Abstract
Objective
We sought to determine the prevalence, clinical features, and laboratory characteristics of polyneuropathies in Waldenström's macroglobulinaemia (WM), a malignant bone marrow disorder with lymphocytes that produce monoclonal IgM.
Methods
We prospectively studied 119 patients with WM and 58 controls. Medical history was taken, and neurological examinations, electrodiagnostic tests, and serum studies were performed by different examiners who were blinded to results except the diagnosis of WM.
Results
Polyneuropathy symptoms, including discomfort and sensory loss in the legs, occurred more frequently (p<0.001) in patients with WM (47%) than in controls (9%). Patients with WM had 35% lower quantitative vibration scores, and more frequent pin loss (3.4 times) and gait disorders (5.5 times) than controls (all p<0.001). Patients with IgM binding to sulphatide (5% of WM) had sensory axon loss; those with IgM binding to myelin associated glycoprotein (MAG) (4% of WM) had sensorimotor axon loss and demyelination. Patients with WM with IgM binding to sulphatide (p<0.005) or MAG (p<0.001) had more severe sensory axon loss than other patients with WM. Demyelination occurred in 4% of patients with WM with no IgM binding to MAG. Age related reductions in vibration sense and sural SNAP amplitudes were similar (∼30%) in WM and controls.
Conclusions
Peripheral nerve symptoms and signs occur more frequently in patients with WM than controls, involve sensory modalities, and are often associated with gait disorders. IgM binding to MAG or sulphatide is associated with a further increase in the frequency and severity of peripheral nerve involvement. Age related changes, similar to those in controls, add to the degree of reduced nerve function in patients with WM.
Keywords: Waldenström's macroglobulinaemia, neuropathy, IgM, monoclonal antibody, gait disorder
Waldenström's macroglobulinaemia (WM) is a chronic neoplastic disorder with malignant bone marrow cells that produce a monoclonal IgM immunoglobulin (IgM M‐protein).1,2,3 Clinical features of WM are due to either infiltration of neoplastic cells or properties of the circulating IgM.1,2,3,4,5,6 Proliferation of neoplastic cells is associated with the prognosis for long term survival and many systemic features of WM, including cytopenia, lymphadenopathy, splenomegaly, and hepatomegaly. Disorders related to the circulating IgM often cause considerable morbidity5,6,7,8 and include amyloidosis, polyneuropathies, and disorders related to hyperviscosity, cryoglobulinaemia, and cold agglutinin.
Although polyneuropathies are rarely a presenting feature in WM,5 they are common and often symptomatic.1 Some polyneuropathies in WM may be related to specific antigenic targets of the monoclonal serum IgM, including myelin associated glycoprotein (MAG) and sulphatide.8,9,10 Other polyneuropathies in WM could be due to direct tumour cell infiltration, deposition of IgM in various tissues, the amount and properties of the circulating monoclonal IgM, or binding to unidentified antigens. Most studies of the types and frequency of polyneuropathies in WM are anecdotal or based on retrospective analysis of patients referred for evaluation of a neuropathy. We report the frequency and the clinical and electrodiagnostic features of polyneuropathies in a large prospective series of ambulatory patients with WM and compare the results with a group of control patients of similar ages.
PATIENTS AND METHODS
Patients with WM and controls were examined during a weekend in April 2003 at a meeting of the International Waldenström's Macroglobulinemia Foundation in Reston, Virginia. A diagnosis of WM in 119 patients was based on a history of WM, but not myeloma, other lymphomas, or monoclonal gammopathy of unknown significance (MGUS), and the presence of an IgM M‐protein in serum testing in our laboratory. Neurological histories, physical examinations (including motor, sensory, gait, and tendon reflex evaluation) and nerve conduction studies were performed by board certified neurologists. Examiners were blinded to the results of other testing, and to clinical information other than a diagnosis of WM. Clinical and electrophysiological assessments were performed in a random order. Strength was classified on a Medical Research Council scale. Deep tendon reflexes were classified as reduced if they were rated as +/− or absent. Assessment of pin sensation was accomplished with a safety pin. Vibration sense was quantitated using a graduated Rydel‐Seiffer 64 Hz tuning fork.11 Proprioception was evaluated at the great toe and graded as normal or abnormal. Tandem gait was measured as the number of heel to toe steps (to a maximum of eight) that the patient could perform along a straight line.
Nerve conduction studies were performed on XlTek EMG machines.11 All subjects had unilateral peroneal motor (recorded from the extensor digitorum brevis) and sural sensory nerve conduction studies. Results were corrected for skin temperature. Additional studies on the opposite leg and the arms were carried out in patients when initial results were abnormal. Normal values were defined based on standards set at the electrodiagnostic laboratory at Washington University. Results were assessed for the presence and severity, of motor and sensory axon loss and for features of demyelination.12 Axon loss was defined as sural sensory nerve action potentials (SNAPs) <5 μV or common peroneal compound motor action potentials (CMAPs) <2 mV. Severe axon loss was defined as absent SNAPs or peroneal CMAPs <1 mV. Demyelination was assessed according to standard criteria,12 except that a focal reduction of ⩾50% in CMAP amplitude was required to be considered conduction block.
The presence and type of serum M‐proteins were analysed by immunofixation methodology. ELISA and Western blot testing for IgM binding to antigens was performed as previously described13,14 on all serums without knowledge of clinical or electrophysiological data. All serums positive for binding to MAG or sulphatide had very high titres of IgM binding greater than 20 000 and showed evidence of clonality, with selective ELISA binding of κ or λ light chains to MAG or sulphatide that corresponded to the class of the serum IgM M‐proteins. Nerve pathology was not available for any patient.
Patients with WM (table 1) were subdivided into WM with IgM binding to MAG (WM‐MAG; n = 5), WM with IgM binding to sulphatide (WM‐S; n = 6), and other patients with WM without IgM binding to MAG or sulphatide. The latter subgroup, with IgM binding to neither identified target antigen, was further subdivided into patients with WM without (WM‐O) and with (WM‐O‐Dem; n = 4) demyelinating features on electrodiagnostic testing. The 58 controls were spouses or siblings of the patients, but were otherwise unselected. Serum IgM levels from our 119 WM patient serums were compared with serum from 39 consecutive patients identified in our neuromuscular clinic as having MGUS with IgM M‐proteins and 171 patients diagnosed with monoclonal macroglobulinaemia, most of whom had WM, at Baylor University Medical Center, Dallas.15
Table 1 Features of WM and control patients.
| Patients with WM | Controls | p | |||||
|---|---|---|---|---|---|---|---|
| Age (years), mean (SE) (range) | 63 (1) (38 to 81) | 61 (1) (43 to 78) | 0.2 | ||||
| Disease duration (years) | 4.3 (0.3) | NA | NA | ||||
| Polyneuropathy | |||||||
| Symptoms in feet | 47% | 9% | <0.001 | ||||
| Previous diagnosis | 22% | 0% | <0.001 | ||||
| Vibration, toes | 4.9 (0.3) | 7.1 (0.4) | <0.001 | ||||
| Age > 60 years | 4.3 (0.4) | 5.8 (0.5) | 0.04 | ||||
| Age < 60 years | 6.1 (0.6) | 8.4 (0.4) | 0.03 | ||||
| Pin loss | 37% | 11% | <0.001 | ||||
| Tandem gait abnormal | 31% | 5% | <0.001 | ||||
| Serum IgM level (g/l) (normal < 2.1) | 20 (1) | NA | NA |
NA, not available; WM: Waldenström's macroglobulinaemia. Data are mean (SE) unless otherwise stated.
Results were compared by Fisher's exact, χ2, two sided Wilcoxon signed ranks or t tests, and expressed as mean (SE).
RESULTS
Serum testing
Monoclonal IgM binding to MAG was found in five patients with WM (4%) and to sulphatide in six (5%). No WM‐O‐Dem patients had IgM binding to sulphated glucuronyl paragloboside (SGPG). The mean serum IgM level in patients with WM (table 1) was more than five times higher (p<0.001) than the IgM levels (4 (1) g/l) in our series of 39 patients with MGUS but similar to levels in patients with monoclonal macroglobulinaemia.15 Total serum IgM was similar in all WM subgroups (table 2).
Table 2 Clinical and electrophysiological features in WM subgroups and controls.
| Patients with WM | Control patients | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WM‐S | WM‐MAG | WM‐O‐Dem | WM‐O | |||||||||||||||
| p versus WM‐O | p versus WM‐O | p versus WM‐O | p versus control | |||||||||||||||
| General features | ||||||||||||||||||
| Patient age (years) | 69 (2) | 0.1 | 65 (5) | 0.7 | 66 (4) | 0.5 | 63 (1) | 0.2 | 61 (1) | |||||||||
| Disease duration (years) | 3.0 (0.8) | 0.4 | 6.4 (2.3) | 0.3 | 4.8 (0.3) | 0.5 | 4.3 (0.3) | NA | NA | |||||||||
| IgM levels (g/l) | 18 (5) | 7 | 18 (3) | 7 | 17 (10) | 4 | 21 (1) | NA | NA | |||||||||
| Examination features | ||||||||||||||||||
| Vibration | ||||||||||||||||||
| Toes | 1.8 (0.8) | 0.02 | 2.2 (1.2) | 0.06 | 0.3 (0.3) | 0.005 | 5.1 (0.3) | <0.001 | 7.1 (0.4) | |||||||||
| Fingers | 10.8 (1.4) | 0.8 | 8.8 (1.4) | 0.1 | 9.8 (0.9) | 0.2 | 11.1 (0.3 | <0.001 | 12.6 (0.3) | |||||||||
| Pin loss | 50% | 0.6 | 100% | 0.02 | 75% | 0.2 | 32% | 0.005 | 11% | |||||||||
| DTRs reduced | 33% | 0.6 | 100% | <0.001 | 100% | <0.001 | 16% | 0.3 | 9% | |||||||||
| Proprioception changes | 17% | 0.9 | 40% | 0.1 | 50% | 0.07 | 9% | 0.6 | 5% | |||||||||
| Toe weakness | 0% | 0.9 | 40% | 0.1 | 50% | 0.06 | 8% | 0.5 | 4% | |||||||||
| Tandem gait changes | 17% | 0.8 | 40% | 1.0 | 67% | 0.5 | 29% | 0.001 | 5% | |||||||||
| Sural sensory conductions | ||||||||||||||||||
| SNAP amp (μV) | 3.0 (1.9) | 0.08 | 0.4 (0.4 | 0.004 | 0 | 0.005 | 6.7 (0.5) | <0.001 | 9.2 (0.6) | |||||||||
| SNAP absent | 67% | 0.005 | 80% | 0.001 | 100% | <0.001 | 14% | 0.08 | 3% | |||||||||
| Peroneal motor conductions | ||||||||||||||||||
| CMAP amp (μV) | 2.1 (0.2) | 0.06 | 0.7 (0.2) | 0.001 | 0.3 (0.1) | <0.001 | 3.6 (0.2) | 0.4 | 3.9 (0.2) | |||||||||
| Nerve CV (m/s) | 47 (2) | 0.3 | 34 (3) | <0.001 | 32 (1) | <0.001 | 49 (1) | 0.08 | 51 (1) | |||||||||
| Distal latency (ms) | 5.2 (0.3) | 0.1 | 11.8 (1.4) | <0.001 | 8.6 (1.6) | 0.008 | 5.2 (0.1) | 1.0 | 5.0 (0.1) | |||||||||
Clinical and electrophysiological features in WM subgroups and controls. WM, Waldenström's macroglobulinaemia; WM‐S, patients with WM with serum monoclonal IgM binding to sulphatide at titres >20 000; WM‐MAG, patients with WM with serum monoclonal IgM binding to myelin associated glycoprotein at titres >20 000; WM‐O‐Dem, patients with WM with demyelinating neuropathies but without serum IgM binding to MAG or sulphatide; WM‐O, other patients with WM without serum IgM binding to MAG or sulphatide; IgM levels, g/dl; amp, amplitude; SNAP, sensory nerve action potential; CMAP, compound motor action potential; nerve CV, nerve conduction velocity; NA, not applicable.
History and physical examination
The mean age of patients with WM was not different from controls (tables 1 and 2). Symptoms in both feet consistent with a polyneuropathy occurred more frequently in patients with WM compared with controls. Most patients with WM with polyneuropathy symptoms (82%) had discomfort such as pain, burning, or paresthesia. More patients with WM than controls had previously been diagnosed with polyneuropathy. There were no differences in patient age (table 2), disease duration (table 2), frequency of neuropathic symptoms, prior diagnoses of neuropathy, or treatment modalities (data not shown) among the four WM subgroups.
Examination features in WM‐O patients
WM‐O patients more frequently had loss of large and small fibre sensory modalities than did controls. Quantitative vibration scores were reduced at the toes and fingers. Pin sensation was reduced more frequently (2.9 times) in the distal legs. Tandem gait abnormalities were more frequent (5.8 times) in WM‐O patients than in controls. Deep tendon reflexes, toe strength, and joint position sense at the toes were not different between the WM‐O and control groups.
Examination features in other WM subgroups compared with WM‐O patients
WM‐S patients had a 65% reduction in quantitative vibration scores at the toes. WM‐MAG patients had more frequent loss of pin sensation and tendon reflexes. WM‐O‐Dem patients had absent tendon reflexes and a 94% reduction of quantitative vibration scores at the toes. For the group of all patients with demyelinating neuropathies (the WM‐MAG and WM‐O‐Dem groups combined) vibration at the toes (p = 0.001), proprioception (p = 0.009), toe weakness (p = 0.005), and tendon reflexes (p<0.001) were all more abnormal than the WM‐O patient group.
Electrodiagnostic studies
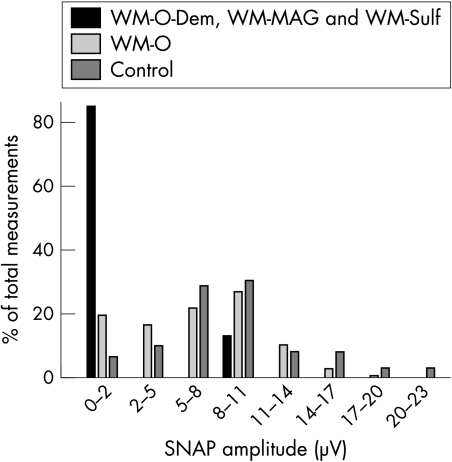
The results of the electrodiagnostic studies are shown in tables 2 and 3 and in fig 1.
Table 3 Associations of SNAP amplitudes with WM patient features.
| Patients with WM: features | SNAP amplitude (μV) | p | ||||
|---|---|---|---|---|---|---|
| Feature present | Feature absent | |||||
| History | ||||||
| Age > 60 years | 5.7 (0.6) | 8.3 (0.7) | 0.007 | |||
| Disease duration > 4 years | 5.5 (0.7) | 6.5 (0.6) | 0.2 | |||
| Treatment modality | ||||||
| Rituximab | 6.7 (0.8) | 6.2 (0.6) | 0.6 | |||
| Cladribine | 5.4 (1.0) | 6.8 (0.5) | 0.2 | |||
| Fludarabine | 6.6 (0.8) | 6.4 (0.5) | 0.8 | |||
| Cyclophosphamide | 7.5 (1.7) | 6.3 (0.5) | 0.4 | |||
| Plasma exchange | 4.9 (1.5) | 6.6 (0.5) | 0.3 | |||
| Clinical examination | ||||||
| Quantitative vibration ⩽ 4 | 4.8 (0.6) | 8.2 (0.6) | < 0.001 | |||
| Pin sensation reduced | 4.5 (0.7) | 7.4 (0.6) | 0.004 | |||
| Proprioception reduced | 3.0 (0.9) | 6.4 (0.5) | 0.01 | |||
| Tandem gait abnormal | 4.3 (0.9) | 7.6 (0.5) | 0.001 | |||
| Laboratory results | ||||||
| Total IgM > 20 g/l | 6.2 (0.6) | 5.7 (0.6) | 0.8 | |||
| Demyelinating features | 0.2 (0.2) | 6.5 (0.5) | <0.001 | |||
| Control patients | ||||||
| Age > 60 | 7.8 (0.7) | 11.0 (1.0) | 0.008 | |||
Figure 1 Distribution of SNAP amplitudes in WM subgroups and controls. SNAP amplitudes in the combined group of WM‐S, WM‐MAG, and WM‐O‐Dem showed more frequent severe axon loss compared with WM‐O patients and controls. The entire distribution of SNAP amplitudes in WM‐O patients was moderately skewed to the left toward reduced levels compared with controls. There were fewer WM‐O patients with higher amplitudes SNAPs and more with lower amplitude SNAPs.
Nerve conduction testing showed axon loss more frequently in patients with WM (48%) (p<0.001) than in controls (19%). There was no difference in the frequency or type of any therapy between WM groups with and without axon loss. Severe axon loss was more frequent (p<0.001) in patients with WM (27%) than in controls (3%).
The overall distribution of SNAP amplitudes was shifted towards lower levels in WM‐O patients compared with controls (fig 1). WM‐O patients had a mean sural SNAP amplitude that was reduced to 73% of control values. Fewer WM‐O patients (p = 0.03) had SNAP amplitudes in the higher ranges (>17 μV) (4%) compared with controls (16%). More WM‐O patients (p = 0.006) had SNAP amplitudes in the lower ranges (<5 μV) (35%) compared with controls (14%). WM‐O patients had a mean CMAP amplitude that was similar to controls.
The WM‐S, WM‐MAG, and WM‐O‐Dem groups all had higher frequencies of severe sensory loss than had the WM‐O group. Mean CMAP amplitudes were reduced in the WM‐S group compared with controls but were not different from WM‐O patients. Mean CMAP amplitudes were reduced in the WM‐MAG and WM‐O‐Dem groups compared with both controls and WM‐O patients. No WM‐S patient had a demyelinating neuropathy. WM‐MAG and WM‐O‐Dem patients had similar features of demyelination, with prolonged distal latencies and slow nerve conduction velocities but no conduction block, and with sensorimotor axon loss.
Other associations among clinical and laboratory features
Relations among clinical features
With greater age, sensory loss was more prominent in both WM and control groups. Quantitative vibration scores in the WM‐O group were 29% lower (p = 0.01) in patients >60 years than in younger patients. Quantitative vibration scores in the control group were 31% lower (p = 0.001) in patients >60 years than in younger patients. The degree of quantitative vibration loss at the toes correlated (r = 0.4, p<0.001) with the severity of tandem gait disorders in patients with WM. Patients with WM with a tandem gait disorder commonly had very reduced quantitative vibration sense (⩽4) at the toes (79%; 27/34). In patients with WM with the most severe gait disorder, who could perform no more than two steps in tandem, quantitative vibration scores (1.9 (0.5)) were 65% lower than other patients with WM (5.4 (0.4); p<0.001). Conversely, in patients with more severe vibratory loss (⩽2), the frequency of tandem gait disorder (61%) was 4.1 times greater (p<0.001) than in the remaining patients with WM (15%). There was no relation between the presence of tandem gait disorders and proprioceptive loss at the toes. Reduced joint position sense at the toes was found in only 29% (10/34) of patients with WM with a tandem gait disorder.
Relations between SNAP amplitudes and clinical and laboratory features
Reduced SNAP amplitudes in patients with WM were associated with several features of the history and examination (table 3). Patients with WM with prominently reduced quantitative vibration scores (⩽4), decreased distal pin sensation or abnormal tandem gait had lower mean sural SNAP amplitudes than other patients with WM. In patients with WM with sensory axon loss (SNAP amplitudes <5 μV), abnormal tandem gait (54%) was 3.8 times more common (p<0.001) than in other patients with WM (14%). The frequency of selective small fibre sensory involvement was similar in patients with WM and controls. Reduced distal pin sensation, with normal vibration and electrodiagnostic studies, were found in 4% of patients with WM and 5% in controls.
Reductions in sural SNAP amplitudes in WM were additive with the effects of age. The mean SNAP amplitudes in WM and controls showed a similar degree of reduction in subjects >60 years compared with those ⩽60 years. Combining the effects of age and the presence of WM, older patients with WM had a mean SNAP amplitude 48% lower than younger controls.
Total IgM levels in serum were not different in patients with WM with reduced sural SNAP amplitudes (18 (2) g/l) compared with those with normal SNAP amplitudes (22 (2) g/l). Disease duration did not differ between patients with reduced and those with normal sural SNAP amplitudes. There was no relation between SNAP amplitudes and prior treatment with rituximab, cyclophosphamide, cladribine, fludarabine, corticosteroids, or plasma exchange.
DISCUSSION
This is the first prospective, controlled study of peripheral nerve involvement in WM. Our results show that, on both clinical and electrophysiological evaluation, WM is associated with an increased degree and frequency of features consistent with polyneuropathy. Vibration and pin sensations were reduced in the lower extremities, and gait was commonly abnormal. Selective small fibre sensory involvement was not increased in patients with WM compared with controls. In electrodiagnostic studies, 48% of patients with WM had axon loss compared with 19% of controls. The frequency of abnormal electrodiagnostic studies in the WM group is similar to previous estimates of polyneuropathy in small series of patients with WM.16,17,18,19 Additional neuropathic changes in patients with WM with ages >60 years can be accounted for by the well documented sensory and axonal loss related to increasing age.11,20,21
The degree of axon loss in WM that exceeds controls probably relates to several factors. Severe axon loss in patients with WM was especially frequent in patients with identified antigenic targets of the IgM M‐protein (the WM‐S and WM‐MAG groups), occurring in 72% of these patients compared with 19% in WM‐O patients (fig 1, table 2). WM‐O‐Dem patients also had severe axon loss. These three groups comprised 40% of all severe neuropathies but only 14% of the total WM population. WM‐O patients more frequently had severe neuropathies than controls (19% v 3%). These data could suggest that approximately 16% of WM‐O patients have an especially severe neuropathy that is due to their disease. However, an alternative interpretation is that most of the WM‐O population have a moderate degree of neuropathic involvement, which produces a general shift in the distribution of nerve function toward more severe involvement (fig 1). A change in the distribution of nerve involvement in the whole WM population is supported by the 75% reduction in the frequency of WM‐O patients with the largest SNAP amplitudes (>17 μV) compared with controls,.
The factors producing axon loss in the WM‐O group remain to be identified. The pattern of sensory axon loss is similar to the polyneuropathy syndromes in the patients with WM with IgM binding to sulphatide, but no IgM binding to sulphated antigens was found in the WM‐O patients. There was no relation of neuropathic symptoms or sensory axon loss to history of drug treatment, disease duration, or IgM level (tables 2 and 3). Some aspects of WM syndromes that have been associated with neuropathy but that were not evaluated in this study include properties of IgM such as cold agglutinin, cryoglobulin, and rheumatoid factor activity. However, these properties of IgM are relatively uncommon in WM,15 and probably do not account for the sensory axon loss found in our patients.
The clinical neuropathic features in patients with WM are predominantly sensory, with distal and symmetric loss of large and small fibre modalities. Strength is often normal. Pin and vibration senses are reduced but joint position is relatively preserved. Tandem gait disorders are common in patients with WM, with severity associated with the degree of vibratory abnormality, but out of proportion to the degree of proprioception loss. The pattern of gait disorder, with the degree of abnormality out of proportion to proprioception loss, is similar to the clinical signs in anti‐MAG and GALOP (gait disorder, autoantibody, late onset polyneuropathy) syndromes.22,23 The pathophysiology underlying the tandem gait disorder in patients with WM, and the relation of disability to specific modalities of sensory loss, requires further study. Peripheral mechanisms may play a role, as the presence of tandem gait disorders in patients with WM is associated with axonal loss, and the degree of abnormality is related to the level of vibration sense loss. An additional central mechanism could be present in our patients with WM, as cerebellar and vestibular functions were not assessed in our evaluations. In any case, tandem gait disorders in WM (and other) polyneuropathies are an important feature of such syndromes as they may be associated with falls and other disturbances in activities of daily living, even when evaluation of standard gait appears intact.24
Demyelination was found in only 8% of WM neuropathy syndromes, with WM‐MAG and WM‐O‐DEM patients similarly prevalent. The electrophysiological changes in both WM‐MAG and WM‐O‐Dem patients, showing prolonged distal latencies and slowed conduction velocities but not conduction blocks, were similar to the patterns previously reported in anti‐MAG antibody associated neuropathy syndromes.25,26,27 Clinical features of patients with demyelinating neuropathies included distal sensory loss and weakness, and gait disorders. Sensory changes were consistently present and included loss of large and small fibre modalities. Tendon reflexes were reduced or absent. Weakness, when present, was predominantly distal, being especially apparent in plantar and dorsal flexion of the toes and ankles.
IgM binding to MAG probably plays a pathogenic role in the production of demyelinating neuropathies.28,29 The cause of the demyelination in WM‐O‐Dem patients, with no identified antigenic targets of the IgM M‐protein, is unclear. IgM binding to myelin antigens other than MAG could occur in this group of patients. We found no evidence of serum IgM binding to myelin protein antigens in myelin by Western blot testing. There was no WM‐O‐Dem serum IgM binding to SGPG, a glycolipid containing sulphated glucuronic acid, a carbohydrate moiety that is thought to be similar to the antigenic target in MAG.30 Detection of any peptide or glycolipid antigenic epitopes for IgM and possible structural similarities to MAG in the WM‐O‐Dem patients might provide clues to tissue targets that are important in the pathogenesis of the IgM antibody related demyelinating neuropathies.
Our data vary from some features of prior studies. IgM M‐proteins in our patients with WM bound to sulphatide (5%) and MAG (4%) less often than the expected frequencies of 28%27 to 62%.8,19 Further, many previously reported patients with IgM M‐protein binding to sulphatide associated with MGUS have had polyneuropathies with both demyelinating and motor features,13,31,32 whereas our patients with WM with monoclonal IgM binding to sulphatide had predominantly sensory involvement and axonal loss without evidence of demyelination. Some differences in frequencies of IgM binding to MAG and sulphatide in WM could be due to the prospective, unselected nature of our series, testing patients who were not specifically referred for neuropathy evaluation. Differences in the frequency of IgM binding to MAG and sulphatide in WM and some patterns of neuropathic changes compared with MGUS patients could also be due to the type, differentiation, or malignancy of the cell populations producing the monoclonal IgM. Variation in the frequency of axonal and demyelinating neuropathies in the presence of IgM binding to sulphatide may be related to biological differences between the cells producing monoclonal IgM and the antigenic tissue targets of the monoclonal IgM in WM compared with MGUS patients.33,34
ACKNOWLEDGEMENTS
The authors thank J Bodnar and B Hansen for helping to gather electrodiagnostic data and with the study design. This study was supported in part by grants from the International Waldenström's Macroglobulinemia Foundation and the Washington University Neuromuscular Research Fund.
Abbreviations
CMAP - compound motor action potential
GALOP - gait disorder, autoantibody, late onset, polyneuropathy
MAG - myelin associated glycoprotein
MGUS - monoclonal gammopathy of unknown significance
SGPG - sulphated glucuronyl paragloboside
SNAP - sensory nerve action potential
WM - Waldenström's macroglobulinaemia
WM‐MAG - WM with IgM binding to MAG
WM‐O - WM with no demyelinating neuropathy and no IgM binding to MAG or sulphatide
WM‐O‐Dem - WM with neuropathy but no IgM binding to MAG or sulphatide
WM‐S - WM with IgM binding to sulphatide
Footnotes
Competing interests: Dr Pestronk has licensed patents for neuropathy related antibody testing to Athena Diagnostics. The other authors have no competing interests
Approval to perform this study was obtained from the Washington University institutional review board. All participants gave informed consent before entering the study
References
- 1.Dimopoulos M A, Panayiotidis P, Moulopoulos L A.et al Waldenström's macroglobulinemia: clinical features, complications, and management. J Clin Oncol 200018214–226. [DOI] [PubMed] [Google Scholar]
- 2.Ghobrial I M, Gertz M A, Fonseca R. Waldenström macroglobulinaemia. Lancet Oncol 20034679–685. [DOI] [PubMed] [Google Scholar]
- 3.Owen R G, Treon S P, Al‐Katib A.et al Clinicopathological definition of Waldenström's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström's Macroglobulinemia. Semin Oncol 200330110–115. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkholm M, Johansson E, Papamichael D.et al Patterns of clinical presentation, treatment, and outcome in patients with Waldenström's macroglobulinemia: a two‐institution study. Semin Oncol 200330226–230. [DOI] [PubMed] [Google Scholar]
- 5.Kyrtsonis M C, Vassilakopoulos T P, Angelopoulou M K.et al Waldenström's macroglobulinemia: clinical course and prognostic factors in 60 patients. Experience from a single hematology unit. Ann Hematol 200180722–727. [DOI] [PubMed] [Google Scholar]
- 6.Stalnikiewicz L, Carrotte‐Lefebvre I, Detourmignies L.et al Prognostic factors in Waldenstrom's macroglobulinemia: description of the complications during the evolution – preliminary results on 101 patients. Semin Oncol 200330216–219. [DOI] [PubMed] [Google Scholar]
- 7.García‐Sanz R, Montoto S, Torrequebrada A.et al Waldenström macroglobulinaemia: presenting features and outcome in a series with 217 cases. Br J Haematol 2001115575–582. [DOI] [PubMed] [Google Scholar]
- 8.Baldini L, Nobile‐Orazio E, Guffanti A.et al Peripheral neuropathy in IgM monoclonal gammopathy and Waldenström's macroglobulinemia: a frequent complication in elderly males with low MAG‐reactive serum monoclonal component. Am J Hematol 19944525–31. [DOI] [PubMed] [Google Scholar]
- 9.Ropper A H, Gorson K C. Neuropathies associated with paraproteinemia. N Engl J Med 19983381601–1607. [DOI] [PubMed] [Google Scholar]
- 10.Vital A, Favereaux A, Martin‐Dupont P.et al Anti‐myelin‐associated glycoprotein antibodies and endoneurial cryoglobulin deposits responsible for a severe neuropathy. Acta Neuropathol 2001102409–412. [DOI] [PubMed] [Google Scholar]
- 11.Pestronk A, Florence J, Levine T.et al Sensory exam with a quantitative tuning fork: rapid, sensitive and predictive of SNAP amplitude. Neurology 200462461–464. [DOI] [PubMed] [Google Scholar]
- 12.Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP). Neurology 199141617–618. [PubMed] [Google Scholar]
- 13.Lopate, G, Parks BJ, Goldstein, JM, et al Polyneuropathies associated with high‐titer anti‐sulfatide antibodies: Characteristics of patients with and without serum M‐proteins. J Neurol Neurosurg Psychiatry 199762581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pestronk A, Li F, Bieser K.et al Anti‐MAG antibodies: major effects of antigen purity and antibody cross‐reactivity on ELISA results and clinical correlation. Neurology 1994441131–1137. [DOI] [PubMed] [Google Scholar]
- 15.Stone M J, McElroy Y G, Pestronk A.et al Human monoclonal macroglobulins with antibody activity. Semin Oncol 200330318–324. [DOI] [PubMed] [Google Scholar]
- 16.Vital A. Paraproteinemic neuropathies. Brain Pathol 200111399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbs H, Arfmann M, Frick E.et al Reactivity of sera and isolated monoclonal IgM from patients with Waldenström's macroglobulinaemia with peripheral nerve myelin. J Neurol 198523243–48. [DOI] [PubMed] [Google Scholar]
- 18.Nobile‐Orazio E. IgM paraproteinaemic neuropathies. Curr Opin Neurol 200417599–605. [DOI] [PubMed] [Google Scholar]
- 19.Nobile‐Orazio E, Marmiroli P, Baldini L.et al Peripheral neuropathy in macroglobulinemia: incidence and antigen‐specificity of M proteins. Neurology 1987371506–1514. [DOI] [PubMed] [Google Scholar]
- 20.Martina I S J, van Koningsveld R, Schmitz P I M.et al Measuring vibration threshold with a graduated tuning fork in normal aging and in patients with polyneuropathy. J Neurol Neurosurg Psychiatry 199865743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson J K. The clinical identification of peripheral neuropathy among older persons. Arch Phys Med Rehabil 2002831553–1558. [DOI] [PubMed] [Google Scholar]
- 22.Nobile‐Orazio E, Meucci N, Baldini L.et al Long‐term prognosis of neuropathy associated with anti‐MAG IgM M‐proteins and its relationship to immune therapies. Brain 2000123710–717. [DOI] [PubMed] [Google Scholar]
- 23.Pestronk A, Choksi R, Bieser K.et al Treatable gait disorder and polyneuropathy associated with high titer serum IgM binding to antigens that copurify with myelin‐associated glycoprotein. Muscle Nerve 1994171293–1300. [DOI] [PubMed] [Google Scholar]
- 24.Stolze H, Petersen G, Raethjen J.et al The gait disorder of advanced essential tremor. Brain 20011242278–2286. [DOI] [PubMed] [Google Scholar]
- 25.Capasso M, Torrieri F, Di Muzio A.et al Can electrophysiology differentiate polyneuropathy with anti‐MAG/SGPG antibodies from chronic inflammatory demyelinating polyneuropathy? Clin Neurophysiol 2002113346–353. [DOI] [PubMed] [Google Scholar]
- 26.Chassande B, Leger J M, Younes‐Chennoufi A B.et al Peripheral neuropathy associated with IgM monoclonal gammopathy: correlations between M‐protein antibody activity and clinical/electrophysiological features in 40 cases. Muscle Nerve 19982155–62. [DOI] [PubMed] [Google Scholar]
- 27.Erb S, Ferracin F, Fuhr P.et al Polyneuropathy attributes: a comparison between patients with anti‐MAG and anti‐sulfatide antibodies. J Neurol 2000247767–772. [DOI] [PubMed] [Google Scholar]
- 28.Nobile‐Orazio E, Francomano E, Daverio R.et al Anti‐myelin‐associated glycoprotein IgM antibody titers in neuropathy associated with macroglobulinemia. Ann Neurol 198926543–550. [DOI] [PubMed] [Google Scholar]
- 29.Monaco S, Bonetti B, Ferrari S.et al Complement‐mediated demyelination in patients with IgM monoclonal gammopathy and polyneuropathy. N Engl J Med 1990322649–652. [DOI] [PubMed] [Google Scholar]
- 30.Ariga T, Kohriyama T, Freddo L.et al Characterization of sulfated glucuronic acid containing glycolipids reacting with IgM M‐proteins in patients with neuropathy. J Biol Chem 1987262848–853. [PubMed] [Google Scholar]
- 31.Kornberg A J, Pestronk A. Antibody‐associated polyneuropathy syndromes: principles and treatment. Semin Neurol 200323181–190. [DOI] [PubMed] [Google Scholar]
- 32.Carpo M, Meucci N, Allaria S.et al Anti‐sulfatide IgM antibodies in peripheral neuropathy. J Neurol Sci 2000176144–150. [DOI] [PubMed] [Google Scholar]
- 33.Lopate G, Pestronk A, Kornberg A J.et al IgM anti‐sulfatide autoantibodies: patterns of binding to cerebellum, dorsal root ganglion and peripheral nerve. J Neurol Sci 1997151189–193. [DOI] [PubMed] [Google Scholar]
- 34.Quattrini A, Corbo M, Dhaliwal S K.et al Anti‐sulfatide antibodies in neurological disease: binding to rat dorsal root ganglia neurons. J Neurol Sci 1992112152–159. [DOI] [PubMed] [Google Scholar]