Autosomal recessive spastic ataxia of Charlevoix‐Saguenay (ARSACS) was originally described among French Canadians in the Charlevoix‐Saguenay‐Lac‐Saint‐Jean region of Quebec (OMIM 270550).1 The gene responsible for ARSACS was identified as sacsin, and frameshift (8585 deletion T, 2805X) and nonsense (C7245T, R2355X) mutations were reported in Quebec.2 Recently, patients with other mutations have been described in countries elsewhere.3 These showed not only the spastic ataxia with peripheral neuropathy recognised in Quebec, but some additional features as well.3 Here we describe a female Japanese ARSACS patient with a novel nonsense mutation (C3774T, Q1198X), which resulted in a shorter truncated protein than those of the French Canadian patients. We report the details of her clinical and genetic data, and discuss the correlation between mutations and phenotypes in ARSACS.
The patient was a 39 year old woman who first walked at 12 months. Her gait was normal in early childhood. A spastic gait started at nine years of age, but she made no complaint about it for many years. After the age of 35 she complained of unsteadiness in her gait and clumsiness in her hands. Her gait disturbance progressed and she visited our clinic at the age of 37.
Consanguinity was not identified in her parents. Family members (parents and one brother) and other relatives have no evident neurological disorder. She had slurred and scanning speech. Horizontal gaze‐evoked nystagmus was observed, and downward nystagmus occasionally appeared in the frontal eye position and with horizontal and upward gaze. Fundoscopy was normal without hypermyelinated retinal fibres. She had a claw hand on the left and an ape hand on the right. Pes cavus was also present. Muscle weakness and atrophy were present in the distal limbs. The Romberg sign was positive and her gait was markedly spastic and ataxic, but she did not need any assistance in walking. Vibration sense was reduced in the distal extremities, but superficial sensation was maintained. Muscle stretch reflexes were increased in both knees, while the right radial and ulnar reflexes and both ankle reflexes were absent. The Babinski sign was positive on both sides. Her verbal IQ was 101, motor IQ 81, and total IQ 92, according to the Wechsler Adult Intelligence Scale–Revised.
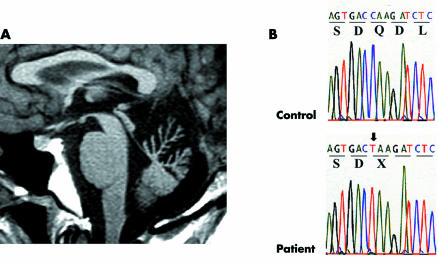
On electroencephalographic study, background activity was 8–9 Hz, and diffuse high amplitude slow waves appeared continuously during and after hyperventilation. Electronystagmography showed marked impairment of optokinetic nystagmus and defective visual suppression of caloric nystagmus. Smooth ocular pursuit was affected, while saccade velocity appeared intact. On nerve conduction studies, distal motor latencies increased, and the amplitude of compound muscle action potential was low, especially in the lower limbs. In contrast, motor conduction velocities were spared except in the right ulnar and left median nerves. Sensory nerve action potentials were not evoked in any of the limbs. Magnetic resonance imaging revealed marked atrophy of the cerebellar vermis limited to the portion above the pyramis (fig 1A).
Figure 1 Brain magnetic resonance imaging and electropherogram of mutation. (A) Sagittal section of T1 weighted image showing marked vermian atrophy limited to the portion above the pyramis. (B) The arrow indicates a homozygous C→T transition at nt3774, leading to the nonsense mutation.
Gene analysis
After informed consent was obtained, genomic DNA was extracted from the patient's leucocytes. Using 30 appropriate primer pairs, the coding exon of the sacsin gene was amplified by polymerase chain reaction (PCR). PCR fragments were sequenced directly on an automated sequencer (ABI PRISM 3100, Applied Biosystems, Foster City, USA). Sequence analysis revealed a homozygous C→T transition at position 3774 (fig 1B), which results in substitution of glutamine 1198 for a stop codon (Q1198X). This mutation was absent in 100 chromosomes of the normal controls.
Comment
We report the 19th sacsin mutation identified to date. Interestingly, all mutations and families show a one to one correspondence, and the same mutation has not been reported among different families. Most mutations are of homozygous nonsense and frameshift type leading to an early stop codon, which suggests loss of function of sacsin as the cause of the disease.3,4
The clinical variation in ARSACS has been pointed out, especially in age of onset, retinal myelination, and mental disturbance.3,4 French Canadian patients show gait disturbance when they begin walking, have prominent retinal hypermyelination, and show no mental disturbance.1 Although our mutation led to an earlier stop codon than mutations in Quebec, our patient had a milder phenotype, with an older age at onset of ataxia and no retinal hypermyelination. In 8534 deletion A, which resulted in the same early stop codon as 8585 deletion T in Quebec, there was dementia but no retinal hypermyelination.4 From these observations, we suggest that the clinical variation in ARSACS is not influenced by the loss of function of sacsin.
Apart for the variations mentioned above, all ARSACS patients have rather uniform clinical features. They have unsteadiness on walking in childhood and became chair bound in adulthood. There is spastic ataxia, and most patients have brisk patellar tendon reflexes, a positive Babinski sign bilaterally, nystagmus, and dysarthria. Pes cavus and disturbed deep sensation are also common. Progression of peripheral neuropathy probably results in the absence of the Achilles tendon reflex and distal muscle atrophy in the course of the disease. Patients with T2902C lack spasticity and brisk patellar tendon reflexes,5 which suggests that the progressive peripheral neuropathy in ARSACS may occasionally mask pyramidal signs.
It is interesting that clinical uniformity arose from mutations distributed widely in the 13299 base pair coding region of sacsin. All frameshift and nonsense mutations in the sacsin gene are located at the N‐terminal side of a leucine zipper motif at the C‐terminal side, dnaJ motif, and HEPN domain.3 Loss of these domains may lead to loss of critical function of sacsin and result in similar phenotypes. Although missense mutations have these domains, phenotypes resemble frameshift and nonsense mutations. As the predicted structure in T2902C,5 missense mutations may lead to a conformational change of sacsin, which causes malfunction of the critical domains. Another possibility is that missense mutations may make sacsin a misfolded protein, which rapidly leads to degradation. We need to examine the degradation speed of the sacsin protein in ARSACS patients. Further studies of the critical domains of sacsin and the conformational change by mutations will clarify the mechanism of ARSACS.
Electronic database information
The nucleotide and the amino acid positions are based on the transcript GenBank accession number NM_014363 and NP_055178.
Acknowledgements
We thank Yuki Watanabe and Takako Sasaki for their technical assistance.
Footnotes
Competing interests: none declared
References
- 1.Bouchard J P, Barbeau A, Bouchard R.et al Autosomal recessive spastic ataxia of Charlevoix‐Saguenay. Can J Neurol Sci 1978561–69. [PubMed] [Google Scholar]
- 2.Engert J C, Berube P, Mercier J.et al ARSACS, a spastic ataxia common in northeastern Quebec, is caused by mutations in a new gene encoding an 11.5‐kb ORF. Nat Genet 200024120–125. [DOI] [PubMed] [Google Scholar]
- 3.Gomez C M. ARSACS goes global. Neurology 20046210–11. [DOI] [PubMed] [Google Scholar]
- 4.Hara K, Onodera O, Endo M.et al Sacsin‐related autosomal recessive ataxia without prominent retinal myelinated fibers in Japan. Mov Disord 200520380–382. [DOI] [PubMed] [Google Scholar]
- 5.Shimazaki H, Takiyama Y, Sakoe K.et al A phenotype without spasticity in sacsin‐related ataxia. Neurology 2005642129–2131. [DOI] [PubMed] [Google Scholar]