variability of the DAT group was not significantly different from that displayed by the controls, except on the divided attention and executive control (set shift/inhibition) tasks. On these task levels, the variability in reaction time performance of the DAT group was significantly greater than the controls. The DLB group showed more attentional variability compared with both the control and DAT groups, regardless of task demands. There was, however, a substantial increase in the intraindividual variability of the DLB group on the executive control and spatial processing task levels (fig 4), with the executive task demands exerting the greatest influence on the degree of variability. The magnitude of the difference between the groups was considerably larger on these task levels, compared with the difference observed on the simple, choice, focused and divided attention tasks. By way of example, figs 5 and 6 show the slower, more variable response pattern exhibited by the DLB group on the executive (set shift/inhibition) task compared with the choice reaction time task, relative to the performance of the DAT and control groups.
Figure 5 Comparative profiles of attentional variability on the choice reaction time task. DAT, dementia of the Alzheimer's type; DLB, dementia with Lewy bodies.
Figure 6 Comparative profiles of attentional variability on the executive 2 reaction time task. DAT, dementia of the Alzheimer's type; DLB, dementia with Lewy bodies.
All groups were more variable on the first and easiest task level: simple reaction time, relative to the more complex choice (t59 = 4.88, p<0.001), focused (t59 = 3.89, p<0.001), and divided attention (t59 = 2.42, p<0.001) tasks. An analysis of covariance was subsequently carried out, controlling for differences in simple reaction time variability across groups on each level of the task. Significant group differences in variability persisted (F2,55 = 25.01, p<0.001), and remained task dependent (F12,330 = 2.94, p = 0.001). The DLB group remained the most variable, the magnitude of which was greatest on the executive and spatial tasks.
Analysis of potential confounding variables
Age and sex were not associated with speed of response, or the degree of intraindividual variability shown by the groups on any level of the task. Patients with dementia who had been prescribed cholinesterase inhibitors were compared with untreated patients on all reaction time tasks. No significant group differences in speed or variability were found. After controlling for the influence of extrapyramidal motor impairment, significant group differences in speed (F2,55 = 15.99, p<0.001) and variability (F2,55 = 21.88, p<0.001) persisted and remained task dependent, as suggested by the significant interaction effects for both mean response time (F14,385 = 1.75, p<0.05) and mean individual variance (F14,385 = 2.13, p = 0.01). The pattern of group differences in response speed did, however, change. Controlling for degree of parkinsonism produced equivalent mean response times in the DAT and DLB groups, but only on tasks that required supervisory attentional control—namely, the divided attention and executive control tasks. Taking into account group differences in mean response time using the coefficient of variation, the DLB group continued to show greater intraindividual variability relative to the DAT and control groups (F2,56 = 13.91, p<0.001). In the DLB group, significant positive correlations were found between response time and attentional variability on all tasks, except the executive control and spatial processing tasks (table 2). In the DAT group, task‐specific correlations were also observed, but the pattern differed from the DLB group. The patients with DAT showed positive correlations between response speed and variability on all tasks, except the divided attention and executive control tasks.
Table 2 Correlations (r) between mean response time and mean individual variance at each task level in the dementia of the Alzheimer's type (DAT), and dementia with Lewy body (DLB) groups.
| DAT | DLB | |
|---|---|---|
| Task | ||
| Simple | 0.47* | 0.71** |
| Choice | 0.59** | 0.62** |
| Focused | 0.66** | 0.48** |
| Divided | 0.32 | 0.57** |
| Executive 1 | 0.22 | −0.28 |
| Executive 2 | 0.33 | 0.04 |
| Spatial 1 | 0.56* | 0.41 |
| Spatial 2 | 0.55* | −0.32 |
*p<0.01; **p<0.001.
The data were also examined with respect to the potential confounding effect of a decline in sustained attention over the duration of the task, due to the fixed order in which the individual task levels were administered. The groups did not differ in their pattern of performance over time across any of the tasks, as evidenced by the non‐significant interaction between group, task and time block, (F28,1176 = 0.77, p = 0.80).
Discussion
Attentional dysfunction is believed to be a prominent and distinguishing neuropsychological feature of DLB,1,8,9,10 but no firm conclusions have been reached regarding the specific nature of the attentional deficit. A breakdown in attentional function is also thought to underpin the tendency to fluctuations,22,23 and may contribute to the development of visual hallucinations,9 emphasising the clinical and diagnostic importance of the study of attention in DLB. Our study is the first of its kind to examine a range of attentional abilities in patients with clinically diagnosed DLB within an integrated methodological framework. This allowed us to examine the influence of specific task demands using a common response format. The only other study to have investigated the individual components of the attentional system in DLB used multiple tests of attention, with varying sensory and motor task parameters, which could have potentially confounded the attentional aspects of performance.9
The current findings confirm that patients with DLB show greater attentional impairment and fluctuations in attention than elderly controls and patients with DAT, matched for stage of illness and severity of dementia. This effect encompassed all aspects of attentional function, ranging from simple processing speed to focused selective attention, divided attention and supervisory attentional control. Nevertheless, task demands did have an effect on the magnitude of the attentional deficit. Increasing the demand on attentional selectivity resulted in a worsening level of performance in DLB, relative to patients with DAT and controls, and this effect was most pronounced when tasks were framed within an executive or spatial context.
At an early stage of the dementing process, executive and spatial impairments feature more prominently in DLB than in DAT,8,9,30,31 consistent with the neuropathological concentration of Lewy bodies in frontal, cingulate and inferior temporal cortex and the more extensive cholinergic depletion of the temporal, parietal and midfrontal cortices in DLB.1,8 By manipulating task conditions to place demands on these compromised cortical functions, we were able to show a relative increase in attentional impairment and variability in DLB, compared with patients with DAT and elderly controls. Interestingly, the executive control demands exerted a greater influence on the degree of attentional variability, relative to the spatial judgement tasks. We speculate that this may be due to the very nature of the cognitive processes underlying executive control. These cognitive processes are responsible for maintaining a goal‐directed course of action, without which lapses of intention and transient fluctuations in performance are more likely to occur. As the data from this and other studies28 show, increases in performance variability are greater in task conditions requiring more active recruitment of executive control processes than in less demanding tasks where executive control is less critical to efficient task performance, particularly in subjects with decreased functional integrity of the prefrontal cortex.
After controlling for differences in mean length of reaction time, significant group differences in the degree of attentional variability remained. Moreover, there was a task‐specific pattern to the correlations between response speed and attentional variability in the dementia groups. The patients with DLB showed greater variability in attentional performance independently of reaction time length, but only on tasks with specific executive and visuospatial processing demands. The cognitive requirements of these tasks made an independent contribution to the degree of attentional variability that could not be attributed to the longer response latencies associated with these tasks. Analyses that controlled for group differences in degree of parkinsonism also showed task‐specific effects. The DLB group continued to show greater impairment and variability in attention, except on tasks that required executive control. Extrapyramidal motor function and executive functions are united via fronto‐striatal pathways. We could speculate that by using the UPDRS score as a covariate and statistically removing variations in striatal involvement, group differences persisted only on the non‐executive task levels, as they are less dependent on the integrity of fronto‐striatal systems.
In terms of sustained attention, the DLB group did not show a greater worsening of performance over time across any of the tasks, including the executive and spatial tasks, relative to the other groups. This finding is of interest for two reasons. Firstly, it suggests that deteriorating sustained attention did not account for the increased attentional impairment and variability evident in the DLB group on the executive and spatial tasks, as these were the last tasks administered. Secondly, while patients with DLB clearly experience a pronounced vigilance deficit, evident as an increase in attentional variability, they do not necessarily exhibit a vigilance decrement (deterioration in performance over time).42
The group differences in attentional function were not attributable to age, sex or medical treatment. The finding of an increase in variability on the simple reaction time task, relative to the more demanding choice, focused and divided attention tasks was somewhat curious and was thought to be an artefact related to the length of the interstimulus interval (ISI). To measure simple reaction time, targets had to appear at random intervals with variable ISIs of 1, 1.5 or 2 s. On all other tasks, the ISI was fixed at 2 s while the task demands were manipulated. The shorter response intervals required by the simple reaction time task may not have allowed sufficient time for the subjects to recover from the processing demands of the previous trial as they were faced with the successive trial. This has been referred to as the “recovery hypothesis”28 and is believed to have confounded performance on the simple reaction time task, producing a relative increase in intraindividual variability. This hypothesis was supported by analyses that controlled for group differences in simple reaction time. The degree of attentional variability was still greatest in the DLB group, beyond that which might be explained by variability attributable to increased impairment at the level of elementary sensorimotor processing.
There is a growing body of neuropsychological, neurochemical and electrophysiological evidence in support of a fundamental disturbance in the central regulation of consciousness in DLB, attributable to a severely compromised ascending cholinergic system.21,22,43 The pathogenesis of the attentional deficit may not, however, be restricted to disturbed consciousness and cholinergic function alone. By showing that task conditions influence the degree of impairment and variability in attentional performance, our findings indicate that there may also be a “top‐down” or cortical contribution to the regulation of attention in DLB, lending support to the hypothesis that cognitive performance in DLB reflects the pathological involvement of both cortical and subcortical regions.44
The findings from this study also make a potentially important contribution to our understanding of the neurocognitive underpinnings of fluctuations in DLB. Fluctuation is indeed a multidimensional phenomenon, characterised by fluctuation not only in attention but also in behaviour, functional abilities and cognitive function more generally. The continuous second‐to‐second attentional variation shown on this and previous reaction time tasks22,23 may reflect a basic level of attentional dysregulation provoked by disruption to the cholinergic system. These “micro‐fluctuations” are not particularly evident at a clinical level and may well occur quite independently of environmental cognitive demands. On another level, by showing that there may be a cortical contribution to attentional processing in DLB, our findings raise the possibility that the gross, clinically observable fluctuations in behaviour and functional ability may be cognitively modulated, depending on situational factors and the degree to which demands are being placed on impaired cortical functions.
Further work is needed in larger, neuropathologically confirmed samples to characterise the nature and genesis of the attentional deficit in DLB, fluctuating attention in particular. Ultimately, this may improve the diagnostic sensitivity of the consensus clinical criteria for DLB.
Acknowledgements
We thank the Austin Hospital Medical Research Foundation (AHMRF) and the Australian Association of Gerontology (AAG) for their financial support.
Abbreviations
CDR - Clinical Dementia Rating
DAT - dementia of the Alzheimer's type
DLB - dementia associated with Lewy bodies
ISI - interstimulus interval
MMSE - Mini‐Mental State Examination
NART - National Adult Reading Test
UPDRS - Unified Parkinson's Disease Rating Scale
Footnotes
Competing interests: None declared.
References
- 1.McKeith I G, Galasko D, Kosaka K.et al Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996471113–1124. [DOI] [PubMed] [Google Scholar]
- 2.Hansen L, Salmon D, Galasko D.et al The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology 1990401–8. [DOI] [PubMed] [Google Scholar]
- 3.McKeith I G, Fairbairn A F, Bothwell R A.et al An evaluation of the predictive validity and inter‐rater reliability of clinical diagnostic criteria for senile dementia of Lewy body type. Neurology 199444872–877. [DOI] [PubMed] [Google Scholar]
- 4.Mega M S, Masterman D L, Benson D F.et al Dementia with Lewy bodies: reliability and validity of clinical and pathologic criteria. Neurology 1996471403–1409. [DOI] [PubMed] [Google Scholar]
- 5.Litvan I, MacIntyre A, Goetz C G.et al Accuracy of the clinical diagnoses of Lewy body disease, Parkinson disease, and dementia with Lewy bodies: a clinicopathologic study. Arch Neurol 199855969–978. [DOI] [PubMed] [Google Scholar]
- 6.Holmes C, Cairns N, Lantos P.et al Validity of current clinical criteria for Alzheimer's disease, vascular dementia and dementia with Lewy bodies. Br J Psychiatr 199917445–50. [DOI] [PubMed] [Google Scholar]
- 7.McKeith I G, Ballard C G, Perry R H.et al Prospective validation of consensus criteria for the diagnosis of dementia with Lewy bodies. Neurology 2000541050–1058. [DOI] [PubMed] [Google Scholar]
- 8.Collerton D, Burn D, McKeith I.et al Systematic review and meta‐analysis show that dementia with Lewy bodies is a visual‐perceptual and attentional‐executive dementia. Dement Geriatr Cogn Disord 200316229–237. [DOI] [PubMed] [Google Scholar]
- 9.Calderon J, Perry R J, Erzinclioglu S W.et al Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared to Alzheimer's disease. J Neurol Neurosurg Psychiatry 200170157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballard C, O'Brien J, Gray A.et al Attention and fluctuating attention in patients with dementia with Lewy bodies and Alzheimer's disease. Arch Neurol 200158977–982. [DOI] [PubMed] [Google Scholar]
- 11.Galasko D, Katzman R, Salmon D P.et al Clinical and neuropathological findings in Lewy body dementias. Brain Cogn 199631166–175. [DOI] [PubMed] [Google Scholar]
- 12.Gnanalingham K K, Byrne E J, Thornton A.et al Motor and cognitive function in Lewy body dementia: comparison with Alzheimer's and Parkinson's diseases. J Neurol Neurosurg Psychiatry 199762243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salmon D P, Galasko D, Hansen L A.et al Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn 199631148–165. [DOI] [PubMed] [Google Scholar]
- 14.van Zomeren A, Brouwer W. Anatomy and physiology of attention. In: van Zomeren A, ed. Clinical neuropsychology of attention. New York: Oxford University Press, 199439–62.
- 15.Barkley R. Critical issues in research on attention. In: Reid Lyon G, Krasnegor N, eds. Attention, memory, and executive function. Baltimore: Paul H Brookes, 199645–56.
- 16.Mirsky A. Disorders of attention: a neuropsychological perspective. In: Reid Lyon G, Krasnegor N, eds. Attention, memory and executive function. Baltimore: Paul H Brookes, 199671–95.
- 17.Ayre G, Ballard C, Pincock C.et al Double dissociation between dementia with Lewy bodies and Alzheimer's disease on tests of attentional and mnemonic function: the role of the basal forebrain. J Psychopharmacol 1998A12(Suppl)A64 [Google Scholar]
- 18.Sahgal A, Galloway P, McKeith I G.et al A comparative study of attentional deficits in senile dementias of Alzheimer and Lewy body types. Dementia 19923350–354. [Google Scholar]
- 19.Sahgal A, Galloway P H, McKeith I G.et al Matching‐to‐sample deficits in patients with senile dementias of the Alzheimer and Lewy body types. Arch Neurol 1992491043–1046. [DOI] [PubMed] [Google Scholar]
- 20.Ballard C G, Aarsland D, McKeith I.et al Fluctuations in attention: PD dementia vs DLB with parkinsonism. Neurology 2002591714–1720. [DOI] [PubMed] [Google Scholar]
- 21.Walker M P, Ayre G A, Ashton C H.et al A psychophysiological investigation of fluctuating consciousness in neurodegenerative dementias. Hum Psychopharmacol 199914483–489. [Google Scholar]
- 22.Walker M P, Ayre G A, Perry E K.et al Quantification and characterisation of fluctuating cognition in dementia with Lewy bodies and Alzheimer's disease. Dement Geriatr Cogn Disord 200011327–335. [DOI] [PubMed] [Google Scholar]
- 23.Walker M P, Ayre G A, Cummings J L.et al Quantifying fluctuation in dementia with Lewy bodies, Alzheimer's disease and vascular dementia. Neurology 2000541616–1624. [DOI] [PubMed] [Google Scholar]
- 24.Byrne E J, Lennox G, Lowe J.et al Diffuse Lewy body disease: clinical features in 15 cases. J Neurol Neurosurg Psychiatry 198952709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballard J. Computerized assessment of sustained attention: a review of factors affecting vigilance performance. J Clin Exp Neuropsychol 199618843–863. [DOI] [PubMed] [Google Scholar]
- 26.Stuss D T, Murphy K J, Binns M A.et al Staying on the job: the frontal lobes control individual performance variability. Brain 20031262363–2380. [DOI] [PubMed] [Google Scholar]
- 27.Salthouse T A. What do adult age differences in the digit symbol substitution test reflect? J Gerontol Psychol Sci 199247121–128. [DOI] [PubMed] [Google Scholar]
- 28.West R, Murphy K J, Armilio M L.et al Lapses of intention and performance variability reveal age‐related increases in fluctuations of executive control. Brain Cogn 200249402–419. [DOI] [PubMed] [Google Scholar]
- 29.Shammi P, Bosman E, Stuss D T. Aging and variability in performance. Aging Neuropsychol Cogn 199851–13. [Google Scholar]
- 30.Mori E, Shimomura T, Fujimora M.et al Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol 200057489–493. [DOI] [PubMed] [Google Scholar]
- 31.Mosimann U P, Mather G, Wesnes K A.et al Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology 2004632091–2096. [DOI] [PubMed] [Google Scholar]
- 32.Hughes C, Berg L, Danzinger W.et al A new clinical scale for the staging of dementia. Br J Psychiatry 1982140566–572. [DOI] [PubMed] [Google Scholar]
- 33.Folstein M, Folstein S, McHugh P. ‘Mini‐mental state': a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 197512189–198. [DOI] [PubMed] [Google Scholar]
- 34.Nelson H E.National Adult Reading Test. Berkshire: NFER‐NELSON, 1982
- 35.McKhann G, Drachman D, Folstein M.et al Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 198434939–944. [DOI] [PubMed] [Google Scholar]
- 36.Lang A E, Fahn S. Assessment of Parkinson's disease. In: Munstat T, ed. Quantification of neurologic deficit. London: Butterworths, 1989 (chapter 21),
- 37.Overall J E, Gorham D R. The Brief Psychiatric Rating Scale. Psychol Rep 196210799–812. [Google Scholar]
- 38.Zigmond A S, Snaith R P. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 198367361–367. [DOI] [PubMed] [Google Scholar]
- 39.Eriksen B A, Eriksen C W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys 197416143–149. [Google Scholar]
- 40.Sharma V, Halperin J H, Newcorn J N.et al The dimension of focused attention: relationship to behaviour and cognitive functioning in children. Perceptual Motor Skills 199172787–793. [DOI] [PubMed] [Google Scholar]
- 41.Stuss D T, Pogue J, Buckle L.et al Characterization of stability of performance in patients with traumatic brain injury: variability and consistency on reaction time tests. Neuropsychology 19948316–324. [Google Scholar]
- 42.Whyte J, Polansky M, Fleming M.et al Sustained arousal and attention after traumatic brain injury. Neuropsychologia 199533797–813. [DOI] [PubMed] [Google Scholar]
- 43.Perry R H, Walker M, Perry E. Dementia with Lewy bodies. A new avenue for research into neurobiological mechanisms of consciousness? In, Fisher E, ed. Progress in Alzheimer's and Parkinson's diseases New York, Plenum Press, 1998 (chapter 65)
- 44.Downes J, Priestley N, Doran M.et al Intellectual, mnemonic, and frontal functions in dementia with Lewy bodies: a comparison with early and advanced Parkinson's disease. Behav Neurol 1998/199911173–83c. [PubMed] [Google Scholar]