Abstract
The objective of this study was to assess different methods of measuring therapy adherence in Parkinson's disease (PD). In a single centre observational study, 112 patients with idiopathic PD were randomised to a crossover trial of active monitoring (n = 69, simple tablet count and electronic monitoring), or to no monitoring (n = 43, control group). All patients completed a self report and visual analogue scale (VAS) indicating therapy intake. In the active monitoring group, 56 (81% of cases) used ⩾80% of their medication, and 13 (19% of cases) used <80%, based on electronic monitoring. Median adherence for self report was 100% (interquartile range (IQR) 100 to 100) and for VAS was 100% (IQR 95 to 100), in both active and control groups. Patients taking ⩾80% of prescribed medication had a median total adherence of 98% (IQR 93 to 101) by electronic monitoring, which was similar to that from other methods: self report 100%, IQR 100 to 100; VAS 100%, IQR 95 to 100; simple tablet count 98%, IQR 89 to 100. Median total adherence in patients taking <80% of medication was significantly lower by electronic monitoring (69%, IQR 44 to 74) than by other methods: self report 100%, IQR 100 to 100; VAS 100%, IQR 95 to 100; and simple tablet count 90%, IQR 78 to 100 (all p<0.0001). Sensitivities of self report (10%), VAS (17%), and simple tablet count (50%) were all low for detecting suboptimal medicine intake. Self report, VAS, and simple tablet counts are insensitive as predictors of suboptimal medicine usage in PD. How patients take their medicines influences interpretation of the therapy response and consequent management decisions, with implications for clinical trial analysis and clinical practice.
Keywords: Parkinson's disease, therapy adherence, method comparison
Consideration of how patients take their medication is vital in understanding the therapeutic response. In Parkinson's disease (PD), an excellent response to levodopa supports the clinical diagnosis,1 while in other disorders poor adherence to drug therapy is frequent and is a major reason for impaired response.2 Evidence for excess medication intake by some PD patients is based largely on self reporting,3 and electronic monitoring (in which bottles with microprocessors record the dates and times of bottle opening) showed that only 1 in 10 patients had complete schedule adherence.4 Simple tablet counts are often used in clinical trials to indicate satisfactory adherence in PD5,6 and other conditions.7 The current study applied multiple techniques of assessing therapy adherence in PD to define sensitivity of these methods against the gold standard of electronic monitoring.8
METHODS
Study population
Consecutive movement disorder clinic patients with idiopathic PD fulfilling UK Brain Bank Criteria were enrolled. Patients provided signed informed consent and the local ethics committee approved the protocol. Patients were taking at least one antiparkinsonism drug but were excluded if use of electronic bottles might adversely affect care.
Study design
A prospective single blind randomised crossover design was undertaken. Two thirds of patients underwent active monitoring, consisting of 2×3 month periods of simple tablet count alone, or simple tablet count concurrent with electronic monitoring, in random order. The remaining one third received no additional therapy monitoring (control group). Data were tested for any order effect on electronic monitoring results (performed first or second in the crossover design). Baseline assessments used the Unified Parkinson's Disease Rating Scale (UPDRS) 1 to 4, Hoehn and Yahr, Schwab and England, Geriatric Depression Scale (GDS), and Mini Mental State Examination (MMSE). Clinical scoring was blind to therapy monitoring method. PD medication was dispensed into separate bottles for each drug type and strength, for electronic monitoring. At 3 months patients completed a validated self report9 of medication intake. They also marked a visual analogue scale (VAS) for each PD drug (range 0 to 120), and graded their accuracy of medicine intake (missed or extra doses). Patients' opinion about being undertreated, overtreated, or "about right" was scored, and the clinician independently recorded their impression of treatment. Patients unable to use the monitoring bottles or who misused them were withdrawn.
Outcome measures
Total adherence (the amount of medication taken compared with the amount prescribed) was estimated by self report, VAS, simple tablet count, and electronic monitoring. Additionally, daily adherence (the percentage of days the correct number of doses was taken) and timing adherence (the percentage of doses taken at the correct time interval) were calculated from electronic monitoring data. Electronic monitoring and matched simple tablet count data were compared by paired t tests. Average medicine intake was used to categorise patients as having "satisfactory adherence" (⩾80% intake) or "underuse" (<80% intake), and electronic and tablet count methods were compared by McNemar's test. Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA) and Statistica (StatSoft, Bedford, UK) software.
RESULTS
Of 135 patients approached, 6 (4%) declined to take part, mainly due to perceived disruption from using electronic monitoring bottles, leaving 129 cases randomised. Seventeen patients (13% of randomised cases) were excluded from analysis, 3 withdrew consent, 9 had problems with the electronic monitoring bottles, 4 patients were lost to follow up, and 1 died, leaving 112 assessable cases. There were no significant differences in baseline characteristics between patients completing versus those dropping out or between patients with active therapy monitoring and controls (table 1).
Table 1 Patient characteristics by group.
| Active therapy monitoring (n = 69) | No additional therapy monitoring (n = 43) | |||
|---|---|---|---|---|
| Male | 57% | 70% | ||
| Age, years | 64 (12) | 63 (8) | ||
| Number prescribed levodopa | 46 (67%) | 30 (70%) | ||
| Average levodopa dose, mg | 526 | 427 | ||
| Number on dopamine agonist | 50 (72%) | 30 (70%) | ||
| Number of PD drugs | 2 (1) | 2 (1) | ||
| Number of PD administrations | 4 (2) | 4 (1) | ||
| Number of PD tablets per day | 9 (5) | 8 (5) | ||
| Number of non‐PD drugs per day | 2 (2) | 3 (3) | ||
| Number of tablets per day | 12 (5) | 11 (5) | ||
| Taking >4 levodopa daily doses | 15 (20%) | 9 (21%) | ||
| Normally use compliance aid | 18 (26%) | 19 (44%) | ||
| Carer helps with medication | 13 (19%) | 8 (19%) | ||
| Duration of PD (years) | 7 (5) | 7 (5) | ||
| UPDRS 2 | 14 (6) | 15 (7) | ||
| UPDRS 3 | 27 (12) | 28 (10) | ||
| Number with dyskinesia | 21 (30%) | 10 (23%) | ||
| Number with “wearing off” | 33 (48%) | 23 (53%) | ||
| Hoehn and Yahr | 2.4 (0.6) | 2.5 (0.6) | ||
| Schwab and England | 78 (13) | 74 (16) | ||
| MMSE | 28 (4) | 28 (3) | ||
| Geriatric depression score | 11 (7) | 10 (8) |
Data are mean (standard deviation) or number (percentage). PD, Parkinson's disease; UPDRS, Unified Parkinson's disease rating scale; MMSE, Mini Mental State Examination. There were no significant differences between groups.
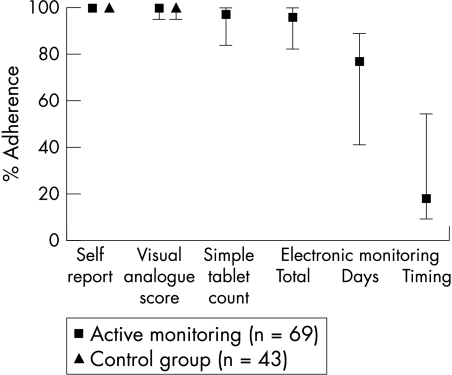
Median adherence rates for self report and VAS were 100% for both actively monitored and control patients (fig 1). More actively monitored patients reported missed doses (39 of 69; 57%) than controls (17 of 43; 40%) but the difference was not significant, and extra doses were reported in 23% of cases for both actively monitored and control patients.
Figure 1 Therapy adherence in 112 patients according to monitoring method. Self report, VAS score, simple tablet count, and total adherence by electronic monitoring all showed high adherence rates. Day and timing adherence rates were lower, indicating irregular and erratic medicine intake. Data are median and interquartile range.
For the 69 active monitoring patients, 111 drugs had matched electronic monitoring and simple tablet count data. The median total adherence measured by simple tablet counts was 97% (interquartile range (IQR) 84 to 100) versus 96% (IQR 86 to 100) using electronic monitoring. Median daily adherence was 77% (IQR 41 to 89) and median timing adherence was 18% (IQR 9 to 54) (fig 1). There was no evidence of any order effect on electronic monitoring results. Of the 69 actively monitored patients, 56 (81%) took ⩾80% and 13 (19%) took <80% of prescribed medication. Considering the <80% group, self report (median 100%, IQR 100 to 100), VAS (median 100%, IQR 95 to 100), and simple tablet counts (median 90%, IQR 78 to 100) all significantly overestimated median total adherence when compared with the result of electronic monitoring (69%, IQR 44 to 74, all p<0.0001). Considering those with ⩾80% adherence, self report (median 100%, IQR 100 to 100), VAS (median 100%, IQR 95 to 100) and simple tablet counts (median 98%, IQR 89 to 100) were not significantly different from electronic monitoring adherence (98%, IQR 93 to 101). Categorisation of medicine intake as ⩾80% or <80% showed a significant difference between electronic monitoring and simple tablet count (p<0.0001, McNemar's test). In those patients who reported suboptimal adherence, electronic monitoring confirmed underuse indicating high specificity (self report 100%; VAS score 97%), but sensitivity was low (self report 10%; VAS score 17%). The broader adherence question of reporting any missed doses increased sensitivity to 77%, but at the expense of low specificity (46%), compared with electronic monitoring. The sensitivity of simple tablet count was 50% and specificity was 76%, compared with electronic monitoring. The proportion of patients with electronic monitoring showing <80% adherence who scored positive on the suboptimal adherence questions was greater (10 of 13 cases; 77%) than in those with good (⩾80%) adherence (36 of 56 cases; 64%) (not significant). Of the 112 patients, 45 (40%) reported never missing or taking an extra dose (from the two adherence questions), which would represent 100% daily adherence in these cases, while in fact no patients achieved this, and only 3 patients (3%) had daily adherence >95%. Median daily adherence of those who reported missed doses was 70% (IQR 36–88) which just reached significance compared with those reporting perfect adherence (81%, IQR 70–89) (p = 0.05).
One quarter of patients reported undertreatment, less than 5% overtreatment, and the remainder regarded their medication level as "about right". Patients taking <80% of their medication (by electronic monitoring), were more likely to report undertreatment (5 of 13; 38%) than those adhering to the prescribed regimen (13 of 56; 23%) (not significant). None of the underusers reported that they were being overtreated. Doctors were more likely than patients to judge that therapy levels were on the side of overtreatment (doctors 10%, patients 6%).
There were no significant differences between the number and types of adverse effects between those with active therapy monitoring and controls.
DISCUSSION
Our finding that self reports, VAS, and simple tablet counts overestimate adherence compared against electronic monitoring in PD is consistent with other diseases.10,11,12,13,14 Electronic monitoring is the established reference technique,8,11,15 and has shown suboptimal intake in PD,4 but ours is the first PD study comparing compliance assessment methods. The results challenge the assumption that symptoms motivate the PD patient to adhere tightly to the drug regimen, which is consistent with other symptomatic diseases.16,17
Although the self report was insensitive in detecting suboptimal PD medication intake, it was highly specific and it also quantified underuse. Our adherence question of missed doses had a similar sensitivity (77%) to the Morisky self report (72%) in depression,10 but neither approach quantifies non‐adherence and the sensitivity is achieved at the expense of specificity. In clinical practice in PD, when self reports or VAS scores are less than 80%, therapy intake is extremely likely to be suboptimum.
Around 40% of our patients declared never missing or taking an extra dose, which compares with 43% in hypertension18 and 30% in HIV positive patients,19 but 3 cases among our 40% declaring perfect adherence used <80% by electronic monitoring, and as in other studies,20 none of our patients in fact had perfect compliance.
Simple tablet counts missed half of undermedicating patients, which has implications for this method in clinical trials.7 In hypertension, "near perfect" pill counts misclassified 22% as having satisfactory adherence13 and in epilepsy, only 13% of patients with suboptimal compliance were detected by pill count,8 with clear implications for therapeutic efficacy.21,22 Additionally, while simple tablet counts are achievable (96% in one study in depression) they are often unreliable (22% of cases).10 We were able to calculate accurate simple tablet counts in only 72% of cases. The complexity of PD therapy influenced this: patients often maintained supplies in more than one location, and sometimes emptied containers before the clinic visit, as experienced elsewhere.23,24,25 Another limitation in these methods is the under‐reporting of excessive medication in those deliberately taking excess therapy.
It was interesting that patients who take less medication than prescribed more frequently reported feeling undertreated. This clinical scenario can lead to a recommendation to increase therapy further, with the potential for further divergence between prescribed and actual medication intake. Unfortunately, simple compliance approaches are unlikely to help; in situations where alternative reasons for poor therapy response (such as development of a Parkinson plus disorder) are not present, techniques such as electronic compliance monitoring may be worthwhile.
ACKNOWLEDGEMENTS
We thank the Neurosciences Foundation (Glasgow) for supporting this project.
Abbreviations
GDS - Geriatric Depression Scale
MMSE - Mini Mental State Examination
PD - Parkinson's disease
UPDRS - Unified Parkinson's Disease Rating Scale
VAS - visual analogue scale
Footnotes
Competing interests: none
References
- 1.Hughes A J, Daniel S E, Kilford L.et al Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry 199255181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urquhart J. Role of patient compliance in clinical pharmacokinetics. A review of recent research. Clin Pharmacokinet 199427202–215. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence A D, Evans A H, Lees A J. Compulsive use of dopamine replacement therapy in Parkinson's disease: reward systems gone awry? Lancet Neurol 20032595–604. [DOI] [PubMed] [Google Scholar]
- 4.Leopold N A, Polansky M, Hurka M R. Drug adherence in Parkinson's disease. Mov Disord 200419513–517. [DOI] [PubMed] [Google Scholar]
- 5.Rascol O, Brooks D J, Brunt E R.et al Ropinirole in the treatment of early Parkinson's disease: a 6‐month interim report of a 5‐year levodopa‐controlled study. 056 Study Group. Mov Disord 19981339–45. [DOI] [PubMed] [Google Scholar]
- 6.Parkinson Study Group Safety and efficacy of pramipexole in early Parkinson disease. A randomized dose‐ranging study. Parkinson Study Group. JAMA 1997278125–130. [DOI] [PubMed] [Google Scholar]
- 7.Hughes D A, Bagust A, Haycox A.et al Accounting for noncompliance in pharmacoeconomic evaluations. Pharmacoeconomics 2001191185–1197. [DOI] [PubMed] [Google Scholar]
- 8.Cramer J A, Mattson R H, Prevey M L.et al How often is medication taken as prescribed? A novel assessment technique. JAMA 19892613273–3277. [PubMed] [Google Scholar]
- 9.Svarstad B L, Chewning B A, Sleath B L.et al The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns 199937113–124. [DOI] [PubMed] [Google Scholar]
- 10.George C F, Peveler R C, Heiliger S.et al Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmacol 200050166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer J A. A systematic review of adherence with medications for diabetes. Diabetes Care 2004271218–1224. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton G A. Measuring adherence in a hypertension clinical trial. Eur J Cardiovasc Nurs 20032219–228. [DOI] [PubMed] [Google Scholar]
- 13.Rudd P, Ahmed S, Zachary V.et al Improved compliance measures: applications in an ambulatory hypertensive drug trial. Clin Pharmacol Ther 199048676–685. [DOI] [PubMed] [Google Scholar]
- 14.Straka R J, Fish J T, Benson S R.et al Patient self‐reporting of compliance does not correspond with electronic monitoring: an evaluation using isosorbide dinitrate as a model drug. Pharmacotherapy 199717126–132. [PubMed] [Google Scholar]
- 15.Lee J Y, Kusek J W, Greene P G.et al Assessing medication adherence by pill count and electronic monitoring in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Am J Hypertens 19969719–725. [DOI] [PubMed] [Google Scholar]
- 16.de Klerk E, van der H D, Landewe R.et al Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout. J Rheumatol 20033044–54. [PubMed] [Google Scholar]
- 17.Milgrom H, Bender B, Ackerson L.et al Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol 1996981051–1057. [DOI] [PubMed] [Google Scholar]
- 18.Morisky D E, Green L W, Levine D M. Concurrent and predictive validity of a self‐reported measure of medication adherence. Med Care 19862467–74. [DOI] [PubMed] [Google Scholar]
- 19.Walsh J C, Horne R, Dalton M.et al Reasons for non‐adherence to antiretroviral therapy: patients' perspectives provide evidence of multiple causes. AIDS Care 200113709–720. [DOI] [PubMed] [Google Scholar]
- 20.Macintyre C R, Goebel K, Brown G V. Patient knows best: blinded assessment of nonadherence with antituberculous therapy by physicians, nurses, and patients compared with urine drug levels. Prev Med 20054041–45. [DOI] [PubMed] [Google Scholar]
- 21.Gordis L. Conceptual and methodological problems in measuring compliance. In: Haynes RB, Taylor DW, Sackett DL, eds. Compliance in health care. Baltimore: John Hopkins University Press, 197923–25.
- 22.Haynes R B, Dantes R. Patient compliance in the design and interpretation of clinical trials. Control Clin Trials 1987812–19. [DOI] [PubMed] [Google Scholar]
- 23.Myers E D, Calvert E J. Information, compliance and side‐effects: a study of patients on antidepressant medication. Br J Clin Pharmacol 19841721–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudd P, Byyny R L, Zachary V.et al Pill count measures of compliance in a drug trial: variability and suitability. Am J Hypertens 19881309–312. [DOI] [PubMed] [Google Scholar]
- 25.Rudd P, Byyny R L, Zachary V.et al The natural history of medication compliance in a drug trial: limitations of pill counts. Clin Pharmacol Ther 198946169–176. [DOI] [PubMed] [Google Scholar]