Abstract
Background
Biomarkers of stroke are an evolving field of clinical research. A serum marker which can differentiate between haemorrhagic and ischaemic stroke in the very early phase would help to optimise acute stroke management.
Objective
To examine whether serum glial fibrillary acidic protein (GFAP) identifies intracerebral haemorrhage (ICH) in acute stroke patients.
Methods
A pilot study assessing 135 stroke patients admitted within six hours after symptom onset. Diagnosis of ICH (n = 42) or ischaemic stroke (n = 93) was based on brain imaging. GFAP was determined from venous blood samples obtained immediately after admission, using a research immunoassay.
Results
GFAP was detectable in the serum of 39 patients (34 of 42 (81%) with ICH, and five of 93 (5%) with ischaemic stroke). Serum GFAP was substantially raised in patients with ICH (median 11 ng/l, range 0 to 3096 ng/l) compared with patients with ischaemic stroke (median 0 ng/l, range 0 to 14 ng/l, p<0.001). Using receiver operating characteristic curve analysis , a cut off point of 2.9 ng/l provided a sensitivity of 0.79 and a specificity of 0.98 for the identification of ICH in acute stroke (positive predictive value 0.94, negative predictive value 0.91; p<0.001).
Conclusions
Serum GFAP can reliably detect ICH in the acute phase of stroke. Further evaluation of the usefulness of GFAP as an early diagnostic marker of ICH is now required, with the aim of optimising cause specific emergency management.
Keywords: biological marker, intracerebral haemorrhage, cerebral ischaemia, acute management
Intracerebral haemorrhage (ICH) accounts for 8–15% of all strokes in Western industrialised countries.1,2 The annual incidence of ICH in Asian countries seems to be two‐ to threefold higher.3 Overall, the prognosis for ICH is poor: 37–47% of patients die within the first year after the event and a substantial proportion of the survivors are left with serious neurological deficits.4,5
Up to now, brain imaging has been indispensable for the reliable differentiation between ICH and cerebral ischaemia in acute stroke.2,6 Consequently, cause specific management of acute stroke patients is not possible in the prehospital setting. For instance, according to current recommendations, raised blood pressure should be treated more aggressively in patients with ICH than in those with ischaemic stroke.6,7,8 Mechanical ventilation is more often required and neurosurgical treatment may be indicated in ICH patients.2,6,9 Thus admission of ICH patients to specialised units may improve their outcome. In addition, very early haemostatic treatment may offer the possibility of arresting haematoma growth, which is mainly responsible for clinical deterioration within the first few hours.10,11,12,13,14 Thus a simple diagnostic test applicable in the prehospital phase would be helpful for triage and to optimise the subsequent hospital management.
Previous studies showed a delayed release of the astrocytic glial fibrillary acidic protein (GFAP) into the serum in patients with ischaemic stroke, reaching maximum concentrations between days 2 and 4.15,16,17 Because of the more sudden disruption of the blood–brain barrier and the resulting brain damage, we hypothesised that GFAP would immediately be detectable in serum in the hyperacute phase of ICH, but not in ischaemic stroke, and thus could be useful as a rapid diagnostic marker for ICH in acute stroke patients.
Methods
Patients
Between November 2002 and June 2004, we prospectively included 135 patients admitted to the stroke unit or the neuro‐intensive care unit of our tertiary care centre of neurology and neurosurgery. Inclusion criteria were the onset of an acute and persisting focal neurological deficit caused by ICH or cerebral ischaemia and admission of the patient to our centre within six hours after symptom onset. Diagnosis of ICH (n = 42) or ischaemic stroke (n = 93) was based on initial or consecutive brain imaging (computed tomography (CT), magnetic resonance imaging (MRI)) showing a parenchymal haematoma or infarction, respectively. Patients with persistent negative findings on repeated brain imaging were excluded. Further exclusion criteria were previous brain injury, a history of intracerebral haemorrhage or cerebral ischaemia, or any other pre‐existing central nervous system disease.
The severity of the neurological deficit on admission to hospital was determined according to the National Institutes of Health stroke scale (NIHSS). Haematoma volume was estimated according to a standard ellipsoid method.18 Infarct size was categorised into three groups for the anterior circulation (less than one third, one third to two thirds, and more than two thirds of middle cerebral artery territory or multiple territories). Volumetric measurements of posterior circulation infarcts were not done. Baseline characteristics of the study population are given in table 1. The study protocol was approved by the ethics committee of the University Hospital. Informed consent for study participation was obtained from patients or their next of kin.
Table 1 Baseline characteristics of the study population.
| Patient characteristics | Ischaemic stroke aetiology (n) | Ischaemic stroke volume (n) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y)* | Female (n)† | Time to admission (h)* | NIHSS* | Large vessel | Cardio‐embolic | Small vessel | Unknown/other | <1/3 MCA | 1/3 to 2/3 MCA | >2/3 MCA | Posterior circulation | ||||
| Ischaemic stroke (n = 93) | 70 (12) | 36 | 1.8 (1.0) | 12 (6) | 19 | 52 | 6 | 16 | 37 | 21 | 28 | 7 | |||
| Haematoma localisation (n) | Haematoma volume (n) | ||||||||||||||
| ICH (n = 42) | Basal ganglia | Lobar | Brain stem | 0 to 20 ml | 21 to 40 ml | 41 to 60 ml | >60 ml | ||||||||
| 67 (11) | 14 | 1.9 (1.4) | 13 (5) | 27 | 10 | 5 | 15 | 11 | 9 | 7 | |||||
Baseline characteristics and clinical data of 93 patients with ischaemic stroke and 42 patients with intracerebral haemorrhage (ICH). For patients with ischaemic stroke, stroke aetiology and categories of infarct size are given. For patients with ICH, haematoma localisation and volume are provided.
Values are mean (SD) or n.
*Mann–Whitney U test; †χ2‐statistics: comparisons of baseline patient characteristics were not significantly different between groups.
ICH, intracerebral haemorrhage; MCA, middle cerebral artery territory; NIHSS, National Institutes of Health stroke scale.
After inclusion into the study, a serum sample (2 ml) was separated from the venous blood sample taken immediately after hospital admission for routine diagnostics (blood was centrifuged for five minutes at 2703×g). Serum samples were stored at −25°C.
GFAP measurement
GFAP was measured using a research prototype test on Elecsys® 2010 (Roche Diagnostics, Mannheim, Germany). The solid phase immunoassay employed one rabbit monoclonal antibody (MAB) (4A11; Research Diagnostics, Flanders, New Jersey, USA) and one rabbit polyclonal antibody (PAB) (Z0334, DakoCytomation, Glostrup, Denmark). The assay format contained three single steps separated by two incubation periods (37°C) and followed by a final incubation period (37°C). In the first step, specimens and standards were incubated with either phosphate buffered saline (PBS) with 0.5% Tween 40 and 1% RPLA4, containing an excess of unmodified MAB, or with PBS only. In the second step, biotinylated MAB and streptavidin coated paramagnetic beads were added. After the second incubation, the PAB labelled with Tris(2,2′‐bipyridyl)ruthenium(III) complex was added to form a sandwich complex that was bound to the beads through biotin–streptavidin interaction. After the final incubation, the GFAP/microparticle activity was measured using electrochemiluminescence, whereby the specific activity was the total activity minus the background (the specific signal was blocked by an excess of unmodified MAB). As a reference, increasing dilutions of human cerebrospinal fluid were used (GFAP was determined according to Missler et al19). The measurement range of the test was from 1.8 to 50 000 ng/l; intra‐assay precision coefficients were 0.85% for negative and 1.6% for positive standards. Using this method, 52 of 55 healthy individuals (95%) tested negative for serum GFAP (<1.8 ng/l), while three were found to have slightly raised values (3.8, 4.4, and 7.2 ng/l, respectively). The present samples were measured in duplicate without prior knowledge of the clinical status of the patients, and the results had a mean standard deviation of 1.31%.
Statistical analyses
Statistical analyses were carried out using the SPSS 10.0 software package (SPSS Inc, Chicago, Illinois, USA). For statistical comparison of GFAP values between patients with ICH and ischaemic stroke, we used the Mann–Whitney U test. Using receiver operating characteristic (ROC) curve analysis, a cut off point providing optimised sensitivity and specificity for the identification of ICH was calculated, and accuracy measures were derived from cross tabulations. We used the χ2 statistic to indicate significant findings. Correlation analysis between GFAP serum concentrations and haematoma volume was undertaken using the non‐parametric Spearman r correlation.
Results
GFAP was detectable in the serum of 39 patients (34 of 42 (81%) with ICH, and five of 93 (5%) with ischaemic stroke). Serum GFAP was substantially raised in patients with ICH (mean (SD), 111.6 (477.5) ng/l; median 11, range 0 to 3096) compared with patients with ischaemic stroke (0.4 (1.9) ng/l; median 0 ng/l, range 0 to 14 ng/l) (p<0.001). Using ROC curve analysis, a cut off point of 2.9 ng/l was found to provide a sensitivity of 0.79 and a specificity of 0.98 for the differentiation of ICH from ischaemic stroke (positive predictive value 0.94, negative predictive value 0.91, p<0.001; area under the curve, 0.89 (95% confidence interval (CI), 0.82 to 0.97), p<0.001). In patients with a moderate to severe initial neurological deficit (NIHSS ⩾6), sensitivity increased to 0.82 (specificity 0.97, positive predictive value 0.94, negative predictive value 0.92, overall accuracy 0.92, p<0.001). Additionally, restricting the analysis to patients admitted within the first three hours after symptom onset (n = 122) gave a sensitivity of 0.75 (specificity 0.98, positive predictive value 0.93, negative predictive value 0.90, overall accuracy 0.91, p<0.001).
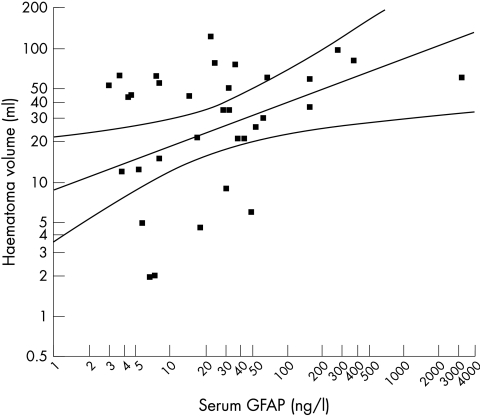
In ICH patients, individual GFAP values were correlated with the corresponding NIHSS score on hospital admission (Spearman r = 0.336, p = 0.042). Furthermore, haematoma volume was also associated with individual GFAP values (Spearman r = 0.462, p = 0.002, fig 1). Of the nine ICH patients with GFAP values ⩽2.9 ng/l (the cut off point), five had small bleeds <6 ml in volume, one had a lobar ICH (22 ml), and three had basal ganglia ICH (16, 29, and 38 ml).
Figure 1 Thirty four of 42 patients with ICH had detectable glial fibrillary acidic protein (GFAP) values. The scatterplot shows serum GFAP concentrations v haematoma volume (Spearman r = 0.462, p = 0.002).
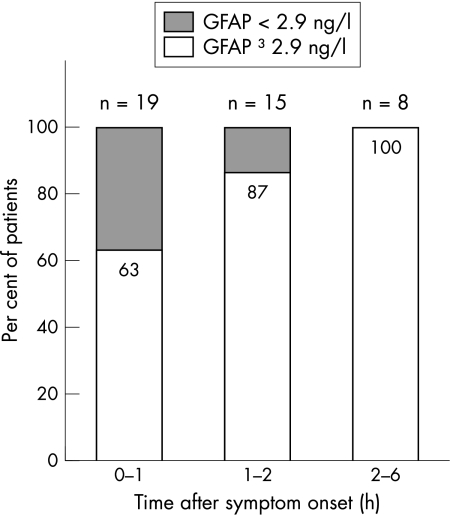
As shown in fig 2, the proportion of ICH patients with a serum GFAP concentration above the cut off point of 2.9 ng/l increased with the time interval elapsing since the onset of neurological symptoms. Within the first hour after symptom onset (mean time interval, 57 minutes), 12 of 19 patients (63%) had GFAP serum values above the cut off point; in the second hour (mean time interval, 1 h 48 min) 13 of 15 patients (87%), and thereafter (mean time interval, 4 h 19 min) all eight ICH patients had serum GFAP values above the calculated cut off point.
Figure 2 Percentage of patients with intracerebral haemorrhage (n = 42) above the cut off point (2.9 ng/l) in relation to the time span elapsed from symptom onset to blood sampling. GFAP, glial fibrillary acidic protein.
Discussion
In this investigation, we found significantly raised serum GFAP values in patients with ICH in comparison to ischaemic stroke within the first six hours after symptom onset. In this time period, GFAP seems to be a serum biomarker capable of detecting ICH with a promising degree of diagnostic accuracy.
Our results indicate that GFAP is not detectable in the serum in the majority of ischaemic stroke patients within the first six hours after symptom onset. This finding fits in well with the previous studies reporting a delayed release of astroglial proteins (S100B and GFAP) into the serum in acute ischaemic stroke, reaching maximum concentrations between day 2 and day 4.15,16,17,20,21,22 This delayed release probably reflects the gradual leakage of these proteins from necrotic cells into the extracellular space and through the blood–brain barrier.21,23,24 On the other hand, acute ICH may cause a more sudden disruption of the blood–brain barrier and rapid destruction of astroglial cells, thus resulting in the earlier appearance of GFAP in the serum. Approximately 75% of our ICH patients admitted within the first two hours had a serum GFAP concentration above the calculated cut off point. Beyond this time point, all ICH patients were GFAP positive (see fig 2). Additionally, haematoma volume and individual NIHSS score were positively correlated with the individual GFAP values (see fig 1). Thus it seems to be plausible that the early serum GFAP release in ICH patients is mainly caused by the direct destruction of brain tissue by haematoma evolution and growth.
The specificity of GFAP for the identification of ICH was high in our sample (0.98). Only two of 93 patients with ischaemic stroke had GFAP values above the calculated cut off point (false positives). We speculate that these two misclassifications may have resulted from an incorrect report of the time of symptom onset, or a recent previous stroke event that had not come to medical attention. In certain circumstances, GFAP could also be detected in healthy subjects in low concentrations (see Methods). In contrast, the sensitivity of GFAP reached only 0.79, with nine ICH patients testing false negative. The majority of these patients had small haematomas with volumes of less than 10 ml and were studied very early after symptom onset (<2 hours). Thus there seems to be the potential for a further increase in the diagnostic accuracy of GFAP testing for ICH. First, a detailed analysis of the GFAP kinetics within the first six hours may help to identify an optimal time window for testing. Second, because the calculated GFAP cut off point is very close to the lower detection limit of the immunoassay used (1.8 ng/l), further improvements to the analytical method may result in better GFAP determination. On the other hand, a negative GFAP test would not be suitable for diagnosing acute cerebral ischaemia. Thus it is important to emphasise that a serum GFAP values above the defined cut off point in the acute phase of stroke indicate intracerebral haemorrhage only, with a very high probability (0.94).
One previous study has also examined serum GFAP in human stroke.16 Patients with ICH were not included, and patients with ischaemic stroke had mean GFAP levels of around 500 ng/l in samples taken 8.4 (5.9) hours after symptom onset. Because our samples were taken on average more than six hours earlier (see table 1), we assume that this discrepancy reflects the delayed leakage of GFAP across the blood–brain barrier in patients with ischaemic stroke. It should also be noted that it has not been possible to determine absolute serum GFAP concentrations accurately until now, making comparisons between studies difficult. Nevertheless, because all our measurements have employed the same test, uncertainties about the “true” absolute values cannot have influenced the predictive value of GFAP for ICH in the present series.
There are several limitations to this pilot study. First, we determined GFAP at the time point of hospital admission, not in the prehospital setting. Additionally, the relation between GFAP positivity and the time elapsed since symptom onset suggest that the earlier GFAP testing is done the less likely is a positive finding (fig 2). This should be further investigated using blood samples obtained in the prehospital setting. Second, the GFAP cut off point we found in the present population may not necessarily be valid in other populations of acute stroke patients. Thus it would be worthwhile to reconfirm the cut off point in separate independent stroke populations and to optimise this for clinical use. Third, as mentioned above we used a research prototype immunoassay for GFAP determination. It is likely that with technical improvement of this test the validity of the determination of serum GFAP values especially close to the lower detection limit will increase further. Fourth, the ability to apply GFAP testing in the prehospital setting requires a point of care test which is not yet available.
Nevertheless, our pilot study indicates that serum GFAP may function as a reliable biomarker for intracerebral haemorrhage in acute stroke. Furthermore, our data may stimulate further research to evaluate the usefulness of GFAP as a surrogate marker for the evolution of brain parenchymal haematomas. This offers the possibility of obtaining additional paraclinical information and optimising the cause specific management of acute stroke patients even in the emergency setting.
Abbreviations
GFAP - glial fibrillary acidic protein
ICH - intracerebral haemorrhage
NIHSS - National Institutes of Health stroke scale
Footnotes
Competing interests: Christian Foerch and Matthias Sitzer are designated as inventors in the European patent application cited below. Ingo Curdt was employed as a scientific trainee by Roche Centralised Diagnostics, 82377 Penzberg, Germany; he is also designated as an inventor in the European patent application cited below. Title of pending patent: “Use of GFAP for identification of intracerebral haemorrhage” (patent application number: 03021571.9; date of filing: 24 September 2003. Bernard Yan, Florian Dvorak, Marcella Hermans, Joachim Berkefeld, Andreas Raabe, Tobias Neumann Haefelin, and Helmuth Steinmetz declare they have no competing interests.
References
- 1.Sudlow C L, Warlow C P. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke 199728491–499. [DOI] [PubMed] [Google Scholar]
- 2.Qureshi A I, Tuhrim S, Broderick J P.et al Spontaneous intracerebral hemorrhage. N Engl J Med 20013441450–1460. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Kutsuzawa T, Takita K.et al Clinico‐epidemiologic study of stroke in Akita, Japan. Stroke 198718402–406. [DOI] [PubMed] [Google Scholar]
- 4.Juvela S, Hillbom M, Palomaki H. Risk factors for spontaneous intracerebral hemorrhage. Stroke 1995261558–1564. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson O G, Lindgren A, Brandt L.et al Prediction of death in patients with primary intracerebral hemorrhage: a prospective study of a defined population. J Neurosurg 200297531–536. [DOI] [PubMed] [Google Scholar]
- 6.Broderick J P, Adams H P, Barsan W.et al Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke 199930905–915. [DOI] [PubMed] [Google Scholar]
- 7.Carlberg B, Asplund K, Hagg E. The prognostic value of admission blood pressure in patients with acute stroke. Stroke 1993241372–1375. [DOI] [PubMed] [Google Scholar]
- 8.Ohwaki K, Yano E, Nagashima H.et al Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke 2004351364–1367. [DOI] [PubMed] [Google Scholar]
- 9.Gujjar A R, Deibert E, Manno E M.et al Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: indications, timing, and outcome. Neurology 199851447–451. [DOI] [PubMed] [Google Scholar]
- 10.Kazui S, Naritomi H, Yamamoto H.et al Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke 1996271783–1787. [DOI] [PubMed] [Google Scholar]
- 11.Brott T, Broderick J, Kothari R.et al Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997281–5. [DOI] [PubMed] [Google Scholar]
- 12.Mayer S A. Ultra‐early hemostatic therapy for intracerebral hemorrhage. Stroke 200334224–229. [DOI] [PubMed] [Google Scholar]
- 13.Mayer S A, Brun N C, Broderick J.et al Safety and feasibility of recombinant factor VIIa for acute intracerebral hemorrhage. Stroke 20053674–79. [DOI] [PubMed] [Google Scholar]
- 14.Mayer S A, Brun N C, Begtrup K.et al Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2005352777–785. [DOI] [PubMed] [Google Scholar]
- 15.Niebroj‐Dobosz I, Rafalowska J, Lukasiuk M.et al Immunochemical analysis of some proteins in cerebrospinal fluid and serum of patients with ischemic strokes. Folia Neuropathol 199432129–137. [PubMed] [Google Scholar]
- 16.Herrmann M, Vos P, Wunderlich M T.et al Release of glial tissue‐specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S‐100B and glial fibrillary acidic protein. Stroke 2000312670–2677. [DOI] [PubMed] [Google Scholar]
- 17.Foerch C, Singer O, Neumann‐Haefelin T.et al Utility of serum GFAP in monitoring acute MCA territorial infarction. Cerebrovasc Dis 200316(suppl 4)45 [Google Scholar]
- 18.Broderick J P, Brott T G, Duldner J E.et al Volume of intracerebral hemorrhage. A powerful and easy‐to‐use predictor of 30‐day mortality. Stroke 199324987–993. [DOI] [PubMed] [Google Scholar]
- 19.Missler U, Wiesmann M, Wittmann G.et al Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin Chem 199945138–141. [PubMed] [Google Scholar]
- 20.Buttner T, Weyers S, Postert T.et al S‐100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke 1997281961–1965. [DOI] [PubMed] [Google Scholar]
- 21.Missler U, Wiesmann M, Friedrich C.et al S‐100 protein and neuron‐specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke 1997281956–1960. [DOI] [PubMed] [Google Scholar]
- 22.Foerch C, Otto B, Singer O C.et al Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke 2004352160–2164. [DOI] [PubMed] [Google Scholar]
- 23.Fassbender K, Schmidt R, Schreiner A.et al Leakage of brain‐originated proteins in peripheral blood: temporal profile and diagnostic value in early ischemic stroke. J Neurol Sci 1997148101–105. [DOI] [PubMed] [Google Scholar]
- 24.Marchi N, Rasmussen P, Kapural M.et al Peripheral markers of brain damage and blood‐brain barrier dysfunction. Restor Neurol Neurosci 200321109–121. [PMC free article] [PubMed] [Google Scholar]