Abstract
Leptin is currently believed to control body composition largely, if not entirely, via hypothalamic receptors that regulate food intake and thermogenesis. Here we demonstrate direct extraneural effects of leptin to deplete fat content of both adipocytes and nonadipocytes to levels far below those of pairfed controls. In cultured pancreatic islets, leptin lowered triglyceride (TG) content by preventing TG formation from free fatty acids (FFA) and by increasing FFA oxidation. In vivo hyperleptinemia, induced in normal rats by adenovirus gene transfer, depleted TG content in liver, skeletal muscle, and pancreas without increasing plasma FFA or ketones, suggesting intracellular oxidation. In islets of obese Zucker Diabetic Fatty rats with leptin receptor mutations, leptin had no effect in vivo or in vitro. The TG content was ≈20 times normal, and esterification capacity was increased 3- to 4-fold. Thus, in rats with normal leptin receptors but not in Zucker Diabetic Fatty rats, nonadipocytes and adipocytes esterify FFA, store them as TG, and later oxidize them intracellularly via an “indirect pathway” of intracellular fatty acid metabolism controlled by leptin. By maintaining insulin sensitivity and preventing islet lipotoxicity, this activity of leptin may prevent adipogenic diabetes.
Leptin, the peptide hormone that controls body composition, is believed to do so largely, if not entirely, via hypothalamic receptors that regulate food intake and body weight (1–3). However, the various leptin receptor isoforms, including the long isoform OB-Rb, are widely expressed throughout the body (4–6), suggesting that leptin may have important actions on extraneural tissues as well. The idea that leptin may directly act on peripheral tissues gains further credence from the extraordinary changes in body fat of normal Wistar rats made chronically hyperleptinemic by infusion of the recombinant adenovirus containing the leptin cDNA (AdCMV–leptin) (7). In these rats, all discernible body fat disappeared within 7 days of this infusion whereas in pairfed normoleptinemic rats only a modest reduction in body fat took place. The magnitude and rapidity of the lipid depletion raised the possibility of a direct hormone-to-cell action of the leptin, in addition to the central effects that occur via the sympathetic nervous system. The goal of this study was to determine if leptin has important effects on cells outside the central nervous system that are involved in regulation of body weight and composition. Obesity is often associated with pancreatic β-cell dysfunction and diabetes, so we selected the pancreatic islets as the target tissue. Moreover, islets are known to express OB-R (6), and they become dysfunctional when they accumulate triglycerides (TG) in excessive amounts (8).
MATERIALS AND METHODS
Animals.
Male Wistar rats were obtained from Charles River Breeding Laboratories. Obese homozygous (fa/fa) Zucker diabetic fatty (ZDF)-drt rats and lean heterozygous (fa/+) ZDF and wild-type (+/+) littermates were bred in our laboratory from [ZDF/Drt-fa (F10)] rats purchased from R. Peterson (University of Indiana School of Medicine, Indianapolis). Male rats from our colony exhibiting the described phenotype (8, 9) were used.
Islet Isolation and Culture.
Pancreatic islets were isolated according to the method of Naber et al. (10) with some modifications and were maintained in suspension culture in 60-mm Petri dishes at 37°C in a humidified atmosphere of 5% CO2 and 95% air (11). The culture medium consisted of RPMI 1640 medium supplemented with 8.0 mM glucose, 10% fetal bovine serum, 200 units/ml penicillin, 0.2 mg/ml streptomycin, and 2% BSA, fraction V (Miles) either with or without 1 mM long chain fatty acid mixture (oleate-to-palmitate, 2:1; sodium salt; Sigma).
Triglyceride and DNA Measurements.
After 3 days in culture, islets were washed twice with Hanks’ balanced salt buffer and suspended in 50 μl of buffer (2 M NaCl/2 mM EDTA/50 mM sodium phosphate, pH 7.4). After sonication, 10 μl of the homogenate was mixed with 10 μl of tert-butyl alcohol and 5 μl of a Triton X-100/methyl alcohol mixture (1:1 vol/vol). For TG measurements of the pancreas, skeletal muscle, and liver, total lipids were extracted from ≈100 mg of tissue as described (11) and frozen under liquid nitrogen. TG was extracted with 30 μl of tert-butyl alcohol and 20 μl of a Triton X-104/methyl alcohol mixture (1:1 vol/vol). TG content was measured by the Sigma Triglyceride (GPO-Trinder) kit (8), and DNA content was measured by a method of Hopcroft et al. (12).
Oxidation and Esterification of [3H]-Palmitate in Pancreatic Islets.
Oxidation and esterification of [3H]palmitate by islets were determined as described (13, 14). Groups of 100–200 islets were incubated in duplicate with 1 mM 9,10-[3H]palmitate for 3 days. Palmitate oxidation was assessed by measuring tritiated water in the medium. Excess [3H]-palmitate was removed by precipitating twice with an equal volume of 10% trichloroacetic acid with 2% BSA. Supernatants in a microcentrifuge tube were placed in a scintillation vial containing unlabeled water and incubated at 50°C for 18 h. Tritiated water was measured as described for use of [3H]-glucose (11). Esterification of [3H]palmitate by islets was determined by measuring [3H]palmitate uptake by islets.
Construction of AdCMV–Leptin.
AdCMV–leptin was prepared as described (7). In brief, a BamHI- and XbaI-restricted leptin cDNA fragment that included 60 bp of a 5′ untranslated region and 76 bp of a 3′ untranslated region was ligated to similarly treated pACCMVpLpA (15). The resulting plasmid was cotransfected with pJM17 (16) into 293 cells by calcium phosphate/DNA coprecipitation to generate the AdCMV–leptin virus (17). Stocks of AdCMV–leptin were amplified and purified as described (17) and stored at −70°C in PBS with 0.2% BSA and 10% glycerol at 1–3 × 1012 plaque-forming units (pfu)/ml. A virus containing the bacterial β-galactosidase (β-gal) gene under control of the CMV promoter (AdCMV–β-gal) was prepared and used as described (18).
Hyperleptinemic Rat Model.
Two milliliters of AdCMV–leptin or AdCMV–β-gal containing a total of 1 × 1012 pfu/ml were infused into Wistar heterozygous lean and homozygous ZDF rats over a 10-minute period. Animals were studied in individual metabolic cages, and food intake and body weight were measured daily. Blood samples were collected from the tail vein in capillary tubes coated with EDTA for leptin assay at the indicated intervals, beginning 3 days after adenovirus infusion. Plasma leptin was assayed using the Linco leptin assay kit (Linco Research Immunoassay, St. Charles, MO). Plasma free fatty acids (FFA) were measured with a colorimetric assay using fatty acyl-CoA synthase, acyl-CoA oxidase, and H2O2-linked dye reagent (Boehringer Mannheim). The β-hydroxybutyrate (β-OH) level was determined spectrophotometrically (19). Urinary ketone (acetoacetic acid) levels were quantified as 0, +1, and +2 by dipstick (Keto-Diastix, Bayer, Elkhart, IN).
Genotyping of ZDF Animals.
Following the method of Phillips et al. (20), total DNA was extracted from rat tails (≈3 mm) by proteinase K digestion followed by phenol/chloroform extraction and ethanol precipitation (21). Primers 5′-GTT TGC GTA TGC AAG TCA CAG-3′ and 5′-ACC AGC AGA CAT GTA TCC GAG-3′ were used to amplify products from 5 ng of genomic DNA at 50°C for 1 min, 30 s and at 68°C for 5 min. PCR products were digested with MspI for 2 h at 37°C and analyzed on a 2% agarose gel.
Statistics.
Significance was analyzed by unpaired t test and one-way ANOVA.
RESULTS
In Vivo Effects of Leptin on TG of Tissues.
In our previous report demonstrating the absence of fat in hyperleptinemic rats (7), lipid measurements in various tissues were not carried out. To quantify the lipid content of tissues, we repeated the study and compared the content of TG and phospholipids in liver, pancreas, and skeletal muscle of hyperleptinemic rats with pairfed controls and free-feeding control animals infused with AdCMV–β-gal. Adipose tissue could not be compared because it could not be identified in the hyperleptinemic rats. As shown in Table 1, the TG content was reduced profoundly in the hyperleptinemic group in every tissue studied. In pancreas, for example, TG content was 4% of the free-feeding control rats and 20% of pairfed controls. A similar reduction in TG content was noted in skeletal muscle and liver. Phospholipids were unchanged (data not shown).
Table 1.
TG content of pancreas, skeletal muscle, and liver of hyperleptinemic rats and pairfed and free-feeding controls
| Tissue | AdCMV–β-gal Wistar | AdCMV–leptin Wistar | Pairfed Wistar* |
|---|---|---|---|
| Pancreas | 8.86 ± 1.86 (3) | 0.47 ± 0.39 (6) | 3.63 ± 2.00 (3) |
| Skeletal muscle | 4.12 ± 1.16 (4) | 0.33 ± 0.12 (4) | 1.31 ± 0.26 (5) |
| Liver | 4.82 ± 1.03 (5) | 0.64 ± 0.19 (4) | 4.59 ± 1.17 (5) |
Data are given as mean ± SEM. AdCMV–leptin infusion resulted in a rise in plasma leptin level from 1.3 ± 0.3 ng/ml before to 12 ± 1.9 ng/ml 3 days after the infusion. Leptin levels were 14.7 ± 2.2 and 13.7 ± 2.1 ng/ml at 7 and 14 days postinfusion, respectively. In the control groups, plasma levels were invariably below 1.5 ng/ml. Total lipids were extracted from ≈100 mg of tissue as described (22) and frozen under liquid nitrogen. TG was extracted with 30 μl of tert-butyl alcohol and 20 μl of a Triton X-104/methyl alcohol mixture (1:1, vol/vol). TG content was measured by a Sigma Triglyceride (GPO-Trinder) kit as described (5). Number of experiments is in parentheses. TG content in milligrams per gram of wet weight of tissue.
Pairfed AdCMV–β-gal-infused Wistar rats did not appear to differ in terms of body fat from intact pairfed rats.
In Vitro Effects of Leptin on TG of Islets.
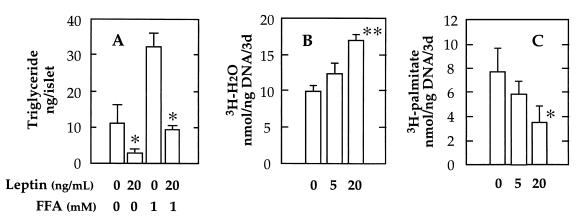
All of the tissues in which depletion of TG had occurred expressed OB-R (4–6, 23), so we undertook to determine if leptin could directly affect the TG content of such cells without mediation via the sympathetic nervous system. We selected the pancreatic islets as the target tissue because they are known to express OB-R (6) and are rendered dysfunctional when they accumulate TG (8). The effect of 20 ng/ml leptin on TG content of islets cultured for 3 days was examined in the absence and in the presence of a 1-mM 2:1 oleate-to-palmitate mixture of FFA. In the absence of FFA, we observed a striking decline in TG content in the leptin-cultured islets from normal Wistar rats (Fig. 1A), which is evidence that endogenous TG were being depleted at an increased rate in the presence of leptin. In the presence of 1 mM FFA but without leptin in the medium, the TG content of islets rose 192%; this rise was markedly reduced when leptin was present in the culture medium. Thus, leptin blocked the de novo formation of TG from exogenous FFA or stimulated the hydrolysis of newly formed TG at a rate that exceeded their esterification, or both.
Figure 1.
Effect of recombinant leptin on triglyceride content (A), oxidation (B), and esterification (C) of [3H]palmitate in islets from normal Wistar rats cultured for 3 days. Data represent the mean ± SEM for three samples. ∗, P <0.05; and ∗∗, P <0.01 vs. O leptin.
To assess more directly the influence of leptin on TG metabolism, we added 1 mM [3H]palmitate to the culture medium and measured the effects of the hormone on oxidation and esterification of the long chain fatty acid, as described (13). As shown in Fig. 1B, a 42% increase in oxidation occurred in islets from Wistar rats when 20 ng/ml leptin was present, and the incorporation of [3H]palmitate into TG was reduced by 54% (Fig. 1C). These effects on FFA metabolism could account for the reduction of the TG content of islets by leptin.
Plasma FFA and β-Hydroxybutyrate Levels in Hyperleptinemic Rats.
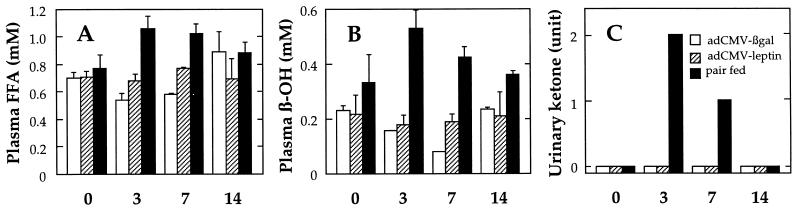
The forgoing results indicate that leptin can reduce the TG content of islet cells by increasing oxidation and reducing esterification of FFA intracellularly. When TG stores are reduced by starvation or insulin deficiency, the FFA are released and delivered to other tissues. This increased lipolysis is reflected by a rise in plasma FFA levels. β-Oxidation of FFA takes place largely in the liver and results in an increase in plasma and urine ketones (24). To determine if the striking leptin-mediated depletion of adipocyte fat involves the classic pathways of lipolysis and ketogenesis, we measured plasma FFA and β-OH levels in rats before and 3, 7, and 14 days after they had been made hyperleptinemic by infusion of AdCMV–leptin (leptin levels 13.7 ± 2.1 ng/ml). Pairfed rats and free-feeding AdCMV–β-gal-infused rats served as controls. As shown in Fig. 2A, FFA levels remained at the prehyperleptinemic baseline levels throughout the 14 days, even at the 3-day postinfusion point before body fat had disappeared. β-OH also failed to rise in the hyperleptinemic rats (Fig. 2B), and daily urine determinations were negative for ketonuria (Fig. 2C). By contrast, plasma FFA, β-OH, and urinary ketones were increased in pairfed controls (Fig. 2 A–C). These results suggest that leptin-induced depletion of TG in white adipocytes involves the same increase in intracellular TG metabolism observed in islets, rather than the export of FFA to other tissues.
Figure 2.
Effect of infusion of AdCMV–leptin on plasma FFA (A) and β-OH (B) and urinary ketones (C) of normal Wistar rats. AdCMV–β-gal-infused rats, open bars; AdCMV–leptin-induced rats, dashed bars; pairfed controls, closed bars. Blood and urine samples were collected before (0) and 3, 7, and 14 days after operations. Data represent the mean ± SEM for four to six samples.
Hyperleptinemic Effects in ZDF Rats.
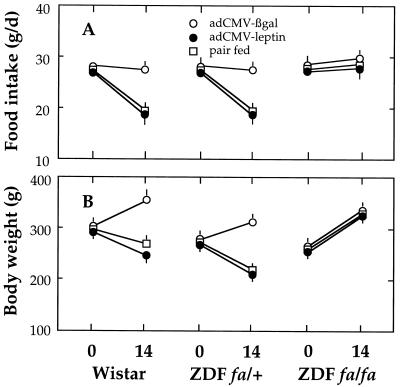
If, in fact, the lipopenic action of leptin is a direct one mediated by leptin receptors, the effect should not occur in cells in which the leptin receptor is defective. The fa mutation in obese Zucker and ZDF consists of a Glu-269 → Pro mutation in the extracellular domain of all OB-R isoforms (20, 25). These fa/fa rats have a phenotype of leptin resistance with obesity despite hyperleptinemia. We therefore tested the effects of infusing AdCMV–leptin into homozygous ZDF rats. The dramatic effects on food intake, body weight, and fat content observed in normal rats did not occur in ZDF rats (Fig. 3), providing further proof of leptin resistance. However, in heterozygous (fa/+) rats, food intake and body weight declined as in Wistar rats.
Figure 3.
Effect of infusion of AdCMV–leptin on food intake (A) and body weight (B) of normal Wistar and heterozygous (fa/+) and homozygous (fa/fa) ZDF rats. AdCMV–β-gal-infused rats, open circles; AdCMV–leptin-induced rats, closed circles; pairfed controls, open squares. Food intake and body weight changes were measured before (0) and 14 days after operations. Data represent the mean ± SEM for four to six samples.
In Vitro Effects of Leptin in Islets of ZDF Rats.
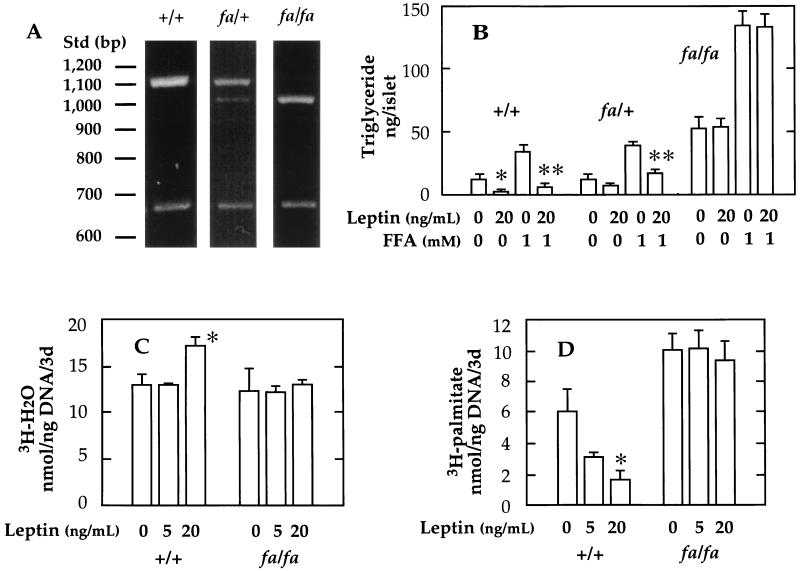
We therefore isolated islets from homozygous and heterozygous OB-R-defective (fa/fa) rats and wild-type controls (+/+) (Fig. 4A) to determine if their cells were unresponsive to the in vitro lipopenic action of leptin demonstrated in islets from normal rats. We had reported that the TG content of islets from obese ZDF rats is extraordinarily high (8) and that the capacity of their islets to esterify FFA to TG is greatly increased (14). These abnormalities are consistent with resistance to the leptin effects identified above. We cultured the islets of obese ZDF rats with 20 ng/ml leptin both with and without 1 mM FFA mixture. As shown in Fig. 4B, the islets of obese rats had a TG content of 52 ng/islet, 6.7 times that of wild-type, lean ZDF (+/+) rats. Leptin in the culture medium did not lower the TG content, and it did not reduce the greatly exaggerated increase in TG content that occurred in the presence of 1 mM FFA. By contrast, wild-type lean ZDF rats (+/+) with two normal OB-R alleles exhibited the full response to leptin (Fig. 4B). Heterozygous ZDF rats (fa/+) responded partially; the TG-lowering effect of leptin was only 38% compared with 66% in the wild-type rats; TG accumulation was reduced 57% in heterozygous islets compared with 83% of the wild-type/wild-type ZDF rats (Fig. 4B). Leptin failed to increase [3H]- palmitate oxidation or reduce its incorporation into TG in islets from homozygous (fa/fa) ZDF rats (Fig. 4 C and D).
Figure 4.
(A) Genotyping of ZDF animals. Effect of recombinant leptin on triglyceride content (B), oxidation (C), and esterification (D) of [3H]-palmitate in islets from wild-type (+/+), heterozygous (fa/+), and homozygous (fa/fa) ZDF rats cultured for 3 days. Lanes in A: 1, wild-type (+/+); 2, heterozygous lean (fa/+); 3, homozygous obese fa/fa rat. A 130-bp MspI fragment in lanes 2 and 3 is not shown. Data in B–D represent the mean ± SEM for three samples. ∗, P <0.05; and ∗∗, P <0.01 vs. O leptin.
DISCUSSION
These results provide new insights into the mechanisms of leptin-induced fat depletion and the pathogenesis of the β-cell lipotoxicity described previously in ZDF rats (8, 9, 14). The results demonstrate that leptin depletes TG in cells with OB-R receptors via a direct mechanism that involves both an increase in FFA oxidation and a decrease in esterification. The ubiquity of the expression of leptin receptor isoforms, including the long isoform OB-Rb (4–6, 23), together with the in vitro demonstration that leptin reduces acetyl CoA carboxylase activity (26), is consistent with extraneural action. Also consistent with this idea are the disappearance within 7 days of all visible body fat in rats made chronically hyperleptinemic by infusion of AdCMV–leptin (7) and the exhibition by pairfed controls of only a modest reduction in body fat. Although the in vitro effect of leptin on TG content was demonstrated only in islets, the fact that TG content in the liver, pancreas, and skeletal muscle also was drastically reduced in hyperleptinemic rats implies that the same direct lipopenic action of leptin may be operative in many other OB-R-expressing tissues. The results further show that this effect requires the presence of normal leptin receptors and that the effect is attenuated if one OB-R allele is abnormal.
It now seems clear that the extraordinarily high TG content and increased esterification of FFA in islets of ZDF rats reported earlier (8, 14) is a reflection of the absence of leptin-mediated inhibition of lipogenesis from FFA. These findings thus provide the first explanation for diabetes in obese ZDF rats by linking the OB-R mutation to the β-cell lipotoxicity. The islets of these fa/fa animals are overloaded with fat at the time that hyperglycemia begins (8). Because maneuvers that reduce islet fat content prevent the diabetes in ZDF rats (27), it has been proposed that the TG accumulation in islets plays a causal role in the β-cell dysfunction (6, 9, 19). Thus, the predisposition to diabetes in the homozygous ZDF rats may reflect the fact that their tissues have been completely “unleptinized” throughout their lives and therefore have accumulated high levels of TG. In normal rats, this accumulation is prevented by the action of leptin. It is expected that any therapy that reduces tissue TG will improve β-cell function and reduce insulin resistance.
In hyperleptinemic rats, every tissue that was examined was lipopenic. We speculate that nonadipocytes normally carry a small quantity of TG, perhaps to provide a reservoir of fuel and a degree of independence from adipocytes or to serve as an intracellular second messenger. This TG storage function is regulated by leptin. In the obesity of ZDF rats, the regulatory control by leptin is absent, and these putative intracellular TG reserves soar to levels over 50 times that of hyperleptinemic rats. This results in insulin resistance, β-cell dysfunction, and diabetes.
Acknowledgments
We thank Drs. Michael S. Brown, Joseph L. Goldstein, and Dan Foster for critically reviewing this paper and Todd Kirchgessner, Ph.D., Bristol–Myers Squibb, Princeton, NJ, for providing recombinant leptin. Kay McCorkle provided technical support, and Sharryn Harris provided secretarial assistance. This work was supported by National Institutes of Health Grants DK02700-37 and P50H2598801, a National Institutes of Health/Juvenile Diabetes Foundation Diabetes Interdisciplinary Research Program, and a Department of Veterans Affairs Institutional Research Support grant.
ABBREVIATIONS
- AdCMV
recombinant cytomegalovirus
- β-gal
β-galactosidase
- TG
triglyceride
- FFA
free fatty acids
- ZDF
Zucker diabetic fatty
- β-OH
β-hydroxybutyrate
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–431. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz M W, Seeley R J, Campfield L A, Burn P, Baskin D G. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaisse C, Halaas J L, Horvath C M, Darnell J E, Stoffel M, Friedman J M. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 4.Tartaglia L A, Dombski M, Weng X, Deng N H, Culpepper J, Devos R, Richards G J, Campfield L A, Clark F T, Deeds J, Muir C, Sanker S, Moriarty A, Moore K J, Smutko J S, Mays G G, Woolf E A, Monroe C A, Tepper R I. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 5.Wang M-Y, Zhou Y-T, Newgard C B, Unger R H. FEBS Lett. 1996;392:87–90. doi: 10.1016/0014-5793(96)00790-9. [DOI] [PubMed] [Google Scholar]
- 6.Kieffer T J, Heller R S, Habener J F. Biochem Biophys Res Commun. 1996;224:522–527. doi: 10.1006/bbrc.1996.1059. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Koyama K, Yuan X, Lee Y, Zhou Y-T, O’Doherty R, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y H, Hirose H, Ohneda M, Johnson J H, McGarry J D, Unger R H. Proc Natl Acad Sci USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Unger R H. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 10.Naber S P, McDonald J M, Jarett L, McDaniel M L, Ludvigsen C W, Lacy P E. Diabetologia. 1980;19:439–444. doi: 10.1007/BF00281823. [DOI] [PubMed] [Google Scholar]
- 11.Milburn J L, Hirose H, Lee Y H, Nagasawa Y, Ogawa A, Ohneda M, BeltrandelRio H, Newgard C B, Johnson J H, Unger R H. J Biol Chem. 1995;170:1295–1299. doi: 10.1074/jbc.270.3.1295. [DOI] [PubMed] [Google Scholar]
- 12.Hopcroft D W, Mason D R, Scott R S. Horm Metab Res. 1985;17:559–561. doi: 10.1055/s-2007-1013606. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Ogawa A, Ohneda M, Unger R H, Foster D W, McGarry J D. Diabetes. 1994;43:878–883. doi: 10.2337/diab.43.7.878. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y H, Hirose H, Zhou Y-T, Esser V, McGarry J D, Unger R H. Diabetes. 1997;46:407–413. doi: 10.2337/diab.46.3.408. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Foix A M, Coats W S, Baque S, Alam T, Gerard R D, Newgard C B. J Biol Chem. 1992;276:25129–25134. [PubMed] [Google Scholar]
- 16.McCrory W J, Bautista D S, Graham F L. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 17.Becker T C, Noel R J, Coats W S, Gomez-Foix A M, Alam T, Gerard R D, Newgard C B. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 18.Herz J, Gerard R D. Proc Natl Acad Sci USA. 1993;90:2812–2817. doi: 10.1073/pnas.90.7.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGarry J D, Guest M J, Foster D W. J Biol Chem. 1970;245:4382–4390. [PubMed] [Google Scholar]
- 20.Phillips M S, Liu Q Y, Hammond H A, Dugan V, Hey P J, Caskey C T, Hess J F. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 21.Blin N, Stafford D W. Nucleic Acids Res. 1976;3:2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bligh EG, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 23.Cioffi J A, Shafer A W, Zupancic T J, Smithgbur J, Mikhail A, Platika D, Snodgrass H R. Nat Med. 1996;2:585–589. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 24.McGarry J D, Foster D W. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- 25.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shima K. Biochem Biophys Res Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- 26.Bai Y L, Zhang S Y, Kim K S, Lee J K, Kim K H. J Biol Chem. 1996;271:13939–13942. doi: 10.1074/jbc.271.24.13939. [DOI] [PubMed] [Google Scholar]
- 27.Ohneda M, Inman L R, Unger R H. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]