Abstract
Objective
To investigate the influence of deep white matter hyperintensities (DWMH) and periventricular white matter hyperintensities (PVWMH) on progression of cognitive decline in non‐demented elderly people.
Methods
All data come from the nested MRI sub‐study of the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). We performed a 3 year follow up study on 554 subjects of the PROSPER study using both repeated magnetic resonance imaging and cognitive testing. Cognitive decline and its dependency on WMH severity was assessed using linear regression models adjusted for sex, age, education, treatment group, and test version when applicable.
Results
We found that the volume of PVWMH at baseline was longitudinally associated with reduced mental processing speed (p = 0.0075). In addition, we found that the progression in PVWMH volume paralleled the decline in mental processing speed (p = 0.024). In contrast, neither presence nor progression of DWMH was associated with change in performance on any of the cognitive tests.
Conclusion
PVWMH should not be considered benign but probably underlie impairment in cognitive processing speed.
Keywords: MRI, aging, cognition, white matter hyperintensities
Increasing age, cerebrovascular disease, and risk factors are associated with presence and severity of white matter hyperintensities (WMH) in the brain.1,2 Although the clinical significance of these WMH remains to be fully elucidated, several cross sectional studies on the topic have found associations between the presence and severity of WMH and deficits in global and selective cognitive functioning.3,4,5,6,7
Findings from longitudinal studies on the role of WMH in the aetiology of cognitive decline are conflicting. Some large longitudinal population based studies have reported that the presence of WMH at baseline is associated with the rate of cognitive decline.8,9,10 However, few studies have examined longitudinal cognitive performance in combination with serial magnetic resonance imaging (MRI) measurements.11,12,13,14 In contrast with single MRI studies, most of these found no association between change in WMH and the course of cognitive functioning.11,12,13
White matter hyperintensities can be localised to two anatomically distinct regions: (a) the area under the cortex (subcortical or deep; DWMH) and (b) the area adjacent to the ventricles (periventricular; PVWMH). The distinction between DWMH and PVWMH is of clinical significance as they have been associated with different clinical consequences.5,15
So far, the progression of different types of WMH (deep and periventricular) in relation to longitudinal cognitive performance has not been studied in a large sample of subjects.14 We undertook a 3 year follow up study with both repeated MRI and cognitive testing to investigate the association between the presence and progression of DWMH and PVWMH and cognitive decline in a large sample of non‐demented elderly.
METHODS
Setting
The data in this report were drawn from the MRI sub study of the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER), a randomised, double blind, placebo controlled trial to test the hypothesis that treatment with pravastatin (40 mg/day) reduces the risk of vascular disease in elderly patients with pre‐existing vascular disease or with significant risk of developing it.16
Subjects
Inclusion and exclusion criteria of the PROSPER study have been described in detail elsewhere.16,17 Briefly, men and women aged 70–82 years were recruited if they had either pre‐existing vascular disease or a raised risk of such disease because of smoking, hypertension, or diabetes. Individuals with poor cognitive function at baseline (Mini Mental State Examination <24) were not eligible to participate. Of the 1100 Dutch participants in the PROSPER study, 646 consented for this sub‐study. From these original 646 subjects, 92 dropped out. Seven participants were claustrophobic during the first MRI and two did not undergo MRI due to technical problems. By the time of follow up, 40 subjects had died, 3 shad a contraindication for MRI, 6 had withdrawn informed consent, and 34 refused a second MRI because of claustrophobia or illness. In total, 554 subjects underwent MRI at baseline and follow up and had annual cognitive evaluation. The age range of the included subjects was 70–82 years at study entry. Loss of participants to follow up was studied. Compared with the follow up participants, those who dropped out performed worse on the Letter Digit Coding Test (LDCT) and Stroop test, had higher total WMH volumes, and were more likely to have a history of myocardial infarction.
Image acquisition
Dual fast spin echo images (echo time 27/120 ms, repetition time 3000 ms, 48 contiguous 3 mm slices without an interslice gap, matrix 256×256, field of view 220, acquisition percentage 80%) and fluid attenuated inversion recovery (FLAIR) images were obtained from all 554 subjects at baseline and after a mean (SD) follow up of 33 (1.4) months. MRI was performed on a clinical system operating at 1.5 T field strength (Philips Medical Systems, Best, The Netherlands).
Image postprocessing
For postprocessing, the Dual Echo MR images were transferred to an offline workstation. Quantification of WMH volumes was performed using semi‐automated software developed in house (Department of Radiology, Division of Image Processing).18 By combining fuzzy clustering, connectivity rules and mathematical morphology, WMH segmentations were generated automatically. WMH were defined as hyperintense lesions on both proton density and T2 weighted images. WMH connected to the lateral ventricles were labelled as PVWMH. WMH not connected to the lateral ventricles were labelled as DWMH (fig 1).
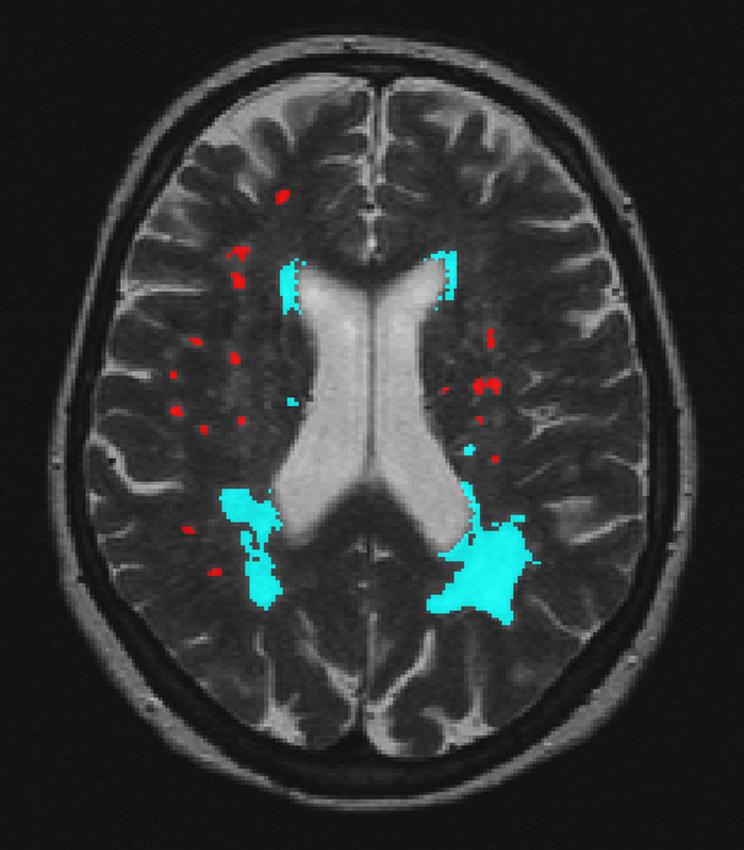
Figure 1 Computerised semiautomatic detection system for quantification of volumes of deep and periventricular white matter hyperintensities. Red, deep white matter hyperintensities; blue, periventricular white matter hyperintensities.
To correct for incidental inclusion of cerebrospinal fluid and grey matter, these automatically generated WMH segmentations were edited manually by two trained raters (DMJvdH and VHtD). Moreover, FLAIR hardcopies were used as a reference to rule out other pathogenesis and the entanglement of WMH with Virchow‐Robin spaces. Infratentorial lesions (brain stem and cerebellum) were excluded.
To prevent the possibility of overreading WMH progression in a direct scan comparison setting, we analysed baseline and follow up MRI in random order. Fifteen MRI scans were segmented twice to assess the intra‐rater and inter‐rater reliability of the volumetric WMH measurements. Intraclass correlation coefficients for PVWMH and DWMH volumes were all >0.99. In addition, eight participants were scanned and rescanned (with repositioning) in one MRI session, a procedure that gives a measure of the reliability of the measurement of WMH change. Using the scan–rescan data, we found intraclass correlation coefficients of 0.90 for DWMH and 0.82 for PVWMH.
Other MRI measurements
Incident brain infarction during follow up was assessed. Infarction was defined as a parenchyma defect seen on a FLAIR scan with the same signal intensity as cerebrospinal fluid and following vascular distribution.
Neuropsychological assessment
Cognitive functioning was assessed using a battery of cognitive function tests administered at baseline and at end of follow up.19 The battery was designed to assess global cognitive functioning with a specific focus on memory and executive functioning. Memory functioning was estimated with the Picture Word Learning Test (PWLT).19 Tests of executive functioning and attention included the LDCT and the abbreviated Stroop Color Word Test.20 The PWLT was derived from the Fifteen Words Test,21 originally described by Rey. In this test, 15 pictures are presented sequentially to the subject, who is asked to recall as many objects as possible. This process is repeated three times and, after 20 minutes, delayed recall is tested. The LDCT we used is a modification of the procedurally identical Symbol Digits Modalities Test, used to measure the speed of processing of general information.19 The Stroop test has often been used to test selective attention and speed of processing; subjects are asked to successively read sheets with (a) colour names, (b) coloured patches and (c) colour names printed in incongruously coloured ink. In the latter, subjects have to name the colour of the word, not the word itself. In our analyses we used the average number of words generated in the three immediate recall conditions (PWLTimm), the number of words generated in the delayed recall condition (PWLTdel), the number of correct digits for the LDCT, and time to complete the colour interference section of the Stroop test.
Statistical analysis
Statistical analyses were performed using SAS (SAS Institute, Cary, NC, USA). Summary statistics of cognitive test results and WMH measurements at baseline and follow up are reported as mean (SD). Baseline WMH measurements were split into three strata reflecting WMH severity (low, intermediate, high; appendix). These strata were specified for both DWMH and PVWMH separately. We then calculated difference scores at each subject's final visit (follow up minus baseline) for each cognitive test. Cognitive decline and its dependency on WMH severity was then assessed by comparison of these difference scores using linear regression models. To investigate whether progression of WMH was accompanied by an alteration in the rate of cognitive decline we repeated this procedure with strata based on change in WMH after 3 years of follow up.
One of the advantages of difference scores is that they are normally distributed, even when the original outcomes are not, thereby fulfilling a major condition for parametric analyses.19 All analyses were adjusted for sex, age, education, treatment group, and test version when applicable. Furthermore, in the second analyses we also adjusted for incident brain infarction of the participants (n = 66) to estimate the independent effect of WMH on cognitive decline. The level of significance was set at p<0.05.
RESULTS
Subjects' characteristics at baseline are shown in table 1. Mean (SD) age of the 554 participants was 75 (3.2) years, and 44% were women. Mean (SD) age at which subjects left school was 15.5 (2.9) years. Table 2 presents the neuropsychological test results and characteristics of WMH for all subjects at baseline and at follow up.
Table 1 Baseline characteristics of study sample (n = 554).
| Men (n = 313) | Women (n = 241) | p | ||||
|---|---|---|---|---|---|---|
| Continuous variates (mean, SD) | ||||||
| Age (years) | 74.5 (3.1) | 75.6 (3.2) | <0.0001 | |||
| Systolic blood pressure (mmHg) | 158.4 (22.6) | 156.7 (20.4) | 0.42 | |||
| Diastolic blood pressure (mmHg) | 85.8 (10.8) | 85.9 (11.4) | 0.97 | |||
| Total cholesterol (mmol/l) | 5.5 (0.8) | 6.1 (0.9) | <0.0001 | |||
| LDL cholesterol (mmol/l) | 3.8 (0.7) | 4.1 (0.8) | <0.0001 | |||
| HDL cholesterol (mmol/l) | 1.2 (0.3) | 1.3 (0.3) | <0.0001 | |||
| Triglycerides (mmol/l) | 1.4 (0.7) | 1.6 (0.6) | 0.02 | |||
| Categorical variates (n,%) | ||||||
| Current smoker | 84 (26.8) | 31 (12.7) | <0.0001 | |||
| History of diabetes | 54 (17.3) | 37 (15.4) | 0.55 | |||
| History of hypertension | 164 (52.4) | 186 (77.2) | <0.0001 | |||
| History of myocardial infarction | 52 (16.6) | 15 (6.2) | 0.0002 | |||
| History of stroke or TIA | 49 (15.7) | 41 (17.0) | 0.67 | |||
| History of any vascular disease | 153 (48.9) | 88 (36.5) | 0.0036 | |||
| Baseline MRI stroke | 122 (39.2) | 91 (38.4) | 0.84 | |||
| Pravastatin | 150 (47.9) | 125 (51.9) | 0.36 | |||
Two sample t test used for continuous variates and χ2 test for categorical variates. †Total, periventricular, and DWMH not normally distributed, Mann‐Whitney test used.
Table 2 Cognitive test results and MRI characteristics of study subjects.
| All subjects (n = 554) | Baseline | Follow up | p | |||
|---|---|---|---|---|---|---|
| Cognitive measures* | ||||||
| Global | ||||||
| Mini Mental State Examination (points) | 28.2 (1.5) | 28.5 (2.0) | 0.0002 | |||
| Memory | ||||||
| Immediate Picture Word Learning (words) | 10.1 (1.8) | 10.2 (2.2) | 0.69 | |||
| Delayed Picture Word Learning (words) | 11.2 (2.6) | 11.1 (3.0) | 0.25 | |||
| Cognitive speed | ||||||
| Letter Digit (digits/minute) | 27.7 (7.1) | 26.3 (7.4) | <0.0001 | |||
| Stroop (seconds) | 55.0 (17.7) | 56.9 (23.3) | 0.074 | |||
| MRI measures† | ||||||
| DWMH (ml) | 1.11 (1.65) | 1.53 (2.16) | <0.0001 | |||
| PVWMH (ml) | 4.12 (8.49) | 5.75 (9.99) | <0.0001 |
Data are presented as means (SD). WMH; white matter hyperintensities. p value from: *paired t test; †Wilcoxon signed rank test.
To determine whether volume of WMH at baseline was related to rate of cognitive decline, the difference in cognitive performance over three years of follow up between WMH severity groups was studied. We found that a higher PVWMH volume at baseline was significantly associated with more time to complete the Stroop test—that is, reduced cognitive speed (table 3). Pairwise comparisons indicated that subjects with intermediate PVWMH load needed more time to complete the Stroop test compared with the subjects with low PVWMH load (mean difference 5.10, 95% confidence interval (CI) 1.91 to 8.29, p = 0.0018). There was also a trend toward those with the highest PVWMH load requiring more time to complete the Stroop compared with the subjects with low PVWMH load (mean difference 3.00, 95% CI −0.46 to 6.45, p = 0.090). Baseline DWMH volume was not associated with change in performance on any of the cognitive tests.
Table 3 Comparison of strata of baseline DWMH and PVWMH with change in cognitive functioning, and of change in total DWMH and PVWMH volumes with change in cognitive functioning.
| Memory | Cognitive speed | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PWLTimm (words) | p | PWLTdel (words) | p | LDCT (digits/min) | p | Stroop (seconds) | p | |||||||||||
| Baseline WMH (ml) | ||||||||||||||||||
| DWMH | ||||||||||||||||||
| Low | −0.03 (0.11) | −0.28 (0.16) | −1.42 (0.26) | 0.57 (0.98) | ||||||||||||||
| Intermediate | 0.03 (0.16) | −0.08 (0.22) | −1.17 (0.37) | 2.12 (1.36) | ||||||||||||||
| High | 0.09 (0.16) | 0.84 | 0.08 (0.23) | 0.43 | −1.44 (0.37) | 0.83 | 0.68 (1.43) | 0.63 | ||||||||||
| PVWMH | ||||||||||||||||||
| Low | 0.09 (0.14) | −0.10 (0.19) | −1.09 (0.31) | −1.74 (1.17) | ||||||||||||||
| Intermediate | 0.02 (0.13) | −0.18 (0.19) | −1.42 (0.31) | 3.36 (1.13) | ||||||||||||||
| High | −0.09 (0.15) | 0.70 | −0.13 (0.21) | 0.96 | −1.63 (0.35) | 0.50 | 1.26 (1.30) | 0.0075a | ||||||||||
| Change in WMH (ml) | ||||||||||||||||||
| DWMH | ||||||||||||||||||
| Low | 0.16 (0.12) | 0.10 (0.18) | −1.50 (0.29) | 0.11 (1.09) | ||||||||||||||
| Intermediate | −0.07 (0.14) | −0.32 (0.19) | −0.91 (0.32) | 0.42 (1.21) | ||||||||||||||
| High | −0.06 (0.16) | 0.37 | −0.14 (0.22) | 0.26 | −1.69 (0.37) | 0.22 | 2.92 (1.39) | 0.25 | ||||||||||
| PVWMH | ||||||||||||||||||
| Low | 0.12 (0.12) | 0.16 (0.17) | −0.98 (0.28) | −1.26 (1.07) | ||||||||||||||
| Intermediate | 0.06 (0.16) | −0.20 (0.23) | −1.63 (0.38) | 2.26 (1.41) | ||||||||||||||
| High | −0.12 (0.14) | 0.39 | −0.37 (0.19) | 0.12 | −1.66 (0.32) | 0.20 | 2.85 (1.22) | 0.024a,b | ||||||||||
Data are presented as means (SE) and overall p‐values are reported. Significant contrasts between low, medium, and high WMH severity groups are indicated by alow–intermediate, blow–high and intermediate–high. All analyses were adjusted for sex, age, education, treatment group and test version when applicable. WMH; white matter hyperintensities. PWLTimm; immediate picture‐word learning. PWLTdel; delayed picture word learning. LDCT; Letter Digit Coding Test. Stroop; 40 item Stroop test.
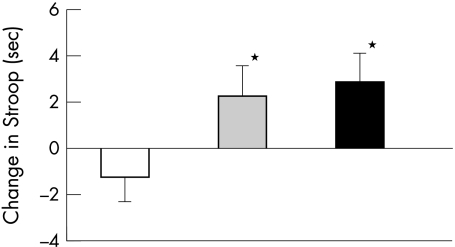
To determine whether progression in WMH volume was associated with rate of cognitive decline, we investigated the difference in cognitive decline between subjects with major, medium, or minor volume changes in WMH after 3 years follow up. A larger progression in total PVWMH volume was consistently associated with more time to complete the Stroop test (table 3; fig 2). Compared with subjects with minor changes in PVWMH, subjects with medium changes needed more time to complete the Stroop test (difference 3.52, 95% CI 0.037 to 7.00, p = 0.048), as did subjects with major changes (difference 4.10, 95% CI 0.91 to 7.30, p = 0.012) (fig 2).
Figure 2 Comparison of strata of 3 year change in PVWMH with 3 year change in performance on Stroop. Mean (SE) raw increase in time to complete the Stroop testare presented. Strata of PVWMH progress: white bar, low; grey bar, intermediate; black bar, high. *p<0.05 compared with low.
After adjustment for incident brain infarction, the association between baseline PVWMH volume and performance on the Stroop test remained significant (p = 0.012), whereas the association between change in PVWMH volume and performance on the Stroop test was no longer significant (p = 0.13). However, when we repeated the latter analyses for subjects with no or minor WMH versus subjects with medium or major WMH we found a significant association between change in PVWMH and change in performance on the Stroop test (p = 0.045).
It could be argued that people with larger brains might have higher absolute WMH volumes. Therefore, we repeated all analyses with WMH volumes expressed as a percentage of parenchyma volume. All observed associations between larger PVWMH volumes and the increased time to complete the Stroop test remained unaltered. Moreover, when we analysed the data with continuous instead of three strata of WMH, both associations between larger PVWMH volumes and the increased time to complete the Stroop test remained, while all other associations were absent.
DISCUSSION
In the present longitudinal study we investigated the role of PVWMH and DWMH in the aetiology of cognitive decline. We found that the volume of PVWMH at baseline was longitudinally associated with reduced mental processing speed. Moreover, we showed that the progression of PVWMH actually paralleled the decline in mental processing speed. This indicates that PVWMH probably causes a decline in mental processing speed.
A number of studies have addressed the association between presence of WMH and cognitive impairment in elderly subjects. Speed of mental processing and attention were found to be mostly affected in the elderly.5,6,7,22,23,24 When type of WMH was taken into account, the presence of PVWMH rather than DWMH was associated with the impairment of cognitive functions, in particular those cognitive functions that involve speed.5 Our longitudinal findings are in line with these cross sectional observations.
There have been few investigation using both repeated cognitive and repeated WMH measurements,11,12,13,14 most of which found no association between WMH progression and course of cognitive functioning.11,12,13 In contrast with these findings, we observed a significant association between the progress in PVWMH volume and the decline in mental processing speed. Methodological differences might explain these contrasting results. Firstly, discrepancies may stem from the use of different scales to assess WMH. The negative studies by Schmidt,11 Wohl,12 and Wahlund13 used visual rating scales, whereas we used a volumetric method to assess WMH. Volumetric WMH measurements are more objective and reliable, and thus provide a more accurate measurement of WMH.25 Visual assessment of WMH progression has limitations and may lead to underestimation.26 This underestimation of the progression of WMH volume might have contributed to inadequate power to detect the effect of WMH progression on cognitive functioning in earlier studies. Secondly, the Schmidt,11 Wohl12 and Wahlund13 studies investigated total WMH in relation to cognitive functioning. As we distinguished between DWMH and PVWMH, it is therefore possible that the previous obscured effect of progress in PVWMH volume on cognitive decline could be revealed in our study.
So far, only Cook et al have observed an association between WMH progression and cognitive decline.14 As in our present study, they used serial, quantitative MRI measurements of both PVWMH and DWMH. They found that, in a sample of 29 healthy elderly subjects, PVWMH progression was associated with performance on a verbal fluency and abstract reasoning task at follow up. Our current findings also suggest that PVWMH but not DWMH are an important factor in cognitive decline. The set of cognitive tests that were used in our investigation are different from the set used by Cook et al; however, both sets included tests that have often been used to measure speed of mental processing (that is, the Stroop and Trailmaking tests). In contrast to our findings, Cook et al found no association between the progression of PVWMH volume and a reduction of mental processing speed. The psychometric properties of the tests (the Stroop is more sensitive to changes in mental processing speed than the Trailmaking test) might account for these different findings. This applies especially for the short version of the Stroop that was used in the present study, because test duration has been shown to have a clearcut effect on age related differences.20 Additionally, in other recent studies, the shorter version of the Stroop appears to be more sensitive.27 The lack of statistical power could also play a role.
Our findings have a strong biological plausibility. White matter tracts support the functioning of the cognitive processes that reside within the different cortical and subcortical brain areas.28 Damage to these tracts results in inefficient neural activity that could lead to, in the first instance, cognitive slowing rather than apparent cognitive dysfunction. Furthermore, reduced mental processing speed is repeatedly observed in multiple sclerosis, which is primarily a white matter disorder.29,30,31
Why PVWMH but not DWMH are associated with cognitive decline is not clear. De Groot et al5 proposed that DWMH might predominantly disrupt the short association fibres, also known as U or arcuate fibres, that link adjacent gyri. Periventricular WMH probably affect the long association fibres that connect the more distant cortical areas. It is relevant that a decrease in cognitive speed has been related to subcortical mechanisms. The ascending fibre system consisting of long white matter tracks to the cortex is thought to underlie attentional mechanisms and the speed and efficiency at which other cognitive tasks are executed. The connections to and from the prefrontal cortex are important.32 Performance on the cognitive tests that are generally used for clinical research depends more on the connection between multiple cortical areas, which are not necessarily adjacent, and thus depends mainly on the long association tracts.
There is a sequence in cognitive decline at old age. In the elderly, reduced cognitive speed is thought to manifest itself first, while other cognitive domains, such as memory, remain relatively intact until the later stages of cognitive decline.32,33,34 Our findings are in line with this sequence. We found that, in a sample of initially cognitive healthy subjects, the progression of PVWMH volume was associated with reduced cognitive speed, but not with memory.
Our study benefits from the large series of baseline and follow up scans and neuropsychological evaluations that were analysed to measure both change in WMH and cognitive functioning over time. Moreover, we used a reliable semiautomated volumetric method of quantifying DWMH and PVWMH. The limitations of this study include the relatively short follow up period. With short follow up periods both cognitive decline and WMH progression are likely to be small, therefore, the association between WMH and cognitive decline might have been underestimated. However, it is therefore even more striking that we found an association between WMH and reduced mental processing speed.
In conclusion, in the present study we found supporting evidence for the role of PVWMH as a causal factor in the decline of cognitive speed. We therefore suggest that PVWMH in elderly subjects should not be considered benign.
ACKNOWLEDGEMENTS
Dr N Schmitz and Dr W M van der Flier reviewed the manuscript and provided valuable suggestions regarding the interpretation of the data. The assistance of R J van Bommel, N M van den Burg, A C G M van Es and A Navabi in data collection is deeply appreciated. This work was supported by an unrestricted research grant from Bristol‐Myers Squibb, USA
Abbreviations
DWMH - deep white matter hyperintensities
FLAIR - fluid attenuated inversion recovery
LDCT - Letter Digit Coding Test
MRI - magnetic resonance imaging
PROSPER - PROspective Study of Pravastatin in the Elderly at Risk
PVWMH - periventricular white matter hyperintensities
WMH - white matter hyperintensities
Appendix
Table A Cutoff values for the strata of baseline and change in WMH volumes.
| Low | Intermediate | High | ||||
|---|---|---|---|---|---|---|
| Baseline WMH | ||||||
| Total WMH | ||||||
| Volume (ml) | 0–1 | >1–5 | >5 | |||
| No. | 209 | 180 | 154 | |||
| DWMH | ||||||
| Volume (ml) | 0–0.5 | >0.5–1.5 | >1.5 | |||
| No. | 275 | 138 | 130 | |||
| PVWMH | ||||||
| Volume (ml) | 0–0.5 | >0.5–3.5 | >3.5 | |||
| No. | 191 | 196 | 156 | |||
| Change in WMH | ||||||
| Total WMH | ||||||
| Volume (ml) | ⩽0.5 | >0.5–2 | >2 | |||
| No. | 213 | 140 | 182 | |||
| DWMH | ||||||
| Volume (ml) | ⩽0.1 | >0.1–0.5 | >0.5 | |||
| No. | 222 | 176 | 137 | |||
| PVWMH | ||||||
| Volume (ml) | ⩽0.4 | >0.4–1.5 | >1.5 | |||
| No. | 230 | 127 | 178 | |||
Footnotes
Competing interests: none
References
- 1.Ylikoski A, Erkinjuntti T, Raininko R.et al White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 1995261171–1177. [DOI] [PubMed] [Google Scholar]
- 2.Awad I A, Spetzler R F, Hodak J A.et al Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke 1981171084–1089. [DOI] [PubMed] [Google Scholar]
- 3.Longstreth W T, Jr, Manolio T A, Arnold A.et al Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke 1996271274–1282. [DOI] [PubMed] [Google Scholar]
- 4.Matsubayashi K, Shimada K, Kawamoto A.et al Incidental brain lesions on magnetic resonance imaging and neurobehavioral functions in the apparently healthy elderly. Stroke 199223175–180. [DOI] [PubMed] [Google Scholar]
- 5.De Groot J C, De Leeuw F E, Oudkerk M.et al Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol 200047145–151. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt R, Fazekas F, Offenbacher H.et al Neuropsychologic correlates of MRI white matter hyperintensities: a study of 150 normal volunteers. Neurology 1993432490–2494. [DOI] [PubMed] [Google Scholar]
- 7.Ylikoski R, Ylikoski A, Erkinjuntti T.et al White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol 199350818–824. [DOI] [PubMed] [Google Scholar]
- 8.Garde E, Mortensen E L, Krabbe K.et al Relation between age‐related decline in intelligence and cerebral white‐matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 2000356628–634. [DOI] [PubMed] [Google Scholar]
- 9.Kuller L H, Shemanski L, Manolio T.et al Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke 199829388–398. [DOI] [PubMed] [Google Scholar]
- 10.De Groot J C, De Leeuw F E, Oudkerk M.et al Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol 200252335–341. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt R, Fazekas F, Kapeller P.et al MRI white matter hyperintensities: three‐year follow‐up of the Austrian Stroke Prevention Study. Neurology. 199913 53132–139. [DOI] [PubMed] [Google Scholar]
- 12.Wohl M A, Mehringer C M, Lesser I M.et al White matter hyperintensities in healthy older adults: a longitudinal study. Int J Geriatr Psychiatry 19949273–277. [Google Scholar]
- 13.Wahlund L O, Almkvist O, Basun H.et al MRI in successful aging, a 5‐year follow‐up study from the eighth to ninth decade of life. Magn Reson Imaging 199614601–608. [DOI] [PubMed] [Google Scholar]
- 14.Cook I A, Leuchter A F, Morgan M L.et al Longitudinal progression of subclinical structural brain disease in normal aging. Am J Geriatr Psychiatry 200412190–200. [PubMed] [Google Scholar]
- 15.O'Brien J, Desmond P, Ames D.et al A magnetic resonance imaging study of white matter lesions in depression and Alzheimer's disease. Br J Psychiatry 1996168477–485. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd J, Blauw G J, Murphy M B.et al The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol. 199915 841192–1197. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd J, Blauw G J, Murphy M B.et al Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 20023601623–1630. [DOI] [PubMed] [Google Scholar]
- 18.van der Flier W M, Middelkoop H A, Weverling‐Rijnsburger A W.et al Interaction of medial temporal lobe atrophy and white matter hyperintensities in AD. Neurology 2004621862–1864. [DOI] [PubMed] [Google Scholar]
- 19.Houx P J, Shepherd J, Blauw G J.et al Testing cognitive function in elderly populations: the PROSPER study. PROspective Study of Pravastatin in the Elderly at Risk. J Neurol Neurosurg Psychiatry 200273385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein M, Ponds R W, Houx P J.et al Effect of test duration on age‐related differences in Stroop interference. J Clin Exp Neuropsychol 19971977–82. [DOI] [PubMed] [Google Scholar]
- 21.Brand N, Jolles J. Learning and retrieval rate of words presented auditorily and visually. J Gen Psychol 1985112201–210. [DOI] [PubMed] [Google Scholar]
- 22.Junque C, Pujol J, Vendrell P.et al Leuko‐araiosis on magnetic resonance imaging and speed of mental processing. Arch Neurol 199 47151–156. [DOI] [PubMed] [Google Scholar]
- 23.Bès A, Gardeur D, Orgogozo J M.et al Leukoaraiosis intensity correlates with hypertension. Neurology 199444A298 [Google Scholar]
- 24.Breteler M M, van Amerongen N M, Van Swieten J C.et al Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke 1994251109–1115. [DOI] [PubMed] [Google Scholar]
- 25.Payne M E, Fetzer D L, MacFall J R.et al Development of a semi‐automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Res 200211563–77. [DOI] [PubMed] [Google Scholar]
- 26.Kapeller P, Barber R, Vermeulen R J.et al Visual rating of age‐related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke 200334441–445. [DOI] [PubMed] [Google Scholar]
- 27.den Hartog H M, Derix M M A, van Bemmel A L.et al Cognitive functioning in young and middle‐aged unmedicated out‐patients with major depression: testing the effort and cognitive speed hypotheses. Psycholog Medie 2003331–9. [DOI] [PubMed] [Google Scholar]
- 28.Mesulam M M. Large‐scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol 199028597–613. [DOI] [PubMed] [Google Scholar]
- 29.Rao S M, Aubin‐Faubert P, Leo G J. Information processing speed in patients with multiple sclerosis. J Clin Exp Neuropsychol 198911471–477. [DOI] [PubMed] [Google Scholar]
- 30.Grigsby J, Kaye K, Busenbark D. Alphanumeric sequencing: a report on a brief measure of information processing used among persons with multiple sclerosis. Percept Mot Skills 199478883–887. [DOI] [PubMed] [Google Scholar]
- 31.Rao S M. White matter disease and dementia. Brain Cogn 199631250–268. [DOI] [PubMed] [Google Scholar]
- 32.Tisserand D J, Jolles J. On the involvement of prefrontal networks in cognitive ageing. Cortex 2003391107–1128. [DOI] [PubMed] [Google Scholar]
- 33.Salthouse T A. Resouce reduction interpretation of cognitive aging. Developmental Review 19888238–272. [Google Scholar]
- 34.Birren J E, Schaie K W.Handbook of the psychology of aging. New York: Van Nostrand Reinhold, 1985