Abstract
Objective
In patients with hemifacial spasm (HFS), abnormal muscle responses (AMR) are frequently present. The objective of this study was to investigate whether the afferent input of AMR is mediated by antidromic facial nerve stimulation or orthodromic trigeminal nerve stimulation.
Methods
AMR in the orbicularis oris muscle were recorded in 28 patients with HFS. When AMR were present, they were recorded after subthreshold stimulation of the facial nerve and weak stimulation delivered to the skin.
Results
AMR were recordable in 24 (86%) of the patients, and usually consisted of the early constant component (mean onset latency, 10.0 ms) and late variable component (35.3 ms), similar to R1 and R2 of the blink reflex. The early or late components of AMR, or both, were frequently elicited after subthreshold stimulation of the facial nerve (43%) and skin stimulation (88%).
Conclusions
AMR are likely to be mediated by trigeminal afferent inputs, rather than antidromic activation of the facial nerve, and are a type of trigeminal reflex.
Keywords: hemifacial spasm, abnormal muscle response, blink reflex, facial nerve, trigenimal nerve
Hemifacial spasm (HFS) is characterised by involuntary clonic or tonic contractions of muscles innervated by the facial nerve, and its prevalence is reported to be approximately 10 cases per 100 000 population.1 It is commonly caused by contact between a blood vessel and the facial nerve at its root exit zone.2,3,4,5 The classic hypothesis is that ectopic firing and ephaptic transmission occur at the site of vascular compression.3,6,7 An alternative and not necessarily contradictory hypothesis is that there is hyperexcitability of facial motor neurones, presumably provoked by excessive afferent activity or by antidromic stimulation or by both.8,9,10,11
Abnormal muscle responses (AMR), and synkinetic responses (SR) of the blink reflexes are frequently present in patients with HFS and useful to confirm the diagnosis.12,13,14,15,16 AMR are elicited by stimulation one branch of the facial nerve on the affected side, causing co‐contraction of muscles innervated by other branches of the facial nerve.14,15,17 SR are evoked from the orbicularis oris (O. oris) muscle in HFS patients, as well as blink reflexes from the orbicularis oculi (O. oculi) muscle, after stimulation of the supraorbital branch of the trigeminal nerve.2,4,6 These abnormal responses result from hyperexcitability of facial nerves/neurones.
Previous studies suggest that AMR is an exaggerated F wave with lateral spread within the facial nucleus,14,15,16,18 but the precise afferent pathway of AMR to facial nucleus is not well understood. To investigate whether AMR is mediated by antidromic facial nerve stimulation or orthodromic trigeminal stimulation, we recorded AMR from the O. oris after facial nerve stimulation of subthreshold intensity and after weak skin stimulation not activating facial nerve branches.
METHODS
Patients
Informed consent concerning this study was obtained from each participant. We studied 28 consecutive patients with idiopathic HFS (4 men and 24 women) prospectively. Mean age was 63 years (range 41 to 86), and the mean duration of illness was 6 years (range 1 to 16). Of the 28 patients, 17 were affected on the left side of the face and 11 on the right side. Eleven patients had never been treated with botulinum toxin type A injection, and the remaining 17 who had received this treatment (mean 3 times; range 1 to 7), were examined more than 4 months after the last treatment. Brain magnetic resonance imaging and magnetic resonance angiography, performed on all the patients, detected nothing suggestive of vascular malformation, tumour, or bony abnormality of the skull. Twenty patients with blepharospasm served as controls.
AMR after facial nerve stimulation
Subjects lay supine with eyes closed. The zygomatic branch of the facial nerve was stimulated at the lower edge of the zygomatic bone, and the compound muscle action potentials (CMAP) of the O. oculi and AMR of the O. oris were simultaneously recorded using surface electrodes placed at a lateral separation of 2–3 cm using a Viking 4 electromyography machine (Nicolet Biomedical Japan, Tokyo, Japan). The stimulus, with duration of 0.2 ms, was given at a frequency <0.5 Hz. Band pass for the recordings was 1 Hz to 3 kHz. The grounding electrode was placed on the forehead. In all the subjects, the stimulus intensity was adjusted to be clearly supramaximum and then subthreshold of the facial nerve. AMR was defined as a clearly identifiable potential on superimposed traces of four consecutive trials with an amplitude exceeding 0.2 mV.
AMR after skin stimulation
Recordings were made simultaneously from the O. oculi and O. oris, and the stimulation of 0.2 ms duration was delivered at the paranasal and temple skin at a frequency <0.5 Hz. Stimulus intensity was set below the motor threshold of the zygomatic branch of the facial nerve, and special care was taken not to elicit the CMAP of the O. oculi and O. oris to confirm that the branches of the facial nerve were not directly stimulated.
SR of blink reflexes
CMAP of the O. oculi and O. oris were simultaneously recorded after supraorbital nerve stimulation with surface electrodes placed on the same sites of the AMR recordings. The stimulus was of 0.2 ms duration with an intensity such that both SR (SR1 and SR2) were just maximum and stable on repeated trials. A frequency of the stimulation was <0.5 Hz.
RESULTS
Frequencies of AMR and SR in patients with HFS or blepharospasm
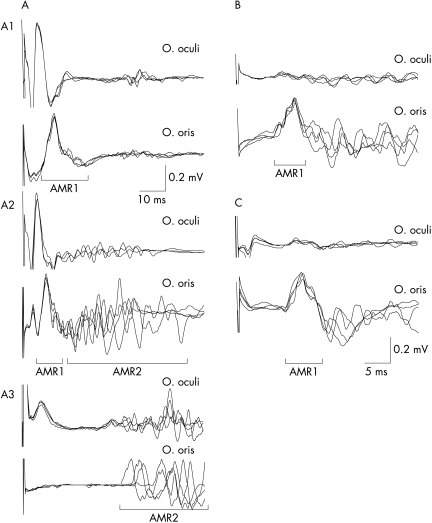
In 28 patients with HFS, AMR after supramaximum stimulation of the zygomatic branch of the facial nerve were recordable in 24 (86%), and SR of blink reflexes were found in 26 (93%) of the patients. AMR or SR or both were elicited in 100% of the HFS patients. In contrast, none of the 20 patients with blepharospasm showed AMR and SR. Typical waveforms of AMR recorded from HFS patients are shown in fig 1A. AMR usually consisted of two components, the early constant component (AMR1) and late variable component (AMR2), partly similar to R1 and R2 of the blink reflex. The mean onset latency was 10.0 ms (range 8.1 to 12.0) for AMR1, and 35.3 ms (range 18.5 to 46.0) for AMR2. Because some of the patients had only one of the two components, we focused on the 24 patients with either AMR1 or AMR2 for later analyses.
Figure 1 Compound muscle action potentials in the orbicularis oculi (O. oculi; upper traces) muscle and abnormal muscle responses (AMR) in the orbicuralis oris (O. oris; lower traces) muscle recorded after supramaximum stimulation at the zygomatic branch of the facial nerve (A), its subthreshold stimulation (B), and paranasal skin stimulation (C). Four successive recordings were superimposed. AMR usually consisted of two components (A2); the early constant component (AMR1) and late variable component (AMR2), but some patients only had either AMR1 (A1) or AMR2 (A3). Note that after motor subthreshold or skin stimulation, there was no compound muscle action potential in the O. oculi and O. oris by direct stimulation.
AMR after subthrehold facial nerve stimulation or after skin stimulation
The frequencies of AMR1 and AMR2 after “subthreshold” stimulation of the zygomatic branch of the facial nerve are shown in table 1. AMR1 were recordable in 43% of the HFS patients and AMR2 in 29%. Fig 1B shows representative waveforms of AMR after “motor subthreshold” stimulation recorded from a HFS patient.
Table 1 Frequencies of abnormal muscle response (AMR) in the orbicularis oris muscle (n = 24).
| Facial nerve stimulation (zygomatic branch) | Skin stimulation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SM | ST | Paranasal | Temple | Either of the two | ||||||
| AMR1* | 96% | 43% | 75% | 42% | 88% | |||||
| AMR2† | 58% | 29% | 63% | 42% | 67% | |||||
*Early component; †late component. SM, supramaximum; ST, subthreshold.
Table 1 also shows the frequencies of AMR1 and 2 after skin stimulation. AMR1 were recordable in 75% of the HFS patients after paranasal skin stimulation, and in 42% after temple skin stimulation. AMR1 could be elicited in up to 88% of the HFS patients after stimulation at either of the two sites of the facial skin. Fig 1C shows typical waveforms of AMR after skin stimulation.
DISCUSSION
Our results confirmed that AMR and SR are very frequently found in patients with HFS; 100% of the HFS patients had AMR or SR or both. These abnormal responses were present in none of the patients with blepharospasm, and their presence strongly supports a diagnosis of HFS rather than blepharospasm.13,14,15,16,17
Our findings show that AMR are frequently elicited after motor subthreshold or weak skin stimulation, suggesting that the afferent pathway of AMR is the trigeminal nerve and that therefore, AMR is a type of trigeminal reflex, rather than F‐wave. The afferent pathway of AMR has not been verified, because all previous studies investigated AMR in HFS patients by the use of supramaximum stimulation only.14,15,16 Motor subthreshold weak stimulation would activate peripheral nerve branches of the trigeminal nerve or skin sensory receptors, and thereby, could elicit trigeminal facial reflexes.
The possibility that a part of facial nerve axons are activated after skin stimulation could not be excluded completely. However, stimulation delivered to the skin with an intensity below motor threshold of the zygomatic branch of the facial nerve is unlikely to predominantly activate facial axons rather than trigeminal axons. Recent evidence shows that in human peripheral nerves, sensory axons have substantially greater persistent Na+ currents than do motor axons, and consequently, firing threshold is significantly lower in sensory than in motor axons.19,20 This is possibly the case for facial and trigeminal axons also. The two components of AMR, AMR1 and AMR2, are partly similar to R1 and R2 of the blink reflex, and this is consistent with a view that AMR is mediated by trigeminal afferents. Furthermore, AMR2 has a long onset latency (mean 35.3 ms), inconsistent with latencies of facial F waves. Finally, subthreshold facial nerve stimulation and skin stimulation delivered to multiple sites elicited similar waveforms of AMR. We suggest that trigeminal afferents are at least largely responsible for triggering AMR in patients with HFS.
This study revealed that the trigeminal facial reflexes are exaggerated in HFS, supporting the idea of hyperexcitability of the facial motor neurones at the level of nucleus.11,14,15 Our results did not show direct evidence of the hyperexcitability of the facial motor neurone pool. However, we think that the presence of the abnormally exaggerated trigeminal facial reflex, which is not evoked in normal subjects, cannot be explained by changes in peripheral facial nerve excitability. Measurements of AMR and SR could provide quantitative information about the hyperexcitability of facial motor neurones in HFS patients, and could be used for objective evaluation of therapeutic intervention, such as treatment with botulinum injection.11 Our findings showed that supramaximum zygomatic branch stimulation most frequently elicits AMR. However, skin stimulation at any site of the face or submaximum stimulation also evokes these abnormal responses, and could be used as an optional procedure of less painful stimulation to prove hyperexcitability of facial motor neurones in HFS.
Abbreviations
AMR - abnormal muscle responses
CMAP - compound muscle action potentials
HFS - hemifacial spasm
O - orbicularis
SR - synkinetic responses
Footnotes
Competing interests: none
References
- 1.Nilsen B, Le K D, Dietrichs E. Prevalence of hemifacial spasm in Oslo, Norway. Neurology 2004631532–1533. [DOI] [PubMed] [Google Scholar]
- 2.Auger R G. Hemifacial spasm: clinical and electrophysiologic observations. Neurology 1979291261–1272. [DOI] [PubMed] [Google Scholar]
- 3.Auger R G, Piepgras D G, Laws E R.et al Microvascular decompression of the facial nerve for hemifacial spasm: clinical and electrophysiologic observations. Neurology 198131346–350. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen V K. Pathophsiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Neurology 198434418–426. [DOI] [PubMed] [Google Scholar]
- 5.Wang A, Jankovic J. Hemifacial spasm: clinical findings and treatment. Muscle Nerve 1998211740–1747. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen V K. Electrophysiology of the facial nerve in hemifacial spasm: ectopic/ephaptic excitation. Muscle Nerve 19858545–545. [DOI] [PubMed] [Google Scholar]
- 7.Sanders D B. Ephaptic transmission in hemifacial spasm: a single‐fiber EMG study. Muscle Nerve 198912690–694. [DOI] [PubMed] [Google Scholar]
- 8.Esteban A, Molina‐Negro P. Primary hemifacial spasm: a neurophysiological study. J Neurol Neurosurg Psychiatry 19864958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Møller A R, Jannetta P J. On the origin of synkinesis in hemifacial spasm: result of intracranial recording. J Neurosurg 198461569–576. [DOI] [PubMed] [Google Scholar]
- 10.Møller A R, Jannetta P J. Hemifacial spasm: results of electrophysiologic recording during microvascular decompression operations. Neurology 198535969–974. [DOI] [PubMed] [Google Scholar]
- 11.Ogawara K, Kuwabara S, Kamitsukasa I.et al Trigeminal afferent input alters the excitability of facial motoneurons in hemifacial spasm. Neurology 2004621749–1752. [DOI] [PubMed] [Google Scholar]
- 12.Møller A R, Jannetta P J. Blink reflex in patients with hemifacial spasm. Observations during microvascular decompression operations. J Neurol Sci 198672171–182. [DOI] [PubMed] [Google Scholar]
- 13.Kim P, Fukushima T. Observations on synkinesis in patients with hemifacial spasm: Effect of microvascular decompression and etiological considerations. J Neurosurg 198460821–827. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa M, Ohira T, Namiki J.et al Abnormal muscle response (lateral spread) and F‐wave in patients with hemifacial spasm. J Neurol Sci 1996137109–116. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa M, Ohira T, Namiki J.et al Electrophysiological investigation of hemifacial spasm after microvascular decompression: F waves of the facial muscles, blink reflexes, and abnormal muscle responses. J Neurosurg 199786654–661. [DOI] [PubMed] [Google Scholar]
- 16.Møller A R, Sen C N. Recordings from the facial nucleus in the rat: signs of abnormal facial muscle response. Exp Brain Res 19908118–24. [DOI] [PubMed] [Google Scholar]
- 17.Tankéré F, Maisonobe T, Lamas G.et al Electrophysiological determination of the site involved in generating abnormal muscle responses in hemifacial spasm. Muscle Nerve 1998211013–1018. [DOI] [PubMed] [Google Scholar]
- 18.Møller A R. Interaction between the blink reflex and the abnormal muscle response in patients with hemifacial spasm: results of intraoperative recordings. J Neurol Sci 1991101114–123. [DOI] [PubMed] [Google Scholar]
- 19.Mogyoros I, Kiernan M C, Burke D. Strength‐duration properties of human peripheral nerve. Brain 1996119439–447. [DOI] [PubMed] [Google Scholar]
- 20.Bostock H, Rothwell J C. Latent addition in motor and sensory fibres of human peripheral nerve. J Physiol (Lond) 1997498277–294. [DOI] [PMC free article] [PubMed] [Google Scholar]