Abstract
Objective
To investigate the ability of patients with myotonic dystrophy type 1 to recognise basic facial emotions. We also explored the relationship between facial emotion recognition, neuropsychological data, personality, and CTG repeat expansion data in the DM‐1 group.
Methods
In total, 50 patients with DM‐1 (28 women and 22 men) participated, with 41 healthy controls. Recognition of facial emotional expressions was assessed using photographs of basic emotions. A set of tests measured cognition and personality dimensions, and CTG repeat size was quantified in blood lymphocytes.
Results
Patients with DM‐1 showed impaired recognition of facial emotions compared with controls. A significant negative correlation was found between total score of emotion recognition in a forced choice task and CTG repeat size. Furthermore, specific cognitive functions (vocabulary, visuospatial construction ability, and speed) and personality dimensions (reward dependence and cooperativeness) correlated with scores on the forced choice emotion recognition task.
Conclusion
These findings revealed a CTG repeat dependent facial emotion recognition deficit in the DM‐1 group, which was associated with specific neuropsychological functions. Furthermore, a correlation was found between facial emotional recognition ability and personality dimensions associated with sociability. This adds a new clinically relevant dimension in the cognitive deficits associated with DM‐1.
Keywords: myotonic dystrophy, emotion recognition, CTG repeat expansion, neuropsychology, personality
The ability to understand non‐verbally expressed emotion is a crucial component in social cognition.1 This ability has been investigated in a number of clinical populations, and studies have shown deficits in facial emotion perception in several neurological and psychiatric disorders.2 Many studies have identified regions in the brain that, when damaged, give rise to emotion processing deficits,3 and a model of a distributed neural system for face perception, including multiple bilateral regions, has been proposed.4 This model contains a core system for visual analysis of faces involving regions in the occipitotemporal visual extrastriate cortex and, additionally, an extended system including limbic regions that are associated with the recognition of facial emotion. The right somatosensory cortex, inferior frontal cortex, basal ganglia, and prefrontal cortex may also be involved in the accurate recognition of emotion.3
Myotonic dystrophy type 1 (DM‐1) is a heritable multisystem disease associated with anomalies regarding brain, cognition and personality.5 It primarily affects bodily muscles, including facial muscles, early in and throughout the disease process.6 The molecular basis of DM‐1 is an unstable CTG repeat expansion located at the 3′ untranslated region of the DMPK gene on chromosome 19.7 The CTG repeat number ranges between 5 and 37 in normal subjects, while in patients with DM‐1, it exceeds 50 but can be up to several thousand units.8 As summarised by Ashizawa,5 the pathology in the central nervous system includes cell loss, pathological tau proteins, focal white matter lesions, atrophy, and reduced blood flow in various cortical and subcortical areas. Neuropsychological studies have revealed cognitive dysfunction involving attention, memory, visuospatial ability, and executive function.9,10 DM‐1 is also associated with avoidant personality traits.9,11
Brain regions activated in facial emotion recognition12,13 are associated with pathology in DM‐1,5 for example, the amygdala, known to be recruited in the recognition of fear.2,14,15 We measured facial emotion recognition ability in patients with DM‐1 and collected information regarding cognition, personality, and CTG repeat expansion size for analysis of possible associations.
METHODS
All participants gave informed consent, and the medical ethics committee at Sahlgrenska Academy, Göteborg University, approved the study.
Subjects
Patients with classical DM‐1 were recruited from the Neuromuscular Center, Sahlgrenska University Hospital. Inclusion criteria were: age 18–65 years with no history of major psychiatric or somatic illness, major brain injury, or alcohol misuse. The diagnosis of DM‐1 was confirmed by an expansion of CTG repeats (>50). Patients with either minimal or congenital DM‐1 were excluded from the study.16 In total, 51 patients at the clinic, meeting the criteria of classical/early adulthood DM‐1, were invited to participate. One patient declined, thus 50 (28 women and 22 men; mean age 42 years, range 23–62) were included in the study. In the DM‐1 group, the range of CTG repeat size was 70–2000. The controls were 41 healthy people (28 women and 13 men; mean age 37 years, range 19–64), recruited by local advertisement at the Sahlgrenska University Hospital. Clinical data from patients with DM‐1 were collected by a self rating procedure designed at the Neuromuscular Center, which aimed to measure the absence or presence of different symptoms. Muscle function was measured by a physiotherapist using Brooke's grading system of mobility.17
Facial emotion recognition tasks
We created two experimental tasks using 28 (2×14) different pictures showing basic emotions from Ekman and Friesen's Pictures of Facial Affect (POFA).18 The stimuli used in these tasks included six men and seven women, expressing basic emotions (happiness, sadness, fear, anger, disgust, surprise) and a neutral state. Facial emotions in POFA, with the highest correct identification rate (>80 %) in Ekman and Friesen's initial studies, were chosen for the tasks.18 In both tasks, each emotion was shown twice. Aspects of the stimuli that might distract subject's focus on facial features, such as hair or jewellery, were masked. A list of the chosen pictures is presented in appendix 1. The order in the two picture series was randomly arranged and thereafter presented in a fixed order for each patient. The free labelling task measured the ability to freely name the correct emotion and incorporated the possibility of identifying difficulties finding the right word for the emotion—that is, alexithymia.19 In the forced choice task, the subjects were provided with fixed response alternatives. This procedure allowed us to investigate whether group differences in former experiment were due to lexical deficits. In both experimental tasks, we manually measured the time interval from exposition of the picture to an answer given by the test person.
The experiment was conducted in a silent room. Both groups performed the two tasks in the same order (free labelling first, followed by forced choice). The experimenter gave instructions verbally, and black and white pictures (90×130 mm) were presented one by one on a computer monitor. The participants sat approximately 600 mm from the screen and were given unlimited time to complete each of the two experimental trials. No feedback was given regarding the correctness of the responses. In both tasks, the participants were presented with photographs of faces of women and men expressing one of six emotional states and a neutral state (a total of 14 presentations). The participants were asked to label the emotion presented in each picture. The appropriateness of the responses on the free labelling task were judged using a list of emotion words and their synonyms derived from a Swedish word and synonym lexicon 20,21 (this list can be obtained from the corresponding author). A different set of photographs was used in the forced choice task, again of 14 photographs showing six emotional states and a neutral state, expressed by women and men. Labels for the seven facial expressions were printed on a sheet of paper, and subjects were asked to select the word that best described the emotion in each photograph. Participants were instructed to have a free choice of any label as many times as needed.
Neuropsychological and personality assessment
The tests were administered in two sessions of approximately 2 hours each. The tests included in the neuropsychological investigation and the function they measured are shown in table 1.
Table 1 Neuropsychological investigations carried out in the study.
| Function measured | Tests used | |
|---|---|---|
| Intelligence | WAIS‐R (Swedish version).22 | |
| Memory | RAVLT (immediate and delayed recall),23 RCFT (immediate and delayed recall).24 | |
| Executive function | COWAT,25 CWT,26 WCST (categories)27 | |
| Verbal function | Vocabulary, WAIS‐R,22 COWAT25 | |
| Visuospatial function | RCFT (copy),24 WAIS‐R block design, 22 WAIS‐R picture arrangement22 | |
| Attention | Digit span (backwards and forwards), WAIS‐R,22 TMT25 | |
| Speed | Digit symbol, WAIS‐R,22 CWT26 |
WAIS‐R, Wechsler Adult Intelligence Scale‐Revised; RAVLT, Rey Auditory Verbal Learning Test; RCFTRey Complex Figure Test; COWAT, Controlled Oral Word Test; WCST, Wisconsin Card Sorting Test; CWT, Colour Word Test; TMT, Trail Making Test.
Personality was assessed with the Temperament and Character Inventory (TCI)28 and results have been presented earlier.11 The personality model includes four temperament dimensions; novelty seeking, harm avoidance, reward dependence, and persistence, and three character dimensions; self directedness, cooperativeness, and self transcendence.
Genetic analysis
DNA was extracted from peripheral blood lymphocytes and analysed for expansions of the CTG repeat in the DMPK gene. The analysis was performed with PCR and Southern blotting using the probe pM10M6.7 The size of CTG expansions was assessed visually from exposed radiographic films.
Statistical analysis
Apart from demographical information, descriptive data are presented as median scores with interquartile ranges. We used nonparametric methods to analyse the data: Mann‐Whitney U test and Spearman's rank correlation test with a Bonferroni‐Holm correction for multiple tests.29 We also conducted multiple regression analyses30 to explore predictors of deficits in facial emotion recognition. Those variables correlating with emotional recognition performance (ρ⩾0.30) were transformed as necessary to approximate normality before being entered into the regressions. SPSS software (version 11.5; Chicago, IL, USA) was used for the data analysis.
RESULTS
Demographic and neuropsychological data
Table 2 shows that patients with DM‐1 were significantly older and had shorter education than the control group. They were also significantly impaired on tests associated with total, verbal and performance IQ.
Table 2 Demographic and neuropsychological background data.
| Variable | Patients with DM‐1 (n = 50) | Healthy controls (n = 41) | p* | |||
|---|---|---|---|---|---|---|
| Age (years) | 41.6 (10.4) | 37.4 (13,6) | <0.05 | |||
| Gender | 22 M, 28 F | 13 M, 28 F | NS† | |||
| Education (years) | 10.7 (2.1) | 13.9 (2.2) | <0.001 | |||
| Age at onset (years) | 26.7 (10.4, 12–50) | – | ||||
| Fatigue | 40 (80%) | – | ||||
| Brooke rating‡ | 0, 8 (1.6, 0–9) | – | ||||
| Information (WAIS‐R) | 19.4 (4.9) | 22.1 (3.2) | <0.01 | |||
| Block design (WAIS‐R) | 19.3 (8.0) | 38.1(6.8) | <0.001 | |||
| Digit symbol (WAIS‐R) | 38.4 (11.4) | 55.4 (11.0) | <0.001 |
Results are presented as means (SD) unless otherwise indicated. *Mann‐Whitney U test; †χ2 test. ‡Brooke's functional test and grading system measuring mobility (hips and legs); in the grading system, zero implies normal function and higher values indicates increasing levels of immobility. Numbers in parentheses are SD and range. NS, not significant.
Performance on facial emotion recognition tasks
In the free labelling task (table 3) the patients with DM‐1 achieved a significantly lower total score than did controls, because of difficulties in recognising expressions signalling anger and surprise. Correspondingly, more patients than controls showed a less than perfect response (a score < 2) on these items. In the forced choice task (table 3) the DM‐1 group again had a lower total score than the control group. The patients had problems with angry facial expressions again, but detection of surprise was unaffected. Instead, the DM‐1 group scored lower on fear and disgust. Hence there were an increased number of patients performing less than perfectly (<2) when exposed to angry, fearful, or disgusted faces. Because major differences were found regarding background data (table 2) we performed an one way covariate analysis of variance, using age, education, and information on the WAIS‐R as covariates. The differences between groups remained (F = 7.3, df = 4, p⩽0.001). We also compared patients with DM‐1 who experienced fatigue and those who did not, and found no significant difference regarding the results on the forced choice task (Mann Whitney U test 190.5, Z = –0.235, p = 0.82). In addition to the increased prevalence of mistakes, the DM‐1 group also took significantly longer to complete each of the two tasks (table 3). An examination of the error pattern in the forced choice task revealed that patients with DM‐1 mostly mistook fear for surprise and disgust for anger—that is, facial configurations were closely related to the chosen emotion. A more diffuse response pattern was found in the DM‐1 patient's interpretation of the facial emotion of anger. There were no gender differences in performance within the DM‐1 and control groups.
Table 3 Performance of patients with DM‐1 and healthy control subjects on emotion recognition tasks.
| Emotions | Patients with DM‐1* (n = 50) | Healthy controls* (n = 41) | p† | Patients with DM‐1 (n = 50)‡ | Healthy controls (n = 41)‡ | p§ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Free labelling task | ||||||||||||
| Total score | 9 (3.00) | 10 (2.50) | <0.001 | – | – | – | ||||||
| Happiness | 2 (0.00) | 2 (0.00) | NS | 0% | 9.5% | NS | ||||||
| Fear | 2 (1.00) | 2 (1.00) | NS | 46% | 33% | NS | ||||||
| Sadness | 1 (1.00) | 1 (1.00) | NS | 56% | 66% | NS | ||||||
| Anger | 2 (1.00) | 2 (0.00) | <0.01 | 48% | 21% | 0.01 | ||||||
| Disgust | 1 (1.00) | 1 (2.00) | NS | 88% | 69% | NS | ||||||
| Surprise | 2 (1.00) | 2 (0.00) | <0.01 | 34% | 12% | <0.05 | ||||||
| Neutral | 0 (1.00) | 1 (1.00) | NS | 86% | 81% | NS | ||||||
| Time (s) | 51 (24) | 44 (18) | <0.05 | – | – | – | ||||||
| Forced choice task | ||||||||||||
| Total score | 12 (2.00) | 14 (1.00) | <0.001 | – | – | – | ||||||
| Happiness | 2 (0.00) | 2 (0.00) | NS | 0% | 0% | NS | ||||||
| Fear | 1 (1.00) | 2 (0.00) | <0.001 | 64% | 21% | <0.001 | ||||||
| Sadness | 2 (0.00) | 2 (0.00) | NS | 14% | 7% | NS | ||||||
| Anger | 2 (1.00) | 2 (0.00) | <0.001 | 46% | 12% | <0.01 | ||||||
| Disgust | 1.5 (1.00) | 2 (1.00) | <0.05 | 50% | 26% | <0.05 | ||||||
| Surprise | 2 (0.00) | 2 (0.00) | NS | 6% | 9% | NS | ||||||
| Neutral | 2 (0.00) | 2 (0.00) | <0.05 | 22% | 7% | NS | ||||||
| Time (s) | 55 (24) | 43 (21) | <0.05 | – | – | – |
*Median (interquartile range); †Mann Whitney U test; ‡% subjects scoring < 2; §χ2 test.
Correlates of facial emotion recognition ability in patients with DM‐1
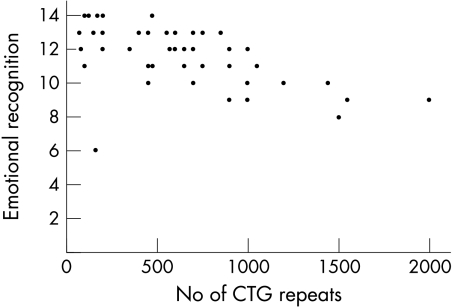
There was a negative correlation (ρ = −0.54, p⩽0.001) between the total score achieved by patients with DM‐1 on the forced choice task and the number of CTG repeats (fig 1). This coefficient rose to ρ = −0.62 (p⩽0.001) when an apparent outlier was removed (fig 1). CTG repeat expansion size was also correlated with latency to complete the forced choice task (ρ = 0.34, p = 0.019). Tables 4 and 5 present the correlation between performance on the forced choice task and the neurocognitive and personality data, respectively, within the DM‐1 group (outlier removed). Ten variables emerged as potential associates of facial emotion recognition (ρ⩾30). Each of these variables plus CTG repeat size was checked for normality using Fisher's test.31 Those that did not conform to normality were transformed according the ladder of powers procedure32 so that they approximated normality, and were then entered in a multiple regression. This model explained 43% of the variance (adjusted R2 = 0.43, p = 0.002). Only the CTG repeat expansion reached the criterion for acceptance in the regression (β = −0.603, p = 0.002). Because the strong influence of the genetic variable might have masked the importance of the neuropsychological variables, we performed a second analysis omitting the genetic data. This also yielded a significant model (adjusted R2 = 0.17, p = 0.03). Only the Rey Complex Figure Test (copy) was significant (β = 0.44, p = 0.003).
Figure 1 Correlation between the forced choice task (total score; maximum score = 14) and CTG triplet repeat expansion.
Table 4 Spearman rank correlations between forced choice task (total score) and neuropsychological data.
| Neuropsychological test | p | |
|---|---|---|
| Information | 0.08 | |
| Digit span | 0.07 | |
| Vocabulary | 0.36† | |
| Arithmetic | 0.22 | |
| Comprehension | 0.31† | |
| Similarities | 0.26 | |
| Picture completion | 0.27 | |
| Picture arrangement | 0.31† | |
| Block design | 0.15 | |
| Digit symbol | 0.30† | |
| WAIS‐R total | 0.24 | |
| WAIS‐R verbal | 0.10 | |
| WAIS‐R performance | 0.07 | |
| Colour Word Test A | −0.42† | |
| Colour Word Test B | −0.29 | |
| Controlled Oral Word Test | 0.021 | |
| Rey Complex Figure Test (copy) | 0.44† | |
| Rey Complex Figure Test (immediate recall) | 0.30 | |
| Rey Complex Figure Test (delayed recall) | 0.30† | |
| Trail Making Test A | –0.32† | |
| Trail Making Test B | –0.27 | |
| Wisconsin Card Sorting Test (categories) | 0.10 | |
| RAVLT (immediate recall) | 0.31 | |
| RAVLT (delayed recall) | 0.17 |
†Significant correlation < 0.05.
Table 5 Spearman rank correlations between forced choice task (total score) and personality dimensions in the Temperament and Character Inventory.
| Dimension | p | |
|---|---|---|
| Novelty seeking | 0.23 | |
| Harm avoidance | 0.06 | |
| Reward dependence | 0.30† | |
| Persistence | 0.02 | |
| Self directedness | 0.21 | |
| Cooperativeness | 0.30† | |
| Self transcendence | –0.03 |
†Significant correlation < 0.05.
DISCUSSION
This study revealed a facial emotion recognition deficit in patients with DM‐1 and the total score of the forced choice task correlated with CTG repeat expansion size, visuoconstructive ability, and sociability related personality dimensions.
As poor eyesight is common in DM‐1, an obvious concern is the possibility that the recognition impairments in patients with DM‐1 were secondary to eye pathology.6 Cataracts might have impaired identification of small facial features. However, when examining the medical records we found that most of the patients with DM‐1 underwent eye checkups regularly and were prescribed visual correction when necessary. We also analysed the performance of the DM‐1 patients on the WAIS‐R picture completion test and compared the results with normative data. This revealed that DM‐1 performance was in the normal range (raw score 15.2, SD 2.26), indicating a basic ability to perform a task strongly associated with visual analyses. However, saccadic eye movements are important for rapid analysis of human facial emotion33 and saccadic problems could influence the correct identification of the emotional facial configuration in patients with DM‐1.6 Indeed, aberrations in saccadic eye movement could possibly explain the longer response time of the patients with DM‐1 compared with controls in this study. In social interplay, a delayed response to facial emotion may be a considerable problem when trying to cope with a rapid flow of visual information, and add a further handicap in correct facial emotion recognition.
CTG repeat expansion size strongly correlated with the total score of facial emotion recognition. Several factors, including cognition, may be affected by CTG repeat expansion size and indirectly influence the ability to recognise facial emotions in DM‐1. It is also possible that the differences seen between the patient and control groups is an effect of deficits shown by patients with extensive CTG repeats (fig 1). Extensive CTG repeat expansion (>1000) has been associated with more severe symptoms, earlier disease onset, and reduced IQ.5 However, our results remained intact when we omitted the nine patients with DM‐1 who had CTG repeats exceeding 1000. This indicates that impairment in facial emotion recognition ability is also found in patients affected with fewer CTG repeats, associated with classical DM‐1, but our data show that these deficits become even more prominent in increasing levels of CTG repeats.
Neuropsychological test performance was moderately and negatively correlated to facial emotion recognition ability. A negative influence of poor cognitive abilities on emotion recognition has been demonstrated previously.34 However, in DM‐1, these associations were rather weak, particularly when compared with the impact of the genetic aberration. This finding suggests that the emotional recognition deficit is not simply a consequence of deficits in neurocognitive ability.
Patients with DM‐1 are low on cooperativeness and empathy.11 Here we found deficits in the ability of patients with DM‐1 to recognise negative emotions and a correlation between facial emotion recognition ability and the sociability dimensions of the TCI (cooperativeness and reward dependence). This association could explain earlier descriptions of patients with DM‐1 as being non‐compliant and indifferent, as summarised by Harper.6 Failure to recognise non‐verbal negative social cues could affect the ability of patients with DM‐1 to adapt to implicit social demand.
In conclusion, this study indicates that there is a reduced emotion recognition ability in DM‐1, adding a new clinically relevant dimension in the cognitive deficits associated with the disease. Note, however, that the data on individual emotions are limited, owing to the small number of exemplars within each category. The overall facial emotion recognition ability was strongly related to the number of CTG repeats. Therefore, deficits in facial emotion recognition should be considered particularly in contact with patients with DM‐1 who have large triplet repeat expansions.
ACKNOWLEDGEMENTS
This research was supported by grants from the Norrbacka, Eugenia, and West Sweden Muscle foundation in Sweden. The authors thank Dr L Samuelsson, Department of Clinical Genetics, Sahlgrenska University Hospital East for the genetic analysis and E Hammarén, Physiotherapist, Neuromuscular Center, Sahlgrenska University Hospital Mölndal for measurement of mobility.
Abbreviations
COWAT - Controlled Oral Word Test
CWT - Colour Word Test
DM‐1 - myotonic dystrophy type 1
POFA - Pictures of Facial Affect
RAVLT - Rey Auditory Verbal Learning Test
RCFT - Rey Complex Figure Test
TCI - Temperament and Character Inventory
TMT - Trail Making Test
WAIS‐R - Wechsler Adult Intelligence Scale‐Revised
WCST - Wisconsin Card Sorting Test
Appendix 1
Photographs chosen from Ekman and Friesen's Pictures of Facial Affect:18 A‐1‐06, PF‐2‐30, JB‐1‐03, MF‐2‐07, EM‐4‐24, JM‐2‐08, JJ‐4‐13, EM‐4‐07, NR‐2‐07, GS‐1‐16, PE‐2‐04, JJ‐5‐13, JB‐1‐16, PF‐2‐12, SW‐1‐16, JM‐1‐04, WF‐3‐11, MO‐2‐11, EM‐2‐04, JM‐3‐11, A‐1‐24, PE‐3‐21, MO‐2‐18, WF‐2‐05, PF‐2‐16, EM‐5‐21, SW‐4‐09 and MF‐1‐06.
Footnotes
Competing interests: none
References
- 1.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci 20034165–178. [DOI] [PubMed] [Google Scholar]
- 2.Posamentier M T, Abdi H. Processing faces and facial expressions. Neuropsychol Rev 20033113–142. [DOI] [PubMed] [Google Scholar]
- 3.Adolphs R. Neural systems for recognizing emotion. Curr Opinion Neurobiol 200212169–177. [DOI] [PubMed] [Google Scholar]
- 4.Haxby J V, Hoffman E A, Gobbini M I. The distributed human neural system for face perception. Trends Cog Sci 20006223–233. [DOI] [PubMed] [Google Scholar]
- 5.Ashizawa T. Mytonic dystrophy as a brain disorder. Arch Neurol 199855291–293. [DOI] [PubMed] [Google Scholar]
- 6.Harper P S. Myotonic dystrophy. In: Major problems in neurology. 3rd ed. London, PA: WB Saunders, 2001
- 7.Brook J D, Mccurrach M E, Harley H G.et al Molecular basis of myotonic dystrophy: Expansion of a trinucleotid (CTG) repeat at the 3'end of a transcript encoding a protein kinase family member. Cell 199268799–800. [DOI] [PubMed] [Google Scholar]
- 8.Ranum L, Day J W. Pathogenic RNA repeats: an expanding role in genetic disease. Trend Genet 200420506–512. [DOI] [PubMed] [Google Scholar]
- 9.Meola G, Sansone V, Perani D.et al Executive dysfunction and avoidant personality trait in myotonic dystrophy type 1 (DM‐1) and in proximal myotonic dystrophy (PROMM/DM‐2). Neuromuscul Disord 200313813–821. [DOI] [PubMed] [Google Scholar]
- 10.Modoni A, Silvestri G, Pomponi M G.et al Characterization of the pattern of cognitive impairment in myotonic dystrophy type 1. Arch Neurol 2004611943–1947. [DOI] [PubMed] [Google Scholar]
- 11.Winblad S, Lindberg C, Hansen S. Temperament and character in patients with classical myotonic dystrophy type 1 (DM‐1). Neuromuscul Disord 200515287–292. [DOI] [PubMed] [Google Scholar]
- 12.Esslen M, Pascual‐Marqui R D, Hell D. Brain areas and time course of emotional processing. Neuroimage 2004211189–1203. [DOI] [PubMed] [Google Scholar]
- 13.Sprengelmeyer R, Rausch M, Eysel U T.et al Neural structures associated with recognition of facial expressios of basic emotions. Proc Biol Sci 19982651927–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermesch P, Sergeant N, Ruchoux M M.et al Specific tauvariants in the brains of patients with myotonic dystrophy. Neurology 199647711–717. [DOI] [PubMed] [Google Scholar]
- 15.Sergeant N, Sablonnaire B, Schraen‐Maschke S.et al Dysregulation of human brain microtubule‐associated mRNA maturation in myotonic dystrophy type 1. Human Mol Genet 2001102143–2155. [DOI] [PubMed] [Google Scholar]
- 16.Brunner H G, Jennekens F G I, Smeets H J M.et al Myotonic dystrophy (Steinert's disease). In: Emery AEH. Diagnostic criteria for neuromuscular disorders. 2nd ed. Royal Society of Medicine Press 199427–29.
- 17.Brooke M H.A clinician's view of neuromuscular diseases. 2nd ed. Baltimore: Williams & Wilkins, 1986
- 18.Ekman P, Friesen W.Pictures of facial affect. Palo Alto: Consulting Psychologists Press, 1976
- 19.Taylor J G, Bagby R M. New trends in alexithymia research. Psychother Psychosom 20047368–77. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous Svenska akademins ordlista över svenska språket. Stockholm: PA Nordstedts och söner. Stockholm 1998
- 21.Walter G.Bonniers synonymordbok Stockholm: Albert Bonniers Förlag. 2000
- 22.Wechsler D, Bartfai A, Nyman H.et alWechsler Adult Intelligence Scale – Revised (WAIS‐R). Stockholm: Psykologiförlaget, 1992
- 23.Schmidt M.Rey Auditory Verbal Learning Test: A handbook. Los Angeles: Western Psychological Services, 1996
- 24.Myers J E, Kelly R M.Rey Osterrieth Complex Figure Test. Stockholm: Psykologiförlaget, 1999
- 25.Lezak M D.Neuropsychological assessment. Oxford University Press 1983
- 26.Smith G J W, Nyman G E, Hentschel U.Manual till CWT – Serialt färgordstest. Stockholm: Psykologiförlaget, 1986
- 27.Grant D A, Berg E A, Nyman H.Wisconsin Card Sorting Test. WCST – revised and expanded. Stockholm: Psykologiförlaget, 1996
- 28.Cloninger R C, Svrakic D M, Przybeck T R. A psychobiological model of temperament and character. Arch of Gen Psychiat 199350975–990. [DOI] [PubMed] [Google Scholar]
- 29.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979665–70. [Google Scholar]
- 30.Miles J, Shelvin M.Applying regression and correlation. A guide for students and research. Thousand Oaks, CA: Sage, 2000
- 31.Pett M A.Nonparametric statistics for health care research. Thousand Oaks, CA: Sage, 1997
- 32.Vellman P F.Data Desk handbook 2 (version 6.0). Ithaca NY: Data Description, 1988
- 33.Adolphs R, Gosselin F, Buchanan T W.et al A mechanism for impaired fear recognition after amygdala damage. Nature 200543368–72. [DOI] [PubMed] [Google Scholar]
- 34.Sachs G, Steger‐Wuchse D, Kryspin‐Exner I.et al Facial recognition deficits and cognition in schizophrenia. Schizophr Res 20046827–35. [DOI] [PubMed] [Google Scholar]