Abstract
Objective
To study cerebrospinal fluid (CSF) and serum samples from 34 consecutive patients suspected of having varicella‐zoster virus (VZV) infection of the central nervous system (CNS).
Population and methods
The patients were divided into three groups. The first group consisted of 27 patients with a rash in one to three dermatomes and clinical suspicion of meningitis and radiculitis; among them, three subgroups were distinguished according to the affected dermatome: ophthalmicus (n = 9), oticus (n = 11) and cervico‐thoraco‐lumbar zoster (n = 7). Four cases of zoster sine herpete (ZSH) were included in the second group: these patients presented with either radiculitis (n = 2) or meningoencephalitis (n = 2), without cutaneous eruption. The third group consisted of three patients with a generalised rash and encephalitis. A polymerase chain reaction (PCR) for VZV DNA and antigen‐driven immunoblots for oligoclonal anti‐VZV antibodies were carried out on all CSF samples.
Results
PCR of the CSF was positive in 44% of the patients from the first group, mainly within the first 7 days after eruption. In addition, intrathecal synthesis of anti‐VZV antibodies was detected in 37% of patients, always after an interval of 7 days (p<0.0001). Among the four patients with ZSH, a positive VZV PCR was detected in three patients and CSF‐specific oligoclonal anti‐VZV antibodies in two. PCR was also positive in the CSF of two of the three patients with generalised rash and encephalitis; local production of anti‐VZV antibodies was seen in a second CSF sample in one patient, and was also present in the third patient.
Conclusion
Amplification of VZV DNA by PCR in the CSF and antigen‐driven immunoblots have important diagnostic value in suspected VZV infection, although their presence depends on the timing of the CSF sampling. VZV is thought to be a causative agent in unexplained cases of meningitis associated with radiculitis or focal CNS symptoms, even in the absence of skin manifestations. In such patients, rapid diagnosis by this combined approach permits early antiviral treatment.
Varicella‐zoster virus (VZV), an exclusively human herpesvirus, causes chickenpox (varicella), becomes latent in the cranial nerve and dorsal root ganglia, and may reactivate decades later in 10–20% of the population to produce shingles (zoster).1 Shingles is characterised by unilateral radicular pain and a vesicular rash that is generally limited to one to three contiguous dermatomes. The annualised incidence of shingles is about 1.5–3 cases per 1000 people, but increases to 11 cases per 1000 in the population >60 years of age.2 VZV can also cause neurological complications, very rarely during the primary infection (most often a varicella cerebellitis) and more often during the reactivation phase. The main complication is post‐herpetic neuralgia, a neuropathic pain syndrome that persists after the dermatomal rash has healed. Acute neurological complications may, however, occur, and affect either the peripheral nervous system (cranial neuropathies, motor radiculopathies of the arm or the leg, bladder and bowel dysfunction) or the central nervous system (CNS; meningitis, myelitis and vasculitic encephalitis). The same neurological complications may be observed in zoster sine herpete (ZSH), which is defined by a dermatomal pain without antecedent rash.3
Cerebrospinal fluid (CSF) analysis is a key tool in the diagnosis of CNS infection with VZV. The amplification of VZV DNA by polymerase chain reaction (PCR) and the detection of intrathecal synthesis (ITS) of anti‐VZV‐specific antibodies are the most reliable ways of establishing a definite diagnosis of VZV CNS infection.4 We present a retrospective study on CSF samples collected from 34 consecutive patients suspected of harbouring CNS VZV infection. Our population included four cases with ZSH and three with disseminated rash with meningoencephalitis. We discuss correlations between the CSF results, the timing of CSF samples and the clinical picture.
Patients
The study population included 34 consecutive patients hospitalised in the Cliniques Universitaires Saint‐Luc, Brussels, Belgium, for suspected VZV‐induced neurological signs and symptoms, or for unexplained meningitis or radiculitis, between February 1993 and July 2004. Tables 1–3 summarise the characteristics of patients in the study.
Table 1 Characteristics of patients with meningoradiculitis with rash.
| n | Age (years)/sex | Zonal area | Associated symptoms | Immuno depression | Date of LP after eruption (days) | Cells/μl (% Ly) | CSF‐specific oligoclonal IgG* | Immunoblotting pattern of VZV antibodies CSF/serum | PCR |
|---|---|---|---|---|---|---|---|---|---|
| Herpes zoster ophthalmicus | |||||||||
| 1 | 50/M | V1 | Diplopia | + | +22 | 14 (98) | + | +++>++ | − |
| 2 | 44/M | V1,V2 | UNK | UNK | +30 | 54 (93) | − | ++>+ | − |
| 3 | 73/M | V1 | Diplopia, neuralgia | − | +18 | 113 (100) | + | ++>+ | − |
| 4 | 72/F | V1 | Diplopia | + | +12 | 270 (99) | + | ++>− | − |
| 5 | 40/F | V1 | Headache | − | +2 | 10 (99) | − | −/− | − |
| 6 | 77/F | V1 | Headache | − | +2 | <6 | − | −/− | + |
| 7 | 61/M | V1 | Diplopia | − | +9 | 80 (99) | − | ++>+ | − |
| 8 | 26/F | V1 | Headache, fever | + | UNK | 166 (72) | − | +/+ | + |
| 9 | 58/M | V1 | Headache | + | +15 | UNK | − | ++>+ | − |
| Herpes zoster oticus | |||||||||
| 10 | 64/M | Oticus | FP | − | +10 | 267 (98) | + | ++>+ | − |
| 11 | 67/M | Oticus | Cerebellar signs, FP | − | +11 | 29 (95) | − | ++>− | + |
| 12 | 17/M | Oticus | FP | − | +5 | 266 (93) | − | +/+ | + |
| 13 | 23/M | Oticus | FP | − | +26 | 12 (ND) | − | −/− | + |
| 14 | 63/M | Oticus | FP | − | +1 | 80 (ND) | + | −/− | + |
| 15 | 57/M | Oticus | FP | + | +3 | <6 | + | −/− | + |
| 16 | 18/M | Oticus | Headache, FP | − | +4 | 127 (96) | − | −/− | + |
| 17 | 51/F | Oticus | FP | − | +7 | 7 (83) | − | −/− | − |
| 18 | 45/F | Oticus | Headache, fever, FP | + | +3 | 6 (ND) | − | −/− | − |
| 19 | 22/F | Oticus | Headache, FP | − | +2 | 246 (96) | − | −/− | + |
| 20 | 46/F | Oticus | FP | + | +9 | 48 (98) | + | +++/+++ | − |
| Cervico‐thoraco‐lumbar zoster | |||||||||
| 21 | 72/F | C5,C6 | Confusion | − | +10 | 196 (97) | + | +++>++ | − |
| 22 | 84/F | C4 | L hemiparesis | − | +1 | <6 | − | −/− | − |
| 23 | 58/F | T 3 | Diffuse hyperesthesia | − | +1 | 13 (98) | − | −/− | − |
| 24 | 71/F | T10, L1 | Headache | − | +4 | 52 (90) | − | ++/++ | + |
| 25 | 21/F | T6,T7 | Headache | − | +6 | 358 (89) | − | ND | + |
| 26 | 73/M | L4,L5 | Epileptic seizure | − | +13 | 46 (98) | + | +++>++ | − |
| 27 | 71/M | T8 | Amnesia | − | +8 | 72 (97) | − | +/+ | + |
CSF, cerebrospinal fluid; F, female; FP, facial palsy; IgG, immunoglobulin G; L, left; LP, lumbar puncture; % Ly, percentage of lymphocytes; M, male; ND, not done; PCR, polymerase chain reaction; R, right; UNK, unknown; VZV, varicella‐zoster virus.
+, present; −, absent; >, higher number of oligoclonal anti‐VZV antibodies in the CSF, indicating an intrathecal synthesis.
*In this column, +, intrathecal synthesis of oligoclonal IgG (patterns II and III); −, a polyclonal IgG background or a mirror pattern (patterns I and IV from Andersson et al5).
Table 2 Characteristics of patients with ZSH infection.
| n | Age (years)/sex | Clinical data | Immuno‐depression | Timing of LP from the first one (days) | CSF pleocytosis | CSF‐specific oligoclonal IgG* | Immunoblotting pattern of VZV antibodies CSF/serum | PCR |
|---|---|---|---|---|---|---|---|---|
| 28 | 40/M | R L5 sciatica | + | − | 47 (97%) | − | −/− | + |
| +2 | 31 (95%) | − | −/− | + | ||||
| 29 | 56/F | Painful cruralgia, fever | − | − | 58 (94%) | − | ++>+ | − |
| 30 | 56/M | Headache, fever, L pyramidal syndrome, vertigo, L INO | − | − | 830 (100%) | − | ND | + |
| +6 | 150 (91%) | + | ND | + | ||||
| +62 | 42 (99%) | + | +++>++ | ND | ||||
| +120 | 88 (100%) | + | ++>+ | − | ||||
| 31 | 59/F | Confusion, headache, hallucinations | + | − | 390 (78%) | + | ++/++ | + |
| +10 | 144 (98%) | + | +++>++ | − |
CSF, cerebrospinal fluid; F, female; IgG, immunoglobulin G; INO, internuclear ophthalmoplegia; L, left; LP, lumbar puncture; M, male; ND, not done; PCR, polymerase chain reaction; R, right; VZV, varicella‐zoster virus; ZSH, zoster sine herpete.
+, present; −, absent; >, higher number of oligoclonal anti‐VZV antibodies in the CSF, indicating an intrathecal synthesis.
*In this column, +, intrathecal synthesis of oligoclonal IgG (patterns II and III); −, a polyclonal IgG background or a mirror pattern (patterns I and IV from Andersson et al5).
Table 3 Characteristics of patients with generalised rash with encephalitis.
| n | Age (years)/sex | Clinical data | Immuno‐depression | Date of LP after eruption (days) | CSF pleocytosis | CSF‐specific oligoclonal IgG* | Immunoblotting pattern of VZV antibodies CSF/serum | PCR |
|---|---|---|---|---|---|---|---|---|
| 32 | 67/M | R hemiparesis, confusion | − | +1 | UNK | + | ND | + |
| +7 | 6 | + | +++>++ | − | ||||
| 33 | 69/F | Confusion, behavioural disorders | + | +1 | <6 | ND | ND | + |
| 34 | 33/M | Dysarthria, cerebellar syndrome | − | +1 | 125 (>50%) | + | ++>+ | ND |
CSF, cerebrospinal fluid; F, female; IgG, immunoglobulin G; LP, lumbar puncture; M, male; ND, not done; PCR, polymerase chain reaction; UNK, unknown; VZV, varicella‐zoster virus.
+, present; −, absent; >, higher number of oligoclonal anti‐VZV antibodies in the CSF, indicating an intrathecal synthesis.
*In this column, +, intrathecal synthesis of oligoclonal IgG (patterns II and III); −, a polyclonal IgG background or a mirror pattern (patterns I and IV from Andersson et al5).
The patients were divided into three groups. The first group consisted of 27 (79%) cases with a rash in one to three dermatomes and clinical suspicion of meningitis and radiculitis. These patients were separated into three subgroups depending on the affected dermatome: ophthalmicus (n = 9, 33%), oticus (n = 11, 41%) and cervico‐thoraco‐lumbar zoster (n = 7, 26%). Clinical signs and symptoms leading to a lumbar puncture were headache and fever (n = 9), diplopia (n = 4), facial palsy (n = 11), confusion (n = 1), immunodepression (n = 7), epileptic seizure (n = 1), amnesic syndrome (n = 1), associated hemiparesis (n = 1) and diffuse hyperaesthesia (n = 1). The second group consisted of 4 (12%) patients with radiculitis (n = 2; ZSH) or meningoencephalitis (n = 2) without cutaneous eruption. The third group consisted of 3 (9%) patients with generalised rash and encephalitis.
Methods
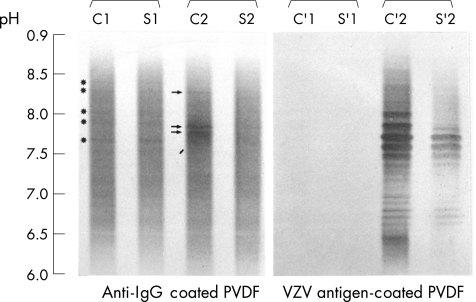
Standard analysis of CSF, including cell count, and measurement of protein, glucose and lactate levels, was carried out in all cases. CSF pleocytosis was defined as a white cell count >5/μl. Antigen‐driven immunoblotting and PCR for VZV DNA were carried out in all cases, according to previously published methods.4 The amplified PCR products came from the Xbal M region of the VZV genome and had 375 base pairs. They were detected by the classic method of electrophoresis. Antigen immunoblotting was carried out after isoelectric focusing of paired CSF and serum samples diluted at the same immunoglobulin G (IgG) concentration. Agarose gels were then blotted on an Immobilon sheet (Millipore, Bedford, MA, USA) coated with a 10‐fold dilution of crude VZV antigens (Whitakker Bioproducts, Walkersville, MD, USA) under a uniform weight for 40 min at 10°C. After washings, immunoblots were briefly fixed in a 0.25% glutaraldehyde buffer, incubated and immunostained with alkaline phosphatase‐conjugate rabbit anti‐human IgG antiserum and its specific substrate (Biorad, Richmond, USA). ITS was considered to be present if oligoclonal anti‐VZV IgG were observed predominantly in the CSF or with a pattern in the CSF different from that in the serum. In contrast, the occurrence of a similar pattern in both the CSF and the serum (“mirror” pattern) or the absence of immunoreactivity in the CSF was thought to indicate the absence of ITS. The intensity of immunostaining was graded by visual inspection as weakly positive (+), positive (++) or strongly positive (+++) and compared with the paired CSF and serum samples (fig 1). The total IgG pattern was simultaneously determined and assessed as recommended by Andersson et al5. The presence of CSF‐specific oligoclonal IgG (patterns II and III) was recorded as positive (+), their absence or a mirror pattern as negative(−; patterns I and IV; tables 1–3).
Figure 1 Typical immunoblots of total immunoglobulin G (IgG) with anti‐IgG‐coated polyvinylidene difluoride (PVDF) membrane (left panel), and antigen‐driven immunoblots of varicella‐zoster virus (VZV)‐specific IgG (right panel) from native cerebrospinal fluid (C) and corresponding serum (S), diluted to the same IgG concentration. One control sample (C1, S1) and samples from patient 21 (C2, S2) are shown. Oligoclonal IgG bands (C2, arrows) and specific anti‐VZV oligoclonal antibodies (C′2) are seen in the CSF from patient 21 (C2, C′2) as a result of intrathecal synthesis. The number of CSF oligoclonal anti‐VZV antibodies exceeds the number of CSF oligoclonal IgG bands and the number of serum oligoclonal antibodies (grading of VZV antibodies was considered to be +++>++). A “mirror” pattern of total IgG is seen in the control sample (C1, S1, *), but no anti‐VZV oligoclonal antibodies are seen (C′1, S′1; grading −/−).
Results
The average age at symptom occurrence was 53 (range 17–84) years, with 14 (42%) patients being older than 60 years. A total of 16 (47%) patients were women. In all, 10 (29%) patients were immunocompromised: seven in the first group (patient 1: radiotherapy for carcinoma of the throat; patients 4 and 9: chronic lymphoid leukaemia; patient 8: systemic lupus erythematosus; patients 15 and 20: HIV seropositive; and patient 18: Crohn's disease); two in the second group (patient 28: systemic lupus erythematosus treated with azathioprine; and patient 31: chronic alcoholism); and one in the third group (patient 33: follicular lymphoma treated with chemotherapy).
First group: dermatomal rash and suspected radiculitis or meningitis
Among the 27 patients, 23 (85%) had CSF pleocytosis. The mean white cell count was 94 (range 0–358) cells/μl, predominantly lymphocytes (range 72–100%). The mean number of cells was lower in the immunocompromised patients than in the immunocompetent ones (72 and 104 cells/μl, respectively); however, this difference was not statistically significant. Lumbar puncture was carried out a mean of 9.0 days (range 1–30) after appearance of the vesicles. We found a shorter delay between the appearance of vesicles and the spinal tap in patients with oticus (mean 7.4 days) and cervico‐thoraco‐lumbar zoster (mean 6.1 days) than in those with ophthalmicus (mean 13.8 days). VZV PCR was positive in 44% of patients in this group (22% in the ophthalmicus subgroup, 64% in the oticus subgroup and 43% in the cervico‐thoraco‐lumbar zoster subgroup). Of the 13 CSF samples collected within the first 7 days after the occurrence of the rash, VZV PCR was positive in 8 (61.5%) cases. By contrast, the PCR was positive in only 3 (25%) of the 12 samples collected after 7 days (Fisher's exact test, p = 0.11). ITS of oligoclonal anti‐VZV IgG was never detected in the 13 “early” CSF samples (<7 days) but was present in 10 of the 12 (83%) samples collected later (Fisher's exact test, p<0.0001). Overall, 37% of the patients in this group showed ITS of anti‐VZV antibodies (fig 1).
Only one patient (patient 22) had a completely normal CSF (no pleocytosis, negative PCR and no ITS of anti‐VZV antibodies): the spinal tap was carried out on day one after eruption, and left hemiparesis was considered to be caused by an ischaemic stroke. By contrast, two patients (patients 6 and 15) showed a positive VZV PCR in the absence of pleocytosis and of ITS of specific antibodies. In both cases, the spinal taps were also carried out early, 2 and 3 days after cutaneous eruption.
Second group: ZSH
Two patients had radiculitis without skin eruption. Patient 28, who had systemic lupus erythematosus, was being treated with azathioprine. This person had headache and right L5 sciatica, but showed no disc herniation on magnetic resonance imaging (MRI) scans of the lumbar spine. A positive VZV PCR was detected in a first CSF sample containing 47 lymphocytes/µl and was confirmed in a second sample collected 2 days later. Patient 29 had painful cruralgia with fever. The CSF sample collected 10 days after the onset of pain also showed mild lymphocytic pleocytosis (58 cells/μl) and ITS of anti‐VZV‐specific antibodies.
Two patients developed meningoencephalitis without antecedent or concomitant rash. Patient 30, a previously healthy man, developed fever, headache and left pyramidal and Claude–Bernard–Horner syndromes over 5 days. A moderate CSF lymphocytic pleocytosis in the first CSF sample led to empirical intravenous treatment with ampicillin. This first sample did not contain specific oligoclonal IgG bands, but these bands were observed in a second sample collected after the final dose of ampicillin. The patient was asymptomatic at that time. A month later, he suddenly had diplopia and vertigo associated with left internuclear ophthalmoplegia. Lesions suggestive of vasculitis were found in the left internal capsule and the floor of the fourth ventricle on MRI of the brain. A third CSF sample collected still showed lymphocytic pleocytosis and showed ITS of anti‐VZV oligoclonal IgG. VZV PCR was negative in this sample, but was retrospectively positive in the first two CSF samples. Recovery was complete after specific treatment with acyclovir. The second patient, a chronic alcoholic woman (patient 31) aged 59 years, showed subacute neurological deterioration with confusion, headache and visual hallucinations (carnival objects). She had no focal neurological deficits and MRI of the brain was normal. CSF analysis showed lymphocytic pleocytosis in two CSF samples collected with an interval of 10 days. VZV PCR was positive in the first sample and oligoclonal anti‐VZV antibodies were present in the second one.
Third group: generalised rash
This group included three patients (patients 32, 33 and 34). The first patient had pneumonia and was treated with antibiotics. He then developed high fever and meningeal signs concomitant with a rash on the left thigh. Confusion and right hemiparesis appeared simultaneously 3 days later, with an extension of the rash to the lower limbs, the buttocks and the thorax. The first CSF sample was positive for VZV DNA and treatment with intravenous acyclovir was started. A second sample, collected 7 days later, was PCR negative, but contained oligoclonal IgG bands and numerous oligoclonal anti‐VZV antibodies. The most cathodic ones were observed only in the CSF and were intrathecally produced. The second patient had a follicular lymphoma that was treated with chemotherapy and he presented with behavioural disorders and confusion, followed by a generalised rash. In this patient, the CSF showed no pleocytosis but VZV DNA was present. Serum was not available for antigen‐driven immunoblotting. The third patient developed gait disturbances and dysarthria 5 days after a typical chickenpox illness. He was diagnosed with post‐varicella cerebellitis. The CSF showed lymphocytic pleocytosis and ITS of anti‐VZV antibodies, but PCR was not carried out. These three patients recovered fully.
Discussion
First group: dermatomal rash and suspected radiculitis or meningitis
Among the 27 patients, lymphocytic pleocytosis was present in the CSF in 23 (85%) patients. The detection of DNA virus by PCR was positive in 12 (44%), notably in 8 (61.5%) of the 13 samples collected within the first 7 days. By comparison, Shoji et al6 reported positive VZV PCR in the CSF of 60% of their cases. The earliest positive PCR was obtained from CSF collected 1 day after the onset of the rash, in a patient with oticus. A higher number of positive PCRs was observed among patients with oticus than among those with ophthalmicus (64% v 22%), but this difference was not significant. A shorter delay in seeking medical advice in patients with facial palsy (mean delay 7.4 days) than in those with isolated shingles in the trigeminal area (mean delay 13.8 days) may partly explain this difference.
CSF‐specific oligoclonal IgG bands were present in 33% of cases, and CSF‐specific oligoclonal anti‐VZV IgG in 37%. We have already reported the presence of oligoclonal IgG antibodies in the absence of oligoclonal IgG bands in various infectious disorders of the CNS. The high resolution of the antigen‐driven immunoblots permits the extraction of specific oligoclonal antibodies from the polyclonal IgG background, as seen in five patients from this cohort (patients 2, 7, 9, 11 and 29).7 In contrast, CSF‐specific oligoclonal IgG may be present because of a chronic coinfection (eg, as in HIV‐seropositive patients 15 and 20) in the absence of detectable intrathecal production of VZV antibodies.
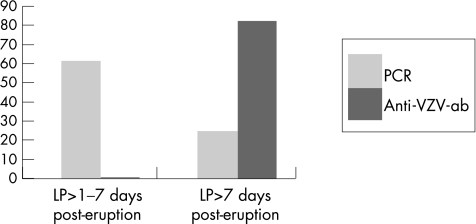
The CSF results were related to the time at which the sample was taken (fig 2). In the course of many viral infections of the CNS, DNA virus is detected during the first 7 days, whereas specific antibodies appear later.4,8,9
Figure 2 Percentage of positive varicella‐zoster virus (VZV) polymerase chain reaction (PCR) and anti‐VZV antibodies (anti‐VZV‐Ab) in the group of patients with dermatomal rash, in relation to the timing of the lumbar puncture (LP). Samples (n = 13) collected within the first 7 days were never positive for anti‐VZV‐Ab, but 8 (61%) were PCR positive. By contrast, 10 of the 12 (83%) samples collected after 1 week contained oligoclonal anti‐VZV‐Ab, of which only 3 (25%) were still PCR positive.
It should be emphasised that these results are not applicable in all cases of zoster, because lumbar puncture was carried out only in patients with neurological complications.
Second group: ZSH
The range of neurological complications of VZV reactivation without cutaneous lesions is as broad as that associated with cutaneous zoster. They are, however, very rare. Several types of neuropathy have been reported, including acute trigeminal neuralgia,10 multiple cranial neuropathies,11 acute polyneuritis,11 cauda equina syndrome with third, sixth and seventh cranial nerves palsies,12 facial palsy,13 recurrent thoracic radicular pain14 and prolonged thoracic radicular pain.15 Diagnosis of the most recent cases has been confirmed by PCR.
Encephalitis resulting from VZV infection is recognised as a vasculopathy that affects large or small vessels.16,17,18 Early cases have been reported after trigeminal rash.19 Afferent trigeminal ganglionic fibres innervating both intracranial and extracranial arteries provide a pathway for viral spread. Brain imaging shows large and small ischaemic or haemorrhagic infarcts of the cortex and subcortical grey and white matter.17 Rare cases of encephalitis without zoster have been reported. Symptoms described are fever, seizures,11,16 mental changes11,20,21 and focal deficits.11,22 Diagnosis of these cases has been made on CSF analysis and by detection of VZV DNA or viral inclusions in histological tissues.
We presented two cases of meningoradiculitis and two of meningoencephalitis without rash. The CSF from the four patients showed pleocytosis, ITS of VZV antibodies and positive VZV PCR. As in cases with radicular rash, the CSF seemed to show a typical pattern of presentation related to the timing of CSF collection. VZV DNA was detected in the first days of disease, followed by the detection of an intrathecal production of anti‐VZV antibodies. The results of both analyses cannot be predicted in these cases because of the absence of skin eruption. Patient 30 is an example of this temporal profile. He underwent four lumbar punctures during his neurological disease: the first two samples contained VZV DNA but no anti‐VZV oligoclonal antibodies, whereas the last two samples showed ITS of anti‐VZV antibodies but a negative PCR. We did not carry out VZV PCR systematically in the CSF from patients with uncomplicated and spontaneously resolving aseptic meningitis; it is possible that VZV may be the cause of a few cases of aseptic meningitis without skin lesions.23
Third group: generalised rash
The incidence of CNS complications of varicella is unknown, but is probably very low. The most common neurological syndrome associated with varicella is cerebellar ataxia.13,24,25,26 This complication typically occurs in children from several days before to 2 weeks after the onset of rash, suggesting an immunological mechanism.26 Complete recovery is the rule. Pleocytosis in the CSF is usually absent. Antibodies to VZV have been shown in the CSF from several patients with disorders of the CNS associated with varicella.27 Viral DNA was detected by PCR in the CSF from three children with post‐varicella cerebellitis.13 Pre‐eruptive cerebellitis occurred in one patient and was confirmed by PCR.24 These cases suggest a relationship between this clinical picture and the presence of VZV in the CNS. In patient 34, the appearance of cerebellar ataxia 5 days after chickenpox seemed to correspond to a post‐infectious disease. CSF analysis, however, disclosed a local production of antibodies to VZV, which was unexpected in this condition. Our report supports the theory of an active viral infection in some cases of post‐varicella cerebellitis.
Meningoencephalitis is a more severe complication of generalised VZV rash occurring in adults. The evolution of the condition is fatal unless it is rapidly diagnosed and treated.28 The CSF is often abnormal (mild to moderate pleocytosis, usually <100 cells/μl, and raised levels of protein). An active viral infection of the CNS is suggested by the presence of VZV DNA in the CSF from these patients.13 Our two patients (patients 32 and 33) also showed positive VZV PCR, confirming the diagnosis and supporting a direct invasion of the CNS by the virus.
In conclusion, our results show a high frequency of lymphocytic pleocytosis, positive PCR and ITS of anti‐VZV antibodies in patients with VZV‐induced neurological diseases. Positive PCR and demonstration of ITS of anti‐VZV antibodies are crucial for a rapid diagnosis, but can be dependent on the timing of samples. It is our practice, when tests are positive, to start treatment with high‐dose, intravenous acyclovir. A vaccine to prevent herpes zoster should decrease the incidence of these VZV‐induced neurological complications.2
Abbreviations
CNS - central nervous system
CSF - cerebrospinal fluid
IgG - immunoglobulin G
ITS - intrathecal synthesis
MRI - magnetic resonance imaging
PCR - polymerase chain reaction
VZV - varicella‐zoster virus
ZSH - zoster sine herpete
Footnotes
Competing interests: None.
References
- 1.Gnann J W, Withley R J. Herpes zoster. N Engl Med 2002347340–346. [DOI] [PubMed] [Google Scholar]
- 2.Oxman M N, Levin M J, Johnson G R.et al A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 20053522271–2284. [DOI] [PubMed] [Google Scholar]
- 3.Lewis G W. Zoster sine herpete. BMJ 19582418–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sindic C J M, Van Antwerpen M P, Goffette S. Clinical relevance of polymerase chain reaction (PCR) assays and antigen‐driven immunoblots for the diagnosis of neurological infectious diseases. Brain Res Bull 200361299–308. [DOI] [PubMed] [Google Scholar]
- 5.Andersson M, Alvarez‐Cermeno J, Bernardi G.et al Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry 199457897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoji H, Honda Y, Murai I. Detection of varicella‐zoster virus DNA by polymerase chain reaction in cerebrospinal fluid of patients with herpes zoster meningitis. J Neurol 199223969–70. [DOI] [PubMed] [Google Scholar]
- 7.Sindic C J M, Van Antwerpen M P, Goffette S. The intrathecal humoral immune response: laboratory analysis and clinical relevance. Clin Chem Lab Med 200139333–340. [DOI] [PubMed] [Google Scholar]
- 8.Monteyne Ph, Laterre E C, Sindic C JM. Encephalitis in immunocompetent patients due to herpes simplex virus type 1 or 2: determination by polymerase chain reaction and detection of intrathecal virus‐specific oligoclonal antibodies, Acta Neurol Belg 199797233–239. [PubMed] [Google Scholar]
- 9.Guillaume B, Sindic C J M, Weber T. Progressive multifocal leukoencephalopathy: simultaneous detection of JCV DNA and anti‐JCV antibodies in the cerebrospinal fluid. Eur J Neurol 20007101–106. [DOI] [PubMed] [Google Scholar]
- 10.Easton H G. Zoster sine herpete causing acute trigeminal neuralgia. Lancet 1970211065–1066. [DOI] [PubMed] [Google Scholar]
- 11.Mayo D R, Booss Varicella zoster‐associated neurologic disease without skin lesions. Arch Neurol 198946313–315. [DOI] [PubMed] [Google Scholar]
- 12.Dueland A N, Devlin M, Martin J R.et al Fatal varicella‐zoster virus meningoradiculitis without skin involvement. Ann Neurol 199129569–572. [DOI] [PubMed] [Google Scholar]
- 13.Puchhammer‐Stockl E, Popow‐Kraupp T, Heinz F X. Detection of varicella zoster virus DNA by polymerase chain reaction in the cerebrospinal fluid of patients suffering from neurological complications associated with chicken pox or herpes zoster. J Clin Microbiol 1991291513–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilden D H, Wright R R, Schneck S A.et al Zoster sine herpete, a clinical variant. Ann Neurol 199435530–533. [DOI] [PubMed] [Google Scholar]
- 15.Amlie‐Lefond C, Mackin G A, Ferguson M.et al Another case of virologically confirmed zoster sine herpete, with electrophysiologic correlation. J Neurovirol 19962136–138. [DOI] [PubMed] [Google Scholar]
- 16.Amlie‐Lefond C, Kleinschmidt‐DeMasters B K, Mahalingam R.et al The vasculopathy of varicella‐zoster virus encephalitis. Ann Neurol 199537784–790. [DOI] [PubMed] [Google Scholar]
- 17.Gilden D H, Kleinschmidt‐Demaster B K, LaGuardia J J.et al Neurological complications of the reactivation of varicella‐zoster virus. N Engl J Med 2000342635–645. [DOI] [PubMed] [Google Scholar]
- 18.Gilden D H, Randall J C, Mahalingam R. VZV vasculopathy and postherpetic neuralgia. Neurology 20056421–25. [DOI] [PubMed] [Google Scholar]
- 19.Pratesi R, Freemon F R, Lowry J L. Herpes zoster ophthalmicus with contralateral hemiplegia. Arch Neurol 197734640–641. [DOI] [PubMed] [Google Scholar]
- 20.Vartdal F, Vandvik B, Norrby E. Intrathecal synthesis of virus‐specific oligoclonal IgG, IgA and IgM in a case of varicella‐zoster meningo‐encephalitis. J Neuro Sci 198257121–132. [DOI] [PubMed] [Google Scholar]
- 21.Gilden D H, Bennett J L, Kleinschmidt‐DeMasters B K.et al The value of cerebrospinal fluid antiviral antibody in the diagnosis of neurologic disease produced by varicella zoster virus. J Neurol Sci 1998159140–144. [DOI] [PubMed] [Google Scholar]
- 22.Nau R, Lantsch M, Stiefel M.et al Varicella‐zoster virus‐associated focal vasculitis without herpes zoster: recovery after treatment with acyclovir. Neurology 199851914–915. [DOI] [PubMed] [Google Scholar]
- 23.Echevarria J M, Martinez‐Martin P, Teller A. Aseptic meningitis due to varicella‐zoster virus: serumantibody levels and local synthesis of specific IgG, IgM and IgA. J Infect Dis 1987155959–967. [DOI] [PubMed] [Google Scholar]
- 24.Barnes D W, Whitley R J. CNS diseases associated with varicella zoster virus and herpes simplex infection. Neurol Clin 19864265–283. [PubMed] [Google Scholar]
- 25.Dangond F, Engle E, Yessayan L. Pre‐eruptive varicella cerebellitis confirmed by PCR. Pediatr Neurol 19939491–493. [DOI] [PubMed] [Google Scholar]
- 26.Rousseva D, Blard J M, Pagès M. Ataxie aiguë au cours d'une varicelle de l'adulte. Rev Neurol 2001157321–322. [PubMed] [Google Scholar]
- 27.Gershon A, Steinberg S, Greenberg S.et al Varicella‐zoster‐associated encephalitis: detection of specific antibody in cerebrospinal fluid. J Clin Microbiol 198012764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boulet E, Blanc P, Testart F.et al Un cas d'encéphalite et de pneumonie varicelleuse chez un adulte sain. La Presse Médicale 1990221055. [PubMed] [Google Scholar]