Abstract
Objective
To describe the neuropsychological characteristics of mild cognitive impairment (MCI) subgroups identified in the Cardiovascular Health Study (CHS) cognition study.
Methods
MCI was classified as MCI‐amnestic type (MCI‐AT): patients with documented memory deficits but otherwise normal cognitive function; and MCI‐multiple cognitive deficits type (MCI‐MCDT): impairment of at least one cognitive domain (not including memory), or one abnormal test in at least two other domains, but who had not crossed the dementia threshold. The MCI subjects did not have systemic, neurological, or psychiatric disorders likely to affect cognition.
Results
MCI‐AT (n = 10) had worse verbal and non‐verbal memory performance than MCI‐MCDT (n = 28) or normal controls (n = 374). By contrast, MCI‐MCDT had worse language, psychomotor speed, fine motor control, and visuoconstructional function than MCI‐AT or normal controls. MCI‐MCDT subjects had memory deficits, though they were less pronounced than in MCI‐AT. Of the MCI‐MCDT cases, 22 (78.5%) had memory deficits, and 6 (21.5%) did not. MCI‐MCDT with memory disorders had more language deficits than MCI‐MCDT without memory disorders. By contrast, MCI‐MCDT without memory deficits had more fine motor control deficits than MCI‐MCDT with memory deficits.
Conclusions
The most frequent form of MCI was the MCI‐MCDT with memory deficits. However, the identification of memory impaired MCI groups did not reflect the true prevalence of MCI in a population, as 16% of all MCI cases and 21.5% of the MCI‐MCDT cases did not have memory impairment. Study of idiopathic amnestic and non‐amnestic forms of MCI is essential for an understanding of the aetiology of MCI.
Keywords: Alzheimer's disease, aging, dementia, mild cognitive impairment, neuropsychology
The term mild cognitive impairment (MCI) is used in clinical research to describe a group of elderly subjects who have cognitive impairments, often involving memory, not of sufficient severity to warrant the diagnosis of dementia. Implicit in the MCI concept is the idea that these subjects are at increased risk of developing Alzheimer's disease.1,2 Although researchers have focused on MCI cases with relatively isolated memory deficits, increasing numbers of studies have shown that performance in cognitive domains other than memory may not be entirely normal.1,3,4,5 Indeed, current studies suggest that there are two forms of MCI, one with predominant impairment of memory (for example, MCI,6 age associated memory impairment (AAMI)7), and the other with a wider range of cognitive impairments (for example, age associated cognitive decline (AACD),8 age related cognitive decline (ARCD)9). Population studies have showed that the cases with predominant impairment of memory constituted a relatively small group compared with all individuals with a much broader form of mild cognitive deficit.10,11,12,13,14,15,16
The most important aspect in the diagnosis of MCI is to separate an age related process (subjects are different from younger individuals, but not from those of the same age) from a pathological state (subjects are different from younger individuals, and from those of the same age), which could be a risk state for dementia. Therefore, how these criteria are operationalised is critical for identifying a “pathological” MCI syndrome, and this may have explained the wide range of estimates of the prevalence of MCI. Among the studies using the AAMI criteria, the MCI prevalence ranged from 7.1% (in subjects aged over 65),17 to 53.8%.18 In other study, the prevalence for AAMI was 18.5%, using cut off points adjusted for age,19 but 40% met the criteria if no age adjustments were made.
Although the MCI syndrome is based on a neuropsychological pattern of impaired and non‐impaired functions, there are still some discrepancies over how to classify these patients. Whether all subjects with memory deficits (memory impaired only and memory associated with other cognitive deficit) should be considered MCI‐amnestic type (MCI‐AT), or whether there should be a clear differentiation between those with an isolated memory deficit and those whose memory deficits are associated with abnormalities in other cognitive domains. For example, studies that have focused on MCI with a predominant memory impairment have also found that these individuals can have verbal fluency,1 receptive language,20 attention, and executive function deficits.3,4,21 Population studies found that that up to 54% of the patients with AAMI met criteria for AACD.22
On the other hand, studies that examined subjects with a much broader cognitive impairment (MCI‐multiple cognitive domain type (MCI‐MCDT)) found that more than 50% of the subjects with MCI‐MCDT can have memory deficits,22 and Schroeder et al showed that 67% of the AACD patients met criteria for AAMI.11 Therefore, a first step in the understanding the relation between memory‐only and memory‐plus deficit cases is to describe the characteristics and the expected proportion of these two MCI subtypes. Second, those cases without any memory deficits—a third MCI group—should be identified and compared with the other two MCI subtypes. Petersen et al proposed classifying MCI patients in three subgroups: amnestic, multiple domains slightly impaired, and single non‐memory domain.23 However, there were no published data to support this classification scheme.
The Cardiovascular Health Study (CHS) cognition study evaluated the cognitive function of 3608 participants to diagnose and classify MCI and dementia (and related disorders) in four American communities, and detailed evaluation of MCI subgroups was conducted in Pittsburgh. The CHS cognition study has identified 6% prevalence for MCI‐AT and 16% for MCI‐MCDT.13 The purpose of the present study was to extend these findings by describing the pattern of neuropsychological defects associated with MCI. In particular, we were interested in examining the patterns of neuropsychological test performance of the MCI‐MCDT participants with and without memory deficits.
Methods
The initial CHS cohort comprised 5201 non‐institutionalised individuals over the age of 65 who were recruited from four communities using the Part A Medicare list (Pittsburgh, Pennsylvania; Sacramento, California; Winston‐Salem, North Carolina; Hagerstown, Maryland) in 1988–89. In 1992–93, the third year of the study, 687 African Americans were added to the study in the same manner. The demographic characteristics of the total CHS cohort have been described previously.24
Beginning in 1988/89, all participants completed the modified Mini‐Mental State Examination (3MSE)25 and the Digit Symbol Substitution Test (DSST)26 at their annual visits, and the Benton Visual Retention Test (BVRT) from 1994 to 1998.27 The Telephone Interview for Cognitive Status (TICS) was used when participants did not come to the clinic.28 Further information on cognition was obtained from proxies using the Informant Questionnaire for Cognitive Decline in the Elderly (IQ CODE),29 and the Dementia Questionnaire (DQ).30 Symptoms of depression were measured with the modified version of the Center for Epidemiology Studies Depression Scale (CES‐D).31 In 1991–94, 3608 participants had magnetic resonance imaging (MRI) of the brain, and repeat MRI in 1997–98. The CHS staff also obtained information from participants and next of kin regarding vision and hearing, the circumstances of the illness, and history of dementia, and functional status, as well as information about pharmaceutical drug use and alcohol consumption.32 Data on instrumental activities of daily living (IADL),33 and activities of daily living (ADL)34 were also collected
The CHS Cognition Study has been described previously in detail, including the methods of classification of dementia and MCI.13,32 Briefly, in 1998–99 the CHS attempted to identify all participants who had either prevalent dementia at the time of MRI of the brain in 1991–94, or subsequent incident dementia before 1998–99. The sample was limited to those participants who had an MRI in 1991–94, and a 3MSE evaluation, for a total of 3608 participants, of whom 3602 were available for the study. Comparison of those who did and did not have MRI has previously been reported.35,36 Participants classified as demented or MCI were reviewed by an adjudication committee comprised of experts in dementia diagnosis, who first classified cases as demented, MCI, or normal, and then adjudicated the specific type of dementia or MCI.13
Clinical examination
Neuropsychological examination
The neuropsychological battery included the following tests:
Premorbid intelligence: American version of the National Reading Test (AMNART)37; Raven's coloured progressive matrices (modified)38;
Memory: California Verbal Learning Test (CVLT)39; modified Rey‐Osterreith figure40;
Visuoperceptual/visuoconstructional: block design (modified from the Wechsler Adult Intelligence Scale–Revised)26; modified Rey‐Osterreith figure40;
Psychomotor speed: Trailmaking A; Baddeley and Papagno divided attention task (single task)43;
Executive functions: Stroop neuropsychological screening test44; Trailmaking B and A/B45; digit spans26; Baddeley and Papagno divided attention task (dual task)43;
Fine motor control: Grooved pegboard test.46
The results of the neuropsychological battery were classified as normal or abnormal (>1.5 SD below individuals of comparable age and education), based on normative data collected from a sample of 250 unimpaired subjects in Pittsburgh. An abnormal domain was considered when two tests of the same domain were impaired.
Neurological examination
The neurological examination included a brief mental status examination, as well as cranial nerve testing, motor tone, abnormal movements, strength, deep tendon reflexes, release signs, plantar response and clonus, cerebellar testing, primary sensory testing, gait, and postural stability. After the mental status examination the neurologist asked the participants about their performance on these tests, and the response was graded on a four point scale. The examiner also completed the Unified Parkinson's Disease Rating Scale (UPDRS),47 and the Hachinski ischaemic scale.48 After completing the neurological examination, the neurologist classified the participant as normal, MCI, or dementia.
Psychiatric examination
Symptoms of depression were measured with the modified version of the Center for Epidemiology Studies Depression Scale (CES‐D) 10‐item version,31 and historical data were available through the CHS. In 1998–99, we administered the Neuropsychiatric Inventory (NPI)49 to expand the psychiatric assessment; historical data were available from 1989 to 1998.
MCI criteria
MCI subjects were diagnosed following the CHS Cognition Study diagnostic criteria for MCI.13 MCI‐AT included subjects with impairments (defined as performance >1.5 SD from controls) in delayed recall of verbal material, non‐verbal materials, or both, and the cognitive deficits must represent a decline from a previous level of functioning. Cognitive functions must otherwise fall within normal limits. This diagnosis did not exclude individuals with mild defects of IADL. The second type, MCI‐MCDT, required impairments in at least one cognitive domain (other than memory), or one abnormal test (which could be a memory test) in at least two domains (defined as performance >1.5 SD) from controls, without sufficiently severe impairment, or loss of activities of daily living to constitute dementia. For example, MCI‐MCDT cases can have an isolated language deficit, or one abnormal memory test with an abnormal visuoconstructional test. These cognitive deficits may or may not affect IADL, but must represent a decline from a previous level of functioning, as detected by the annual 3MSE, BVRT, and DSST scores, or reported in the IQCODE, and DQ.
Subjects were classified as probable MCI when there were no psychiatric, neurological (for example, cerebrovascular disease, history of head trauma encephalopathy, infectious diseases, developmental disabilities), or systemic illnesses that may cause cognitive deficits. Participants with Parkinson's disease and MCI were not included in this study. Subjects were classified as possible MCI when there were comorbid conditions that can cause cognitive deficits, or when they were unable to complete the neuropsychological battery. Subjects were considered to have had a complete neuropsychological assessment when they had completed a minimum of five neuropsychological tests in three cognitive domains.
The diagnosis of MCI was done by an adjudication committee which examined the longitudinal data, as well as the neuropsychological, neurological, psychiatric, and systemic information. The adjudication committee had access to all neuropsychological information. The tests used to determine the pattern of impairment are shown below. Subjects with normal neuropsychological performance and reports of mild IADL problems were considered normal.
Of the 927 participants in the Pittsburgh sample, 552 were classified as normal, 193 as demented, and 159 as MCI. Twenty three subjects died within the first year following their 1992–94 MRI, and insufficient cognitive data were available for their clinical classification. Of these 159 MCI participants, 130 were alive in 1998–99, and 88 had a complete neuropsychological evaluation. Of the 552 normal participants, 439 were alive in 1998–99 and 374 completed the neuropsychological examination. The subtypes of 130 adjudicated MCI cases included 10 probable MCI‐AT, 30 possible MCI‐AT, 30 probable MCI‐MCDT, and 80 possible MCI‐MCDT cases. Of the 42 cases classified as possible MCI because of incomplete data, 67% had a comorbid condition which may have affected their cognitive performance (MCI‐MCDT, 19/30 (63%); MCI‐AT, 9/12 (75%)).
Because the purpose of this study was to describe the cognitive characteristics of MCI subgroups, we evaluated the neuropsychological performance of all subjects at the Pittsburgh site who met criteria for probable MCI.13 First, we compared the subjects based on the MCI‐AT and MCI‐MCDT classification. The second classification rule that was applied to the data focused on the extent of memory loss in the MCI‐MCDT subjects. Based on their performance on the memory tests (>1.5 SD), the MCI‐MCDT subjects were grouped into those with and those whose without memory deficits.
Statistical analysis
Certain of the neuropsychological test scores were combined to produce meaningful values for analysis. The score on the modified Boston Naming Test was the sum of the spontaneous correct responses and the correct responses following a semantic cue. Word generation using letter cues was calculated as the average number of words generated per minute. The word generation using animal types (“subcategories”) was also calculated as the average number of words generated per minute. This allowed direct comparison of word generation across all three conditions (that is, letter cues, category cue, subcategories). The scores on the Trailmaking Test (parts A and B) were recalculated as the number of seconds required to complete each correct connection, and we also calculated the ratios of B:A as times. An “interference score” was calculated for the Stroop neuropsychological screening test by dividing the difference between the interference condition by the baseline condition, and the baseline condition (that is, (I−B)/B), which is a more direct measure of the Stroop effect.
The neuropsychological test data were transformed into standardised scores using routine procedures. The subjects were stratified by age (±80 years) and education (±high school education), and the individual test scores were z transformed, based on the appropriate mean and standard deviation from the control subjects. All scores were then converted into T scores (T = z*10+50), resulting in a mean T of 50 for the control subjects, with a standard deviation of 10.50
The neuropsychological data were further reduced to Domain T scores using the test scores available to the adjudication committee. The individual age and education adjusted z scores were averaged (using the appropriate sign), and these composite standard scores were then transformed into T scores. The test scores contributing to each of the five domain scores are listed in table 2.
Table 2 Neuropsychological characteristics of normal subjects and mild cognitive impairment subgroups (raw scores).
| Variable | Normal | MCI‐AT | MCI‐MCDT | IC* | ||||
|---|---|---|---|---|---|---|---|---|
| Number of cases | 374 | 10 | 28 | |||||
| 3MSE | 96.0 (4.6) | 92.6 (6.2) | 88.2 (7.3) | a,b,c | ||||
| Digit symbol substitution test | 46.8 (12.3) | 38.4 (10.1) | 29.2 (12.1) | a,b,c | ||||
| Premorbid intelligence‡ | ||||||||
| American National Reading Test | 118.3 (8.5) | 121.2 (7.4) | 107.1 (12.4) | b,c | ||||
| Raven's coloured progressive matrices | 27.2 (5.2) | 22.0 (5.3) | 20.0 (4.8) | a,b,c | ||||
| Memory | ||||||||
| California Verbal Learning Test | ||||||||
| Trial 1 | 5.8 (2.0) | 2.8 (1.7) | 3.8 (2.1) | a, b | ||||
| Trial 5 | 10.2 (2.7) | 5.1 (2.8) | 7.3 (2.8) | a,b,c | ||||
| Trials 1 to 5 | 43.3 (10.1) | 24.1 (7.1) | 31.2 (11.3) | a, b | ||||
| Free recall (short) | 8.0 (3.9) | 2.0 (2.5) | 4.7 (2.4) | a,b,c | ||||
| Free recall (long)† | 8.7 (2.9) | 2.0 (2.1) | 5.4 (2.7) | a,b,c | ||||
| Intrusions | 2.1 (3.3) | 3.0 (1.8) | 2.7 (2.7) | d | ||||
| Discriminability index | 89.7 (8.2) | 75.0 (13.2) | 81.7 (8.2) | a,b,c | ||||
| Modified Rey‐Osterreith figure | ||||||||
| Immediate recall | 15.4 (4.8) | 7.3 (4.7) | 10.5 (4.5) | a,b | ||||
| Delayed recall† | 14.7 (4.8) | 4.8 (4.1) | 8.6 (4.6) | a,b,c | ||||
| Visuoconstructional | ||||||||
| Modified Rey‐Osterreith figure† | 22.3 (2.1) | 21.1 (4.0) | 18.7 (3.4) | b,c | ||||
| Block design† | 11.4 (4.8) | 9.7 (3.8) | 3.5 (2.9) | b,c | ||||
| Language | ||||||||
| Boston Naming† | 26.9 (2.6) | 26.4 (1.1) | 23.1 (4.0) | b,c | ||||
| Letters (F±S)† | 25.9 (9.3) | 25.3 (9.2) | 17.0 (8.1) | b,c | ||||
| Category | ||||||||
| Animals† | 15.6 (4.8) | 11.6 (3.1) | 11.1 (4.4) | a,b | ||||
| Birds | 9.7 (3.8) | 7.8 (3.8) | 6.4 (2.6) | b | ||||
| Dogs | 7.0 (3.2) | 5.2 (3.0) | 5.1 (2.3) | b | ||||
| Psychomotor speed | ||||||||
| Trailmaking A (in seconds)† | 45.6 (17.5) | 60.8 (29.3) | 74.9 (27.0) | a,b,c | ||||
| Baddeley and Papagno (single task‐cancellation)†§ | 67.6 (17.4) | 88.0 (41.7) | 92.9 (23.4) | a,b | ||||
| Executive functions | ||||||||
| Trailmaking B (in seconds) | 107.5 (49.3) | 166.7 (95.1) | 204.0 (63.9) | a,b | ||||
| Trailmaking A/B† | 2.5 (2.0) | 2.6 (0.95) | 3.0 (0.96) | d | ||||
| Stroop test (interference)† | 2.31 (1.8) | 2.8 (2.2) | 4.2 (2.1) | b | ||||
| Digit spans | ||||||||
| Forward | 6.4 (1.2) | 6.2 (1.1) | 5.9 (1.0) | b | ||||
| Backward | 4.4 (1.2) | 3.7 (0.95) | 3.4 (1.1) | b | ||||
| Baddeley and Papagno (dual task)†§ | 76.9 (18.4) | 82.8 (28.7) | 98.3 (18.6) | b,c | ||||
| Fine motor control‡ | ||||||||
| Grooved pegboard (seconds) | ||||||||
| Dominant† | 112.0 (33.1) | 119.1 (19.6) | 166.1 (74.8) | a,b,c | ||||
| Non‐dominant† | 123.4 (33.1) | 126.6 (23.7) | 183.9 (97.2) | a,b,c |
Values are mean (SD).
*ANOVA intergroup comparisons (IC): a, MCI‐AT different from normal; b, MCI‐MCDT different from normal; c, MCI‐AT different from MCDT; d, no statistical differences. All p values <0.05, see text for details.
†Measures used to create composite scores.
‡Premorbid intelligence and fine motor control tests were not used to classify MCI cases.
§Per cent change: single v dual tasks.
MCI‐MCDT, multiple cognitive deficits type of mild cognitive impairment; MCI‐AT, amnestic type of mild cognitive impairment; 3MSE, modified Mini‐Mental State Examination.
Results
The demographic and psychometric data from the MCI patients and the normal control subjects are shown in table 1. There was a greater proportion of MCI‐AT subjects with more than high school education than normal controls. By contrast, MCI‐MCDT subjects had lower level of education than normal controls or MCI‐AT subjects. There was a greater proportion of African Americans with MCI‐MCDT than normal controls and MCI‐AT. MCI‐MCDT subjects had higher UPDRS scores than normal controls or MCI‐AT (table 1).
Table 1 Demographic and neurological characteristics of the Pittsburgh mild cognitive impairment subgroups.
| Variable | Normal | MCI‐AT | MCI‐MCDT | IC* | ||||
|---|---|---|---|---|---|---|---|---|
| Number of subjects | 374 | 10 | 28 | |||||
| Age (years) | 79.5 (3.7) | 79.9 (3.4) | 79.7 (5.7) | d | ||||
| Education level | ||||||||
| >high school (%): | 230 (61.5%) | 8 (80%) | 9 (32%) | b,c | ||||
| <high school (%): | 144 (38.5%) | 2 (20%) | 19 (68%) | |||||
| Sex | ||||||||
| Male (%): | 142 (38%) | 6 (60%) | 13 (46%) | d | ||||
| Female (%) | 232 (62%) | 4 (40%) | 15 (54%) | |||||
| Race | ||||||||
| White (%): | 306 (82%) | 7 (70%) | 12 (43%) | b,c | ||||
| African American (%): | 68 (18%) | 3 (30%) | 16 (575) | |||||
| Hachinski Ischemic Scale closest to NP | 1.0 (1.1) | 1.7 (1.2) | 1.1 (0.9) | d | ||||
| UPDRS closest to NP | 4.1 (4.4) | 6.1 (5.4) | 8.7 (5.7) | b,c | ||||
| CES‐D score >7 closest to NP (%) | 88 (24%) | 3 (305) | 9 (32%) | d |
Values are mean (SD) or n (%).
*2 and one way analysis of variance intergroup comparisons (IC): a, MCI‐AT different from normal; b, MCI‐MCDT different from normal; c, MCI‐AT different from MCI‐MCDT; d, no statistical differences. All p values <0.05, see text for details.
CES‐D, Center for Epidemiological Studies Depression Scale; MCI‐MCDT, multiple cognitive deficits type of mild cognitive impairment; MCI‐AT, amnestic type of mild cognitive impairment; NP, neuropsychological assessment; UPDRS, Unified Parkinson's Disease Rating Scale.
Neuropsychological characteristics of MCI
Table 2 shows the raw scores of normal, MCI‐AT, and MCI‐MCDT subjects. The asterisk indicates the measures used to create composite scores in each domain, and the tests used to determine the pattern of impairment by the adjudication committee. Table 3 shows the composite T scores for the MCI patients and controls. The data for the MCI subjects are broken down in two ways; first, we compared subjects with MCI‐AT and MCDT, and second, we compared the performance of MCI‐AT to MCI‐MCDT with and without memory deficits (table 3).
Table 3 Domain T scores for mild cognitive impairment subgroups.
| Controls v MCI‐AT v MCI‐MCDT | Controls v MCI‐AT v MCDT with memory deficits v MCDT without memory deficits | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | MCI‐AT | MCI‐MCDT | IC* | MCI‐MCDT with memory deficits | MCI‐MCDT without memory deficits | IC* | ||||||||
| Number of cases | 374 | 10 | 28 | 22 | 6 | |||||||||
| Memory | 50.2 (6.7) | 29.3 (6.7) | 38.9 (6.7) | a,b,c | 36.7 (5.6) | 46.5 (7.7) | e | |||||||
| Visualconstructional | 50.0 (7.8) | 45.7 (10.9) | 35.4 (9.9) | b,c | 37.9 (6.9) | 35.4 (8.0) | f | |||||||
| Language | 49.9 (9.3) | 45.9 (4.7) | 40.6 (7.0) | b,c | 38.7 (6.3) | 43.5 (8.6) | e | |||||||
| Psychomotor speed | 49.9 (7.0) | 45.2 (8.3) | 36.5 (9.0) | b,c | 37.9 (14.0) | 33.0 (14.3) | f | |||||||
| Executive functions | 50.2 (8.9) | 47.8 (7.7) | 46.0 (7.1) | d | 44.0 (7.5) | 42.3 (6.3) | g | |||||||
| Fine motor control | 49.6 (8.2) | 49.4 (5.3) | 32.9 (22.7) | b,c | 38.7 (16.5) | 28.9 (14.5) | f, h | |||||||
Values are mean (SD).
*Multivariate analysis of variance intergroup comparisons (IC):
Controls v MCI‐AT v MCI‐MCDT: a, MCI‐AT different from normal; b, MCI‐MCDT different from normal; c, MCI‐MCDT different from MCI‐AT; d, no statistical differences.
Controls v MCI‐MCDT with and without memory deficits: e, MCI‐MCDT with memory deficits different from MCI‐MCDT without memory deficits, MCI‐AT, and normal; f, MCI‐MCDT with and without memory deficits different from MCI‐AT and normal; g, MCI‐MCDT without memory deficits different from normal and MCI‐AT; h, MCI‐MCDT with memory deficits different from MCI‐MCDT without memory deficits.
MCI‐MCDT, multiple cognitive deficits type of mild cognitive impairment; MCI‐AT, amnestic type of mild cognitive impairment.
Two multivariate analyses of variance (MANOVA) were completed, using the LSD test (p<0.05) to test between group differences on each of the domain T scores. The first analysis compared the subject's performance as a function of the CHS grouping. There was a main effect of group (F(12,682) = 17.94, p<0.001, η2 = 0.24), and there were specific deficits in performance as shown with the domain scores. Specifically, the MCI‐AT subjects, as expected, had memory domain scores that were significantly lower than those of the controls or the MCI‐MCDT group. The MCI‐AT subjects were not significantly different from the controls on any other domain scores. By contrast, the MCI‐MCDT group was impaired on all other domain scores, and actually performed significantly worse than the MCI‐AT group in the visuoconstruction/visuospatial, language, and fine motor control domains (table 3).
The MANOVA comparing the MCI‐MCDT subjects broken down by memory scores also had a significant effect of group (F(11,593) = 18.000, p<.001, η2 = 0.24). The pattern of spared and impaired functions was different from that observed in the MCI‐MCDT classification. Of the MCI‐MCDT cases, 22 (78.5%) had memory deficits, and six (21.5%) did not. The MCI‐MCDT with memory disorders had more language deficits than the MCI‐MCDT without memory disorders. By contrast the MCI‐MCDT without memory deficits had more fine motor control deficits than the MCI‐MCDT with memory deficits. Visuoconstructional, executive functions, and psychomotor speed function were equally impaired in both forms of MCI‐MCDT compared with normal controls and MCI‐AT. The MCI‐MCDT without memory deficits had worse executive functions than normal controls and MCI‐AT subjects.
Discussion
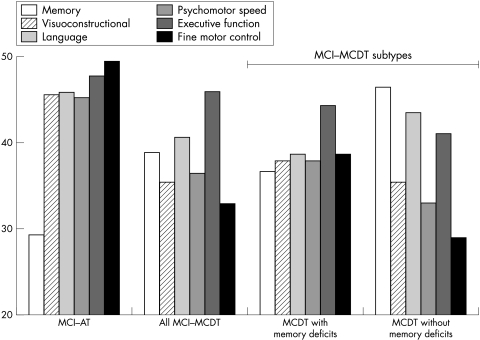
The findings from this study are directly relevant to our understanding of the symptom profile and nosology of MCI. The fact that subjects with MCI are sometimes grouped as having only memory deficit, and sometimes as having memory plus deficits in other areas of cognition can have a significant impact in the clinical characterisation of the MCI syndrome.11,12,16,22 The definitions used here separated the MCI cases differently. In the present study MCI‐AT included only those cases with an idiopathic amnestic disorder. In fig 1, we plot the domain scores by MCI subgroup, and this clearly shows the focal nature of the impairment in MCI‐AT. By contrast, we used a separate classification for those MCI cases who had impairments in multiple domains, or in a single non‐memory domain (that is, MCI‐MCDT). This group clearly has a more diffuse and less well defined pattern of defects. However, when the MCI‐MCDT group was broken down as a function of memory impairment, distinctions were found within that group. The MCI‐MCDT cases with memory loss were impaired in all functions compared with normal controls and MCI‐AT, except in executive functions. Thus, while they had memory deficits, the range of cognitive impairments suggests a very different pattern of neuropathological damage than would be expected in the focal amnestic group (MCI‐AT). As a group, the MCI‐MCDT cases without memory defects present with a less well defined pattern of impairment, but with sparing of memory, language, and executive functions (that is, they have T scores >40).
Figure 1 The domain scores by mild cognitive impairment subgroup. MCDT, multiple cognitive deficits type of mild cognitive impairment; MCI, mild cognitive impairment; MCI‐AT, amnestic type of mild cognitive impairment.
These results make clear that the classification of “MCI‐amnestic”, as it is usually considered, is not sufficient to capture the precise nature of the syndrome (and the underlying pathological damage), as the majority of the MCI cases fell in the category of MCI‐MCDT, and 16% of all MCI cases did not have any memory impairment. In addition, our results support the view that there should be an MCI‐amnestic group with a focal memory disorder and without impairments in other domains. This group should be differentiated from the “MCI‐MCDT with memory disorder”, which was the most frequent form of MCI (56% of all MCI cases), and which represented a group of individuals with a memory deficit and impaired function in other domains. Finally, we believe that the term “MCI‐MCDT without memory impairment” should be used to characterise the remaining patients. While some might argue that this is a semantic distinction of no practical importance, we suggest that to the extent that the pattern of neuropsychological dysfunction is related to the underlying neuropathological abnormality, then these distinctions between subtypes are of the highest importance. As a consequence, studies of the natural history, treatment response, and risk for dementia will provide more meaningful data when attention is paid to the full range of neuropsychological abnormalities in MCI.
The definition of MCI types is based, to some degree, on the same neuropsychological tests used in the analysis. Therefore, any studies of the neuropsychological test profiles of MCI are not independent of the subgroup classification. It is also clearly the case that the breadth of the neuropsychological evaluation will provide better detail to aid in the classification of the subjects—focusing on memory tests with minimal evaluation of other functions could bias the classifications in a way different from a test battery that covers more cognitive domains. The composition of the neuropsychological battery is, therefore, a critical factor in the diagnosis of MCI. The battery must be sensitive enough to detect MCI subgroups as well as the possible contributions of other disease processes (for example, cerebrovascular disease, metabolic disease) to the manifestation of the syndrome. Although there is little agreement about the composition of the optimal test battery, it should be able to clearly identify cognitive domains (memory, language, attention/executive functions, visuoperceptual, and visuoconstructional), and should include multiple measures for each domain/construct, to avoid being responsive only to dementia severity. The test battery should also take into account the variability in the cognitive performance often seen in population studies, which is usually greater than that seen in referral clinics, where subjects seek evaluation for the presence of cognitive disorders.
The manner in which the MCI subjects are subclassified has implications for the cognitive profile of the group and thus for our inferences about the aetiology and possible clinical course of the disorder. When we simply asked whether the subject had a mild memory disorder, we identified 32 such cases (84%); six (16%) did not have a memory impairment. However, memory impaired cases can present with an isolated memory deficit (see fig 1), or in combination with deficits in other domains, the most frequent form of MCI identified in this study. Inspection of the domain T scores, however, revealed that when the MCI‐MCDT subjects were classified on the basis of their memory performance, the memory impaired subjects were significantly different from controls in all domains measured.
The qualitative and quantitative integrity of the MCI‐AT group compared with the MCI‐MCDT memory impaired classification, suggests that the former represents an even more focal pattern of cognitive impairment. MCI‐AT subjects had a greater volume loss in the hippocampus, amygdala, and entorhinal cortex, as well as in the dorsolateral prefrontal cortex and the superior temporal and parietal cortices.51 By contrast, MCI‐MCDT subjects had less atrophy of the anterior mesial temporal lobe (only hippocampus volume loss), and more atrophy in the secondary association cortices (that is, frontal‐temporal‐parietal lobes) than MCI‐AT subjects. By requiring that the memory defect exists in isolation, we gain the opportunity to evaluate a more restricted neurobehavioural syndrome (and, by extension, a more specific neuropathological/aetiological basis).
More than 20% of the MCI‐MCDT cases had a relative preservation of their memory functions. However, there were a few cases with isolated non‐memory domain impairments: one patient had mild deficits in language function, and two had mild defects in visuoconstructional functions. It is important to note that the sample sizes of these subgroups are quite small, and consequently these results must be interpreted cautiously. Nevertheless, these findings provided support for the guidelines for a non‐memory MCI group proposed by Petersen et al.23 Although, as a group, the MCI‐MCDT had normal executive functions (T scores >40), there were five subjects (18%) whose executive domain was impaired (four MCI‐MCDT with memory deficits and one without). However, the deficits in the executive function domain were always associated with an abnormal test score in another cognitive domain.
The development of specific neuropsychological criteria for MCI types will then make it possible to evaluate incidence, prevalence, and determinants of the MCI types within and between populations. The brain morphology changes as measured by MRI or specific brain function abnormalities may further improve the description and could be an independent corroboration of MCI types. The classification of MCI types would then be useful to improve prediction of the subsequent risk of dementia and the type of dementia. Large sample sizes and longer follow up will be required to test the predictive power of classification of MCI types on predicting dementia. Finally, a classification of the type of MCI may provide a better approach to evaluating the efficacy of therapeutic options, especially for testing the efficacy of treatments in preventing the conversion from MCI to dementia. Although the MCI classification that we have used13 was developed from a small number of subjects, the cohort was well defined and the participants very carefully evaluated; nevertheless, independent corroboration is essential.
Acknowledgements
The research reported in this paper was supported by contracts N01‐HC‐85079 through N01‐HC‐85086, N01‐HC‐35129, and N01‐HC‐15103 from the National Heart, Lung, and Blood Institute, and grants AG15928 and AG20098 from the National Institute on Aging. JTB was supported by a Research Scientist Development Award ‐ Level II (MH07033).
Abbreviations
AACD - age associated cognitive decline
AAMI - age associated memory impairment
ARCD - age related cognitive decline
CES‐D - Center for Epidemiological Studies Depression Scale
CHS - Cardiovascular Health Study
IADL - instrumental activities of daily living
MCI‐MCDT - multiple cognitive deficits type of mild cognitive impairment
MCI - mild cognitive impairment
MCI‐AT - amnestic type of mild cognitive impairment
UPDRS - Unified Parkinson's Disease Rating Scale
3MSE - modified Mini‐Mental State Examination
Footnotes
Competing interests: none declared
References
- 1.Petersen R C, Smith G E, Waring S C.et al Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 199956303–308. [DOI] [PubMed] [Google Scholar]
- 2.Morris J C, Storandt M, Miller J P.et al Mild cognitive impairment represents early‐stage Alzheimer disease. Arch Neurol 200158397–405. [DOI] [PubMed] [Google Scholar]
- 3.Hanninen M A, Hallikainen M, Koivisto K.et al Decline of frontal lobe functions in subjects with age‐associated memory impairment. Neurology 199748148–153. [DOI] [PubMed] [Google Scholar]
- 4.Hanninen T, Hallikainen M, Koivisto K.et al A follow‐up study of age‐associated memory impairment: neuropsychological predictors of dementia. J Am Geriatr Soc 1995431007–1015. [DOI] [PubMed] [Google Scholar]
- 5.Rasquin S M C, Lodder J, Visser P J.et al Predictive accuracy of MCI subtypes for Alzheimer's disease and vascular dementia in subjects with mild cognitive impairment: a 2‐year follow‐up study. Dement Geriatr Cogn Disord 200519113–119. [DOI] [PubMed] [Google Scholar]
- 6.Petersen R C, Smith G E, Waring S C.et al Aging, memory, and mild cognitive impairment. Int Psychogeriatr 19979(suppl 1)65–69. [DOI] [PubMed] [Google Scholar]
- 7.Crook T H, Bartus R T, Ferris S H.et al Age‐associated memory impairment: proposed diagnostic criteria and measures of clinical change. Report of a National Institute of Mental Health Work Group. Dev Neuropsychol 19862261–276. [Google Scholar]
- 8.Levy R. Aging‐associated cognitive decline. From the Aging‐Associated Cognitive Decline Working Party. Int Psychogeriatr 1994663–68. [PubMed] [Google Scholar]
- 9.American Psychiatric Association DSM‐IV: Diagnostic and statistic manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association, 1994
- 10.Bowen J, Teri L, Kukull W.et al Progression to dementia in patients with isolated memory loss. Lancet 1997349763–765. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder J, Kratz B, Pantel J.et al Prevalence of mild cognitive impairment in an elderly community sample. J Neural Transm Suppl 19985451–59. [DOI] [PubMed] [Google Scholar]
- 12.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment. A population‐based validation study. Neurology 20015637–42. [DOI] [PubMed] [Google Scholar]
- 13.Lopez O L, Jagust W J, DeKosky S T.et al Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study. Part 1. Arch Neurol 2003601385–1389. [DOI] [PubMed] [Google Scholar]
- 14.Unverzagt F W, Gao S, Baiyewu O.et al Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology 2001571655–1662. [DOI] [PubMed] [Google Scholar]
- 15.Larrieu S, Letenneur L, Orgogozo J M.et al Incidence and outcome of mild cognitive impairment in a population‐based prospective cohort. Neurology 2002591594–1599. [DOI] [PubMed] [Google Scholar]
- 16.Ganguli M, Dodge H H, Shen C.et al Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology 200463115–121. [DOI] [PubMed] [Google Scholar]
- 17.Coria F, Gomez de Caso J A, Mingues L.et al Prevalence of age‐associated memory impairment and dementia in a rural community. J Neurol Neurosurg Psychiatry 199356973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koivisto K, Reinikainen K J, Hanninen T.et al Prevalence of age‐associated memory impairment in a randomly selected population from eastern Finland. Neurology 199545741–747. [DOI] [PubMed] [Google Scholar]
- 19.Barker A, Jones R, Jennison C. A prevalence study of age‐associated memory impairment. Br J Psychiatry 1995167642–648. [DOI] [PubMed] [Google Scholar]
- 20.Lambon‐Ralph M A, Patterson K, Graham N.et al Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer's disease: a cross‐sectional and longitudinal study of 55 cases. Brain 20031262350–2362. [DOI] [PubMed] [Google Scholar]
- 21.Collie A, Maruff P, Currie J. Behavioral characterization of mild cognitive impairment. J Clin Exp Neuropsychol 200224720–733. [DOI] [PubMed] [Google Scholar]
- 22.Richards M, Touchon J, Ledesert B.et al Cognitive decline in ageing: are AAMI and ACD distinct entities? Int J Geriatr Psychiatry 199914534–540. [DOI] [PubMed] [Google Scholar]
- 23.Petersen R C, Doody R, Kurz A.et al Current concepts in mild cognitive impairment. Arch Neurol 2001581985–1992. [DOI] [PubMed] [Google Scholar]
- 24.Fried L P, Borhani N O, Enright P.et al The Cardiovascular Health Study: design and rationale. Ann Epidemiol 19911263–276. [DOI] [PubMed] [Google Scholar]
- 25.Teng E L, Chui H C. The modified mini‐mental state (3MS) examination. J Clin Psychiatry 198748314–318. [PubMed] [Google Scholar]
- 26.Wechsler D.Wechsler adult intelligence scale–revised. New York: The Psychological Corporation, 1981
- 27.Benton A L. The visual retention test as a constructional praxis task. Confin Neurol 1967291–16. [DOI] [PubMed] [Google Scholar]
- 28.Gallo J J, Breitner J C S. Alzheimer's disease in the NAS‐NRC Registry of ageing twin veterans, IV. Performance characteristics of a two‐stage telephone screening procedure for Alzheimer's dementia. Psychol Med 1995251211–1219. [DOI] [PubMed] [Google Scholar]
- 29.Jorm A F, Jacomb P A. The informant questionnaire on cognitive decline in the elderly (IQCODE): socio‐demographic correlates, reliability, validity and some norms. Psychol Med 1989191015–1022. [DOI] [PubMed] [Google Scholar]
- 30.Kawas C, Segal J, Stewart W F.et al A validation study of the dementia questionnaire. Arch Neurol 199451901–906. [DOI] [PubMed] [Google Scholar]
- 31.Fried L P, Kronmal R A, Newman A B.et al Risk factors for 5‐year mortality in older adults: the cardiovascular health study. JAMA 1998279585–592. [DOI] [PubMed] [Google Scholar]
- 32.Lopez O L, Kuller L H, Fitzpatrick A.et al Evaluations of dementia in the cardiovascular health cognition study. Neuroepidemiology 2003221–12. [DOI] [PubMed] [Google Scholar]
- 33.Fillenbaum G G. Screening the elderly: a brief instrument activities of daily living. J Am Geriatr Soc 198533698–706. [DOI] [PubMed] [Google Scholar]
- 34.Katz S, Ford A B, Moskowitz R W.et al The index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963185914–919. [DOI] [PubMed] [Google Scholar]
- 35.Bryan R N, Wells S W, Miller T J.et al Infarct‐like lesions in the brain: prevalence and anatomic characteristics at MR imaging of the elderly – data from the Cardiovascular Health Study. Radiology 199720247–54. [DOI] [PubMed] [Google Scholar]
- 36.Longstreth W T, Manolio T A, Arnold A.et al Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. Stroke 1996271274–1282. [DOI] [PubMed] [Google Scholar]
- 37.Nelson H E.National adult reading test. Windsor, England: NFER‐Nelson, 1982
- 38.Raven J C.Coloured progressive matrices. Los Angeles: Western Psychological Services, 1956
- 39.Delis D C, Kramer J H, Kaplan E.et alThe California verbal learning test. New York: Psychological Corporation, 1987
- 40.Saxton J A, Becker J T, Wisniewski S. The ROCF and dementia. In: Knight JA, editor. The handbook of Rey‐Osterrieth complex figure usage: clinical and research applications. Lutz, FL: Psychological Assessment Resources Inc, 2003569–582.
- 41.Huff F J, Collins C, Corkin S.et al Equivalent forms of the Boston naming test. J Clin Exp Neuropsychol 19868556–562. [DOI] [PubMed] [Google Scholar]
- 42.Benton A L. Differential behavioral effects in frontal lobe disease. Neuropsychologia 1968653–60. [Google Scholar]
- 43.Baddeley A D, Della Sala S, Papagno C.et al Dual task performance in dysexecutive and non‐dysexecutive patients with a frontal lesion. Neuropsychology 199711187–194. [DOI] [PubMed] [Google Scholar]
- 44.Trenerry M R, Crosson B, DeBoe J.et alSTROOP neuropsychological screening test. Odessa, FL: Psychological Assessment Resources, 1989
- 45.Reitan R M. Validity of the Trail Making test as an indicator of organic brain damage. Percep Mot Skills 19588271–276. [Google Scholar]
- 46.Klove H. Clinical neuropsychology. In: Forster FM, editor. Medical Clinics of North America. New York: WB Saunders, 1963 [PubMed]
- 47.Fahn S, Elton R I. UPDRS Development Committee: Unified Parkinsons Rating Scale. In: Fahn S, Marsden CD, Caine D, Goldstein M, editors. Recent developments in Parkinson's disease. Florham Park: MacMillan Healthcare Information, 1987153–163.
- 48.Hachinski V C, Iliff L D, Zihka E.et al Cerebral blood flow in dementia. Arch Neurol 197532632–637. [DOI] [PubMed] [Google Scholar]
- 49.Cummings J L, Mega M, Gray K.et al The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994442308–2314. [DOI] [PubMed] [Google Scholar]
- 50.Heaton R K, Grant I, Matthews C G. Differences in neuropsychological test performance associated with age, education, and sex. In: Grant I, Adams MK, editors. Neuropsychological assessment of neuropsychiatric disorders. New York: Oxford University Press, 1986100–120.
- 51.Bell‐McGinty S, Lopez O L, Cidis‐Meltzer C.et al Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol 2005621393–1397. [DOI] [PubMed] [Google Scholar]