Abstract
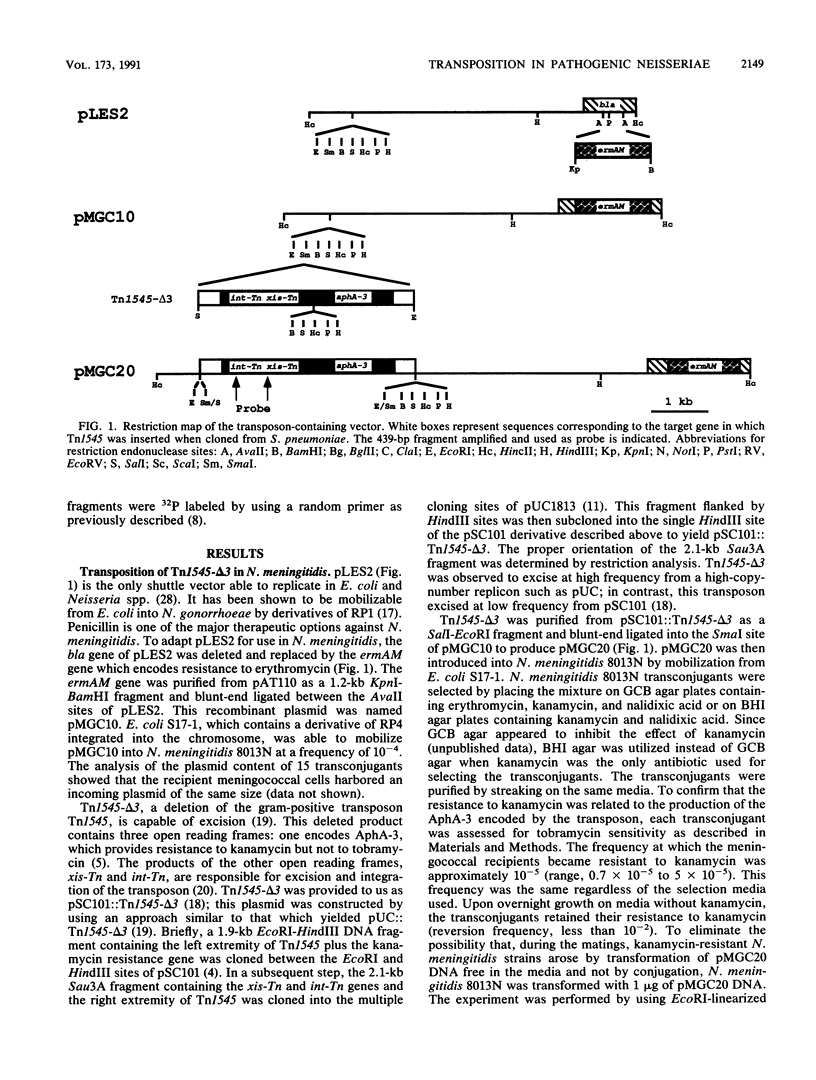
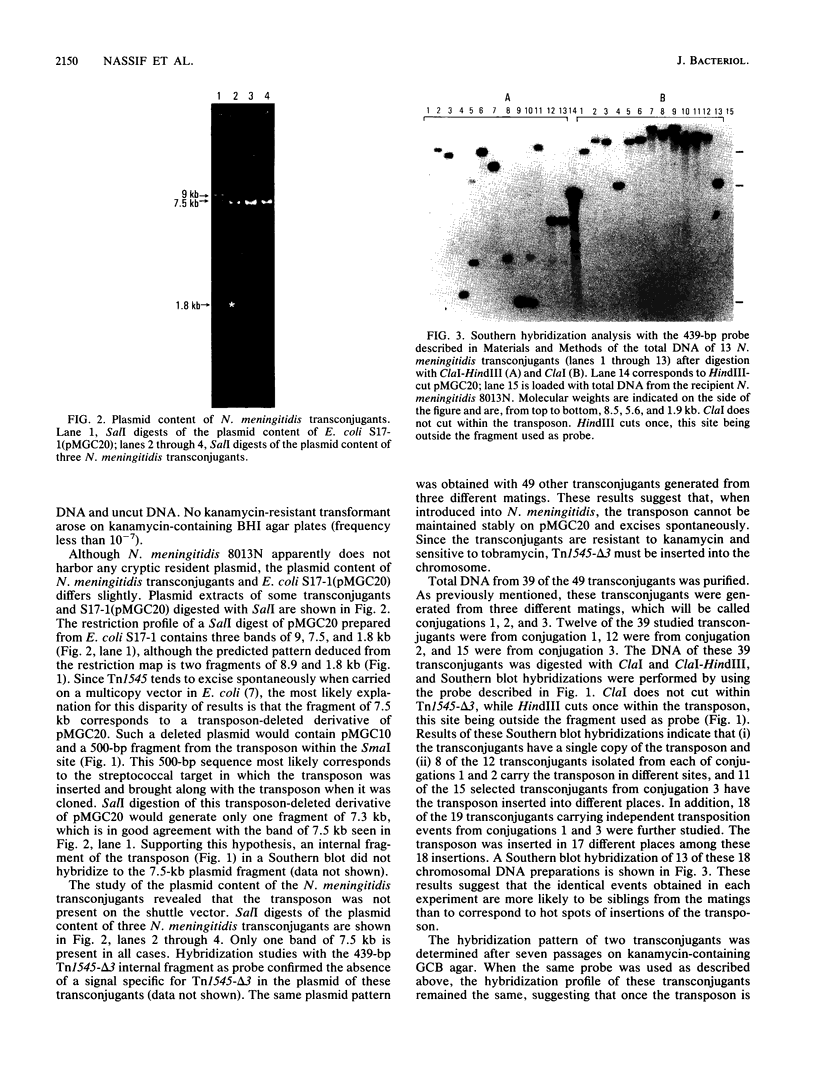
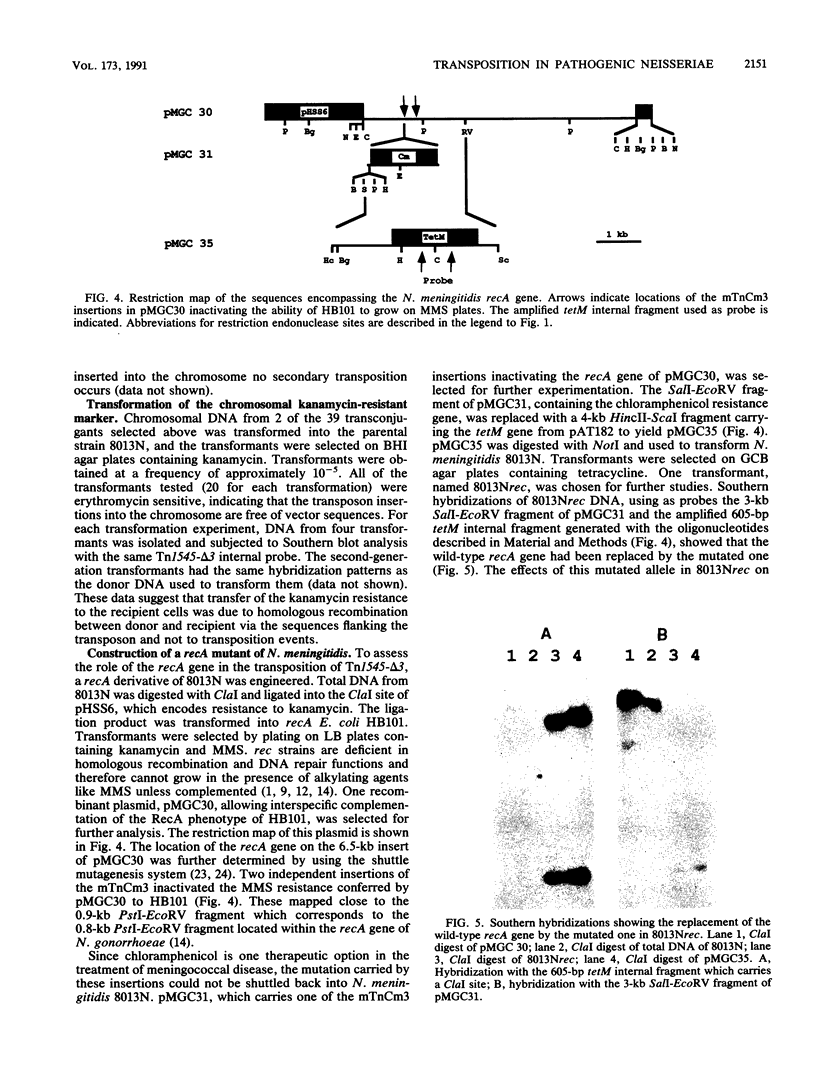
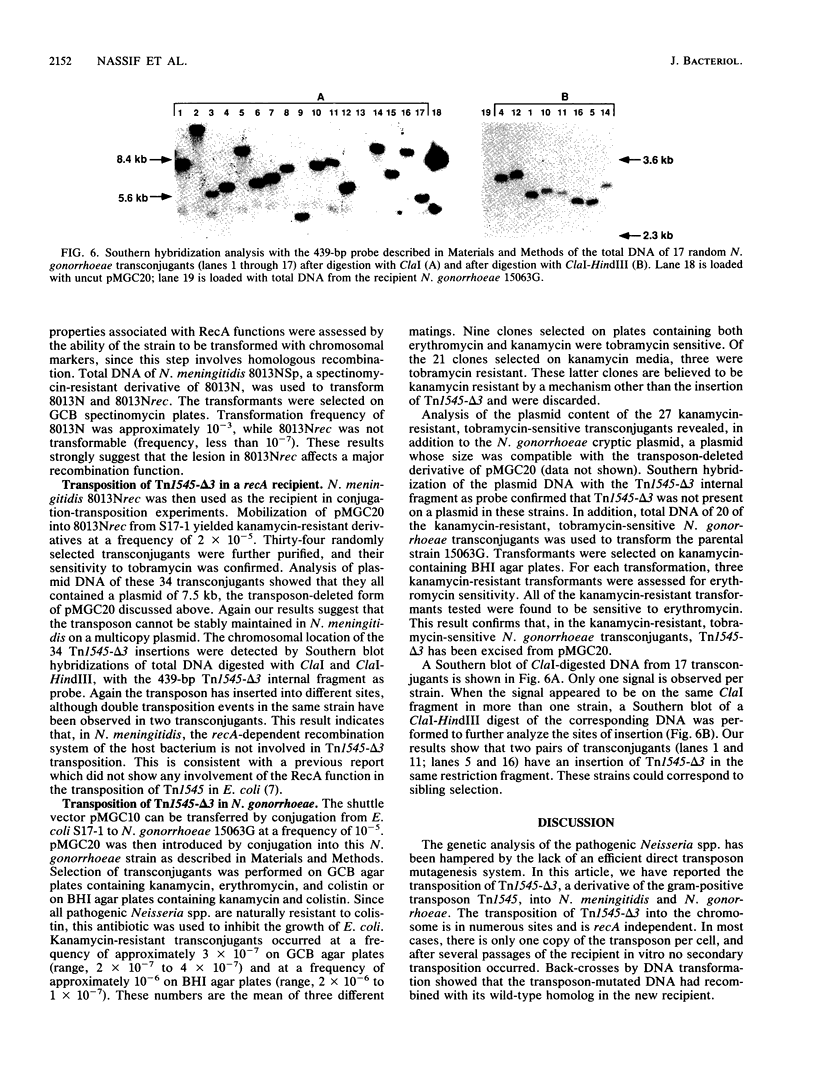
The ability to study the virulence of pathogenic Neisseria spp. has been greatly limited by the absence of genetic tools which allow the construction of defined mutants. We have engineered a transposon system which allows random mutagenesis of the Neisseria genome at relatively high frequency. Tn1545-delta 3 is a 3.4-kb derivative of the gram-positive transposon Tn1545 encoding resistance to kanamycin. Tn1545-delta 3 was subcloned into an erythromycin-resistant derivative of the mobilizable shuttle vector pLES2 to yield the plasmid pMGC20. This latter plasmid was introduced by conjugation from Escherichia coli S17-1 into Neisseria meningitidis 8013N and Neisseria gonorrhoeae 15063G. Kanamycin-resistant 8013N and 15063G transconjugants appeared at frequencies of 10(-5) and 10(-6), respectively. Restriction enzyme analysis and Southern blot hybridization of these transconjugants showed that, in Neisseria spp., the transposon excised spontaneously from pMGC20 and integrated into chromosomal DNA. Our studies revealed that (i) transposition of Tn1545-delta 3 was in numerous, apparently distinct sites, (ii) in most cases, for each transconjugant a single copy of Tn1545-delta 3 was integrated into the chromosome, and (iii) several passages on selective media did not induce secondary transposition. The kanamycin resistance marker expressed by the transconjugants was subsequently transformed into a parental background without change in the chromosomal location of the transposon. To assess the role of the general recombination system in the transposition of Tn1545-delta 3, the recA gene of N. meningitidis has been cloned and a rec derivative of 8013N has been engineered. Similar results were obtained when this latter strain was used as recipient, suggesting that recA function were not required for Tn1545-delta 3 transposition in N. meningitidis. Transposition with Tn1545-delta 3 may be an important technique for mutagenesis of the pathogenic neisseriae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Better M., Helinski D. R. Isolation and characterization of the recA gene of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):311–316. doi: 10.1128/jb.155.1.311-316.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collatz E., Carlier C., Courvalin P. Characterization of high-level aminoglycoside resistance in a strain of Streptococcus pneumoniae. J Gen Microbiol. 1984 Jul;130(7):1665–1671. doi: 10.1099/00221287-130-7-1665. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Tn1545: a conjugative shuttle transposon. Mol Gen Genet. 1987 Feb;206(2):259–264. doi: 10.1007/BF00333582. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Transposable multiple antibiotic resistance in Streptococcus pneumoniae. Mol Gen Genet. 1986 Nov;205(2):291–297. doi: 10.1007/BF00430441. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Mekalanos J. J. Effect of a recA mutation on cholera toxin gene amplification and deletion events. J Bacteriol. 1986 Mar;165(3):723–731. doi: 10.1128/jb.165.3.723-731.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Stephens D. S., Spellman P., Morse S. A. Transposition of Tn916 to different sites in the chromosome of Neisseria meningitidis: a genetic tool for meningococcal mutagenesis. Mol Microbiol. 1990 May;4(5):729–735. doi: 10.1111/j.1365-2958.1990.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Kay R., McPherson J. Hybrid pUC vectors for addition of new restriction enzyme sites to the ends of DNA fragments. Nucleic Acids Res. 1987 Mar 25;15(6):2778–2778. doi: 10.1093/nar/15.6.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Falkow S. Cloning of the recA gene of Neisseria gonorrhoeae and construction of gonococcal recA mutants. J Bacteriol. 1987 Feb;169(2):790–795. doi: 10.1128/jb.169.2.790-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Martin P., Trieu-Cuot P., Courvalin P. Nucleotide sequence of the tetM tetracycline resistance determinant of the streptococcal conjugative shuttle transposon Tn1545. Nucleic Acids Res. 1986 Sep 11;14(17):7047–7058. doi: 10.1093/nar/14.17.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piffaretti J. C., Arini A., Frey J. pUB307 mobilizes resistance plasmids from Escherichia coli into Neisseria gonorrhoeae. Mol Gen Genet. 1988 May;212(2):215–218. doi: 10.1007/BF00334687. [DOI] [PubMed] [Google Scholar]
- Poyart-Salmeron C., Trieu-Cuot P., Carlier C., Courvalin P. The integration-excision system of the conjugative transposon Tn 1545 is structurally and functionally related to those of lambdoid phages. Mol Microbiol. 1990 Sep;4(9):1513–1521. doi: 10.1111/j.1365-2958.1990.tb02062.x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Seifert H. S., Ajioka R. S., Paruchuri D., Heffron F., So M. Shuttle mutagenesis of Neisseria gonorrhoeae: pilin null mutations lower DNA transformation competence. J Bacteriol. 1990 Jan;172(1):40–46. doi: 10.1128/jb.172.1.40-46.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H. S., Chen E. Y., So M., Heffron F. Shuttle mutagenesis: a method of transposon mutagenesis for Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Feb;83(3):735–739. doi: 10.1073/pnas.83.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senghas E., Jones J. M., Yamamoto M., Gawron-Burke C., Clewell D. B. Genetic organization of the bacterial conjugative transposon Tn916. J Bacteriol. 1988 Jan;170(1):245–249. doi: 10.1128/jb.170.1.245-249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. C., Silver L. E., Clark V. L., Young F. E. Construction and characterization of a new shuttle vector, pLES2, capable of functioning in Escherichia coli and Neisseria gonorrhoeae. Gene. 1983 Nov;25(2-3):241–247. doi: 10.1016/0378-1119(83)90228-7. [DOI] [PubMed] [Google Scholar]
- Trieu-Cuot P., Poyart-Salmeron C., Carlier C., Courvalin P. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 1990 Jun 25;18(12):3660–3660. doi: 10.1093/nar/18.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]