Abstract
Vascular endothelial growth factor (VEGF) is implicated in motor neurone degeneration. In normal individuals, hypoxia is known to induce an overexpression of VEGF, as measured in CSF. We show that patients with ALS do not manifest this VEGF overexpression in the presence of hypoxia. Although VEGF gene expression is mainly stimulated by hypoxia, we have measured lower VEGF levels in cerebrospinal fluid (CSF) from hypoxaemic patients with amyotrophic lateral sclerosis (ALS) than in CSF from normoxaemic patients with ALS. In contrast, hypoxaemic neurological controls displayed higher levels than normoxaemic neurological controls. There was a negative correlation between VEGF levels and the severity of hypoxaemia in patients with ALS, suggesting deregulation of VEGF in ALS.
Keywords: amyotrophic lateral sclerosis, VEGF, hypoxia, motor neurone degeneration
Amyotrophic lateral sclerosis (ALS) is a degenerative motor neurone disorder that leads relentlessly to death within 3–5 years of onset. Epidemiological studies have revealed a high incidence of this disease in patients who experience hypoxic conditions, for example during heavy exercise.1 Moreover, progression of the disease is soon marked by respiratory muscle weakness, leading first to nocturnal desaturation and then to alveolar hypoventilation.2 Evidence for a potential implication of VEGF polymorphysm as a risk factor for developing ALS in mice and human was shown by Lambrechts et al.3 At risk haplotypes are associated with lower VEGF expression in patients with ALS and controls, suggesting a quantitative and/or qualitative defect of this neurotrophic factor.3 Expression of the VEGF gene is mainly stimulated by hypoxia through the binding of hypoxia inducible factor (HIF) to a defined hypoxia response element (HRE) in the promoter region. Deletions in the HRE region provoked motor neurone degeneration in a mouse model with pathological features similar to those seen in human ALS.4 Reduced availability of VEGF gives rise to selective motor neurone degeneration via neurotrophic and angiogenic mechanisms.5 Recently, intracerebroventricular delivery or intraperitoneal injections of VEGF have been shown to improve motor performance and significantly prolong survival in two animal models of ALS.6,7 We previously demonstrated lower VEGF levels in CSF in patients with early ALS compared with controls.8 In light of the central role of hypoxia in the regulation of VEGF, we decided to investigate VEGF levels in CSF and plasma as a function of hypoxaemia in patients with sporadic ALS, and compare them with neurological control subjects.
PATIENTS AND METHODS
Patients were referred to our hospital for diagnosis. Plasma and cerebrospinal fluid (CSF) from patients with ALS and control subjects were obtained with informed consent as part of the diagnostic evaluation and/or a research protocol. The characteristics of the 20 sporadic patients with ALS and the 20 neurological controls are summarised in table 1. At the time of lumbar puncture, 10 patients fulfilled the El Escorial diagnostic criteria for definite ALS (six in the group of hypoxaemic patients, four in the non‐hypoxaemic group), and 10 for probable ALS. Neurological controls included degenerative disorders: Parkinson's disease, supranuclear palsy, hydrocephaly, spinocerebellar ataxia, neuropathy, Alzheimer's disease, Lewy body disease, and chorea. The causes of hypoxaemia were predominantly apnoea syndrome, restrictive syndrome (interstitial syndrome), or mixed restrictive and chronic obstructive pulmonary disease, confirmed by pulmonary function testing. Patients with inflammatory or neoplastic diseases were excluded. A normal electrophoretic analysis (total protein, serum albumin in plasma, total protein in CSF) was performed to exclude malnutrition (table 1). The postcentrifugation blood supernatants and the CSF samples were stored in a refrigerator at −80°C, and the maximum time limit for the storage of the samples never exceeded 3 hours. VEGF levels in CSF and plasma were respectively determined using a chemiluminescent assay (QuantiGlo; R&D Systems; 1.76 pg/ml detection limit) and an ELISA test (Quantikine; R&D Systems), as previously described.8 These assays measure unbound VEGF with high intra‐assay and interassay precision, and recognise recombinant VEGF as well as VEGF165 (the major and most potent natural isoform). All VEGF measurements were carried out on the same day and with the same kit.
Table 1 Clinical and biological characteristics.
| Patients with ALS | Neurological controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Hypoxaemic | Normoxaemic | Hypoxaemic | Normoxaemic | |||||
| No. of patients | 10 | 10 | 10 | 10 | ||||
| Age (years) | 64.4 (31–78) | 67.5 (57–77) | 73.4 (63–84) | 67 (28–80) | ||||
| Sex ratio | 6 F/4 M | 3 F/7 M | 1 F/9 M | 3 F/7 M | ||||
| Disease duration DD (months) | 20.6 (9–30) | 17.7 (9–27) | ||||||
| Onset form (DD)* | 7 B (19.4 (7.1)); 3 S (20.6 (7.64)) | 4 B (19.75 (8.1)); 6 S (14.6 (6.2)) | ||||||
| El Escorial category | 6 definite/4 probable | 4 definite/6 probable | ||||||
| Total serum protein (g/l) | 70 (64–77) | 72 (61–82) | 70.8 (56–80) | 70.9 (65–76) | ||||
| Serum albumin (g/l) | 43 (35–58) | 45.4 (38–56) | 39 (36–42) | 41.3 (35.2–48.5) | ||||
| Total CSF protein (g/l) | 0.54 (0.3–0.8) | 0.48 (0.4–0.6) | 0.57 (0.4–0.8) | 0.64 (0.46–1) | ||||
| PaO2 mean level (mm Hg) | 73.5 (67–77)† | 100.5 (96–103)† | 70 (64–74)§ | 95 (94–101)§ | ||||
| Mean nocturnal SaO2 level (%) | 88 (80–91) | 91.2 (87–96) | 81.3 (66–91) | 90 (88–92) | ||||
| SaO2 90 (% of night time ⩽90%) | 4 (2–9)† | 0 (0–0) | 3 (1–5)§ | 0 (0–1) | ||||
| PaCO2 mean level (mm Hg) | 43.7 (39–45.9) | 39 (28.2–45) | 41.6 (26.4–47.8) | 41.5 (38–46) | ||||
| CSF VEGF level (pg/ml) | 3 (2.2–4.7)†‡ | 6 (5.2–7)† | 9.5 (8.2–10.7)‡§ | 5.5 (3.25–7.75)§ | ||||
| Plasma VEGF level (pg/ml) | 167 (120–368) | 154 (110–255) | 266 (240–583) | 224 (131–323) | ||||
Clinical and biological characteristics of the four groups of subjects. Data are stated as median values and (first and third quartiles), except *mean (SD). B, bulbar; S, spinal; CSF, cerebrospinal fluid; VEGF, vascular endothelial growth factor. †Significant difference (p<0.05) between hypoxaemic and normoxaemic patients with ALS; ‡significant difference (p<0.05) between hypoxaemic patients with ALS and hypoxaemic neurological controls; §significant difference (p<0.05) between hypoxaemic and normoxaemic neurological controls.
Diurnal hypoxaemia was assessed by three morning arterial blood gas analyses for each patient. Patients and controls were considered to be hypoxaemic if their oxygen arterial blood pressure (PaO2) was below the age dependent minimum reference value in each arterial blood gas sampling (table 1). Nocturnal hypoxaemia was assessed by nocturnal oxymetry and expressed as a percentage of night time level with an oxygen saturation (SaO2) <90% (SaO2 90, table 1). They were considered to be hypoxaemic if their SaO2 90 was ⩾2%, as previously described.9 Statistical analysis included analysis of variance on ranks using the Bonferroni post hoc test, a χ2 tes,t and Spearman's rank correlation coefficient.
RESULTS
A significant difference in CSF VEGF levels was observed when comparing the four groups (F = 13.7, p = 0.0001; table 1). Each group contained 10 subjects. The first group comprised the hypoxaemic patients with ALS, the second the normoxaemic patients with ALS, the third the hypoxaemic neurological controls, and the fourth the normoxaemic ones (table 1). The post hoc test showed that CSF VEGF levels were significantly: (a) lower in hypoxaemic patients with ALS than in both normoxaemic patients with ALS (p = 0.002) and hypoxaemic neurological controls (p = 0.0001) and (b) higher in hypoxaemic than in normoxaemic controls (p = 0.04). There was no significant difference between CSF VEGF levels in normoxaemic patients with ALS and normoxaemic neurological controls.
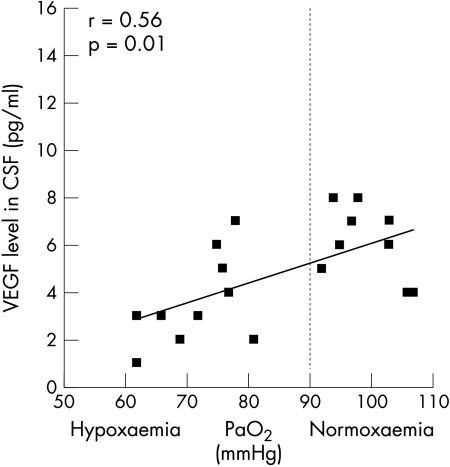
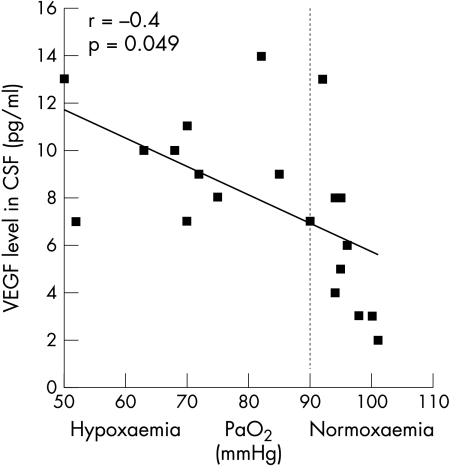
In patients with ALS, we noted a significant positive correlation between VEGF levels in CSF and PaO2 (r = 0.56, p = 0.01) (fig 1) and SaO2 (r = 0.41, p = 0.042). The opposite response appeared in neurological controls, with a significant negative correlation between VEGF levels in CSF and PaO2 (r = −0.4, p = 0.049) (fig 2) and a tendency towards negative correlation for mean nocturnal SaO2 (r = 0.23, p = 0.1). No correlation was found between PaCO2 and VEGF levels in CSF. Plasma levels did not reach the significance level. Equally, we found no correlation between VEGF levels in plasma and those in CSF. CSF VEGF levels did not correlate linearly with age, clinical presentation or disease duration.
Figure 1 CSF levels of vascular endothelial growth factor (VEGF) in patients with ALS according to the level of hypoxaemia (PaO2), displaying a positive correlation between VEGF levels in CSF and PaO2 levels (r = 0.56, p = 0.01).
Figure 2 CSF levels of vascular endothelial growth factor (VEGF) in neurological controls according to the level of hypoxaemia (PaO2), displaying a negative correlation between VEGF levels in CSF and PaO2 levels (r = −0.4, p = 0.05).
DISCUSSION
We observed a paradoxical response, with a lack of VEGF upregulation in hypoxaemic patients with ALS compared with neurological controls; the latter displayed increased levels of VEGF in CSF during hypoxaemia. A positive correlation was found between VEGF levels in CSF and the severity of hypoxaemia in hypoxaemic patients with ALS, with the opposite correlation in hypoxaemic neurological controls. From these results, we attempted to assess how VEGF rates should be considered with respect to hypoxaemia, which was not performed in previous studies.8,10,11 In our first paper,8 low levels of VEGF were observed in the CSF of patients with sporadic ALS within the first year post‐onset.8 Of the 24 patients with ALS, the five patients with hypoxaemia (PaO2 73 mmHg) also showed a trend towards lower levels; however, these did not reach significance because of the small sample size.7
The abnormal VEGF levels in our hypoxaemic patients with ALS suggest a central role of VEGF and hypoxia in the occurrence or worsening of ALS. The involvement of VEGF in the pathogenesis of ALS should be viewed more as deregulation of the hypoxia response mechanisms than simply as a decrease in the VEGF baseline level. VEGF is expressed by numerous cells types in central nervous system, including endothelial cells and microglia.5 It is known to have a direct neurotrophic effect on motor neurones in vitro by improving survival in hypoxaemic or excitotoxic conditions.5 It was recently demonstrated that hypoxic induction of VEGF protein is impaired at a very early stage in mutant SOD1 mice.12 Nevertheless, the paradoxical decrease in VEGF165 dependent neuroprotection in hypoxic patients with ALS may reflect a variety of different physiopathological mechanisms that are still unknown. VEGF receptors could be involved, as the reduction of the VEGF receptor, fetal liver kinase (Flk‐1), by antisense nucleotides induces motor neurone death in rat spinal cords exposed to hypoxia.12 The abnormal response to hypoxia in ALS could also be related to excessive repression of HRE, in the VEGF gene promoter, which is normally stimulated by hypoxia through binding of HIF and leads to the expression of the VEGF gene. Furthermore, the behaviour of other regulation or protective factors (HIF, nuclear factor κ B, erythropoietin during hypoxaemia in ALS has not yet been studied.
In our study, CSF measurements of VEGF seemed more efficient in predicting hypoxia than did plasma measurements. This could be explained by the greater number of factors influencing VEGF levels in plasma wcompared with CSF, such as hypoxaemia regulation mechanisms in neurological controls and amyotrophy in patients with ALS.9
Increased plasma VEGF levels correspond to a normal hypoxia response mechanism,3,13 as displayed by the hypoxaemic neurological controls, who had higher plasma levels (mean 266 pg/ml; range 240–583]) compared with the normoxaemic controls (mean 224 pg/ml; range 131–323). The lack of significant increase in plasma VEGF levels in the hypoxaemic patients with ALS, who showed similar levels to the normoxaemic patients, suggest a general (plasma and CSF) deregulation of VEGF response to hypoxia in ALS. The deregulation was more obvious and significant in CSF possibly because there were fewer factors influencing the VEGF levels in CSF and because of the highest sensitivity of motor neurones to hypoxia.5,13 The effect of the circulatory VEGF on blood brain barrier permeability seems therefore insufficient to compensate for the CNS VEGF decrease in ALS.
The respiratory state of hypoxaemic patients with ALS was mostly represented by mild daytime hypoxaemia (PaO2 67–77 mm Hg) with a slight increase in PaCO2 (43.7 mm Hg in hypoxic patients with ALS versus 39 mm Hg in normoxic patients with ALS, p = 0.1), in accordance with the moderate disease duration (20.6 months; table 1). This observation supports the involvement of VEGF deregulation in the initial stages of the disease, and not as the consequence of the decreasing number of motor neurones. All the hypoxaemic patients with ALS also presented significant nocturnal desaturation compared with non‐hypoxaemic patients. Respiratory disorders observed in ALS are characterised by early repeated intermittent acute hypoxaemia episodes during the night.2 We believe that non‐invasive ventilation should be introduced in this early stage of the disease in order to limit motor neurone destruction.14
Evidence for a VEGF deregulation in response to hypoxia in patients with ALS lead us to support the use of VEGF replacement therapy as a neuroprotective factor.5 Additional studies are required in order to assess the potential therapeutic effects of VEGF.
ACKNOWLEDGEMENTS
This work was supported by grants from the Association pour la Recherche sur la SLA (ARS). The authors wish to thank G Marchandise for performing the VEGF measurements, M Khirredine and E Pélécanos for collecting the data, and Dr D Fraser (Biotech Communication, Damery, France) for proofreading the manuscript.
Footnotes
Competing interests: none
References
- 1.Chio A, Benzi G, Dossena M.et al Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain 2005128472–476. [DOI] [PubMed] [Google Scholar]
- 2.Lyall R A, Donaldson N, Polkey M I.et al Respiratory muscle strength and ventilatory failure in amyotrophic lateral sclerosis. Brain 20011242000–2013. [DOI] [PubMed] [Google Scholar]
- 3.Lambrechts D, Storkebaum E, Morimoto M.et al VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motorneurons against ischemic death. Nat Genet 200334383–394. [DOI] [PubMed] [Google Scholar]
- 4.Oosthuyse B, Moons L, Storkebaum E.et al Deletion of the hypoxia–response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet 200128131–138. [DOI] [PubMed] [Google Scholar]
- 5.Van den Bosh L, Storkebaum E, Vleminckx V.et al Effects of VEGF on motor neuron degeneration. Neurobiol Dis 20041721–28. [DOI] [PubMed] [Google Scholar]
- 6.Storkebaum E, Lambrechts D, Dewerchin M.et al Treatment of motor neuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci 2005885–92. [DOI] [PubMed] [Google Scholar]
- 7.Zheng C, Nennesmo I, Fadeel B.et al Vascular endothelial growth factor prolongs survival in a transgenic mouse model of ALS. Ann Neurol 200456564–567. [DOI] [PubMed] [Google Scholar]
- 8.Devos D, Moreau C, Lassalle P.et al Low levels of VEGF in CSF from early ALS patients. Neurology 2004622127–2129. [DOI] [PubMed] [Google Scholar]
- 9.Hukins C A, Hillman D R. Daytime predictors of sleep hypoventilation in Duchenne muscular dystrophy. Am J Respir Crit Care Med 2000161166–170. [DOI] [PubMed] [Google Scholar]
- 10.Nygren I, Larson A, Johansson A.et al VEGF is increased in serum but not in spinal cord from patients with amyotrophic lateral sclerosis. Neuroreport 2002132199–2201. [DOI] [PubMed] [Google Scholar]
- 11.Ilzecka J. Cerebrospinal fluid VEGF in patients with ALS. Clin Neurol Neurosurg 2004106289–293. [DOI] [PubMed] [Google Scholar]
- 12.Shiote M, Nagano I, Ilieva H.et al Reduction of a vascular endothelial growth factor, fetal liver kinase 1, by antisense oligonucleotides induces motor neuron death in rat spinal cord exposed to hypoxia. Neuroscience 2005132175–178. [DOI] [PubMed] [Google Scholar]
- 13.Schulz R, Hummel C, Heineman S.et al Serum levels of VEGF are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med 200216567–70. [DOI] [PubMed] [Google Scholar]
- 14.Pinto A C, Evangelista T, Carvalho M.et al Respiratory assistance with a non–invasive ventilator (Bipap) in MND/ALS patients: survival rates in a controlled trial. J Neurol Sci 1995129(suppl)19–26. [DOI] [PubMed] [Google Scholar]