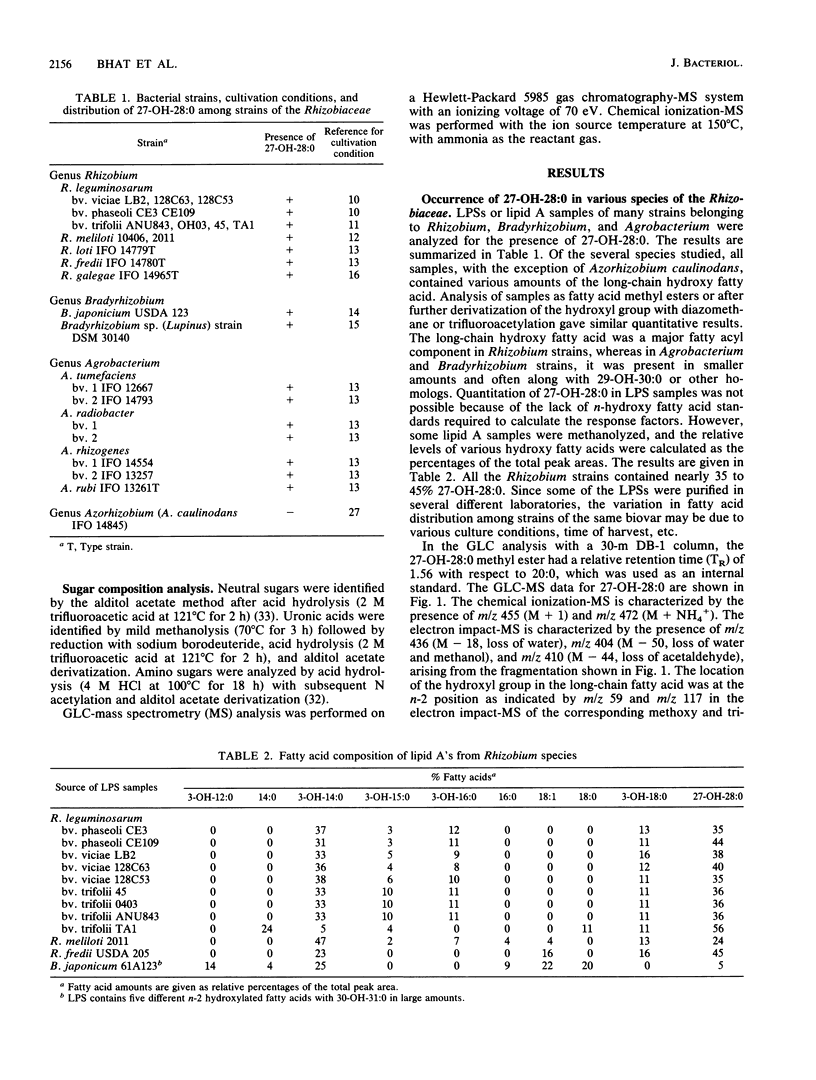
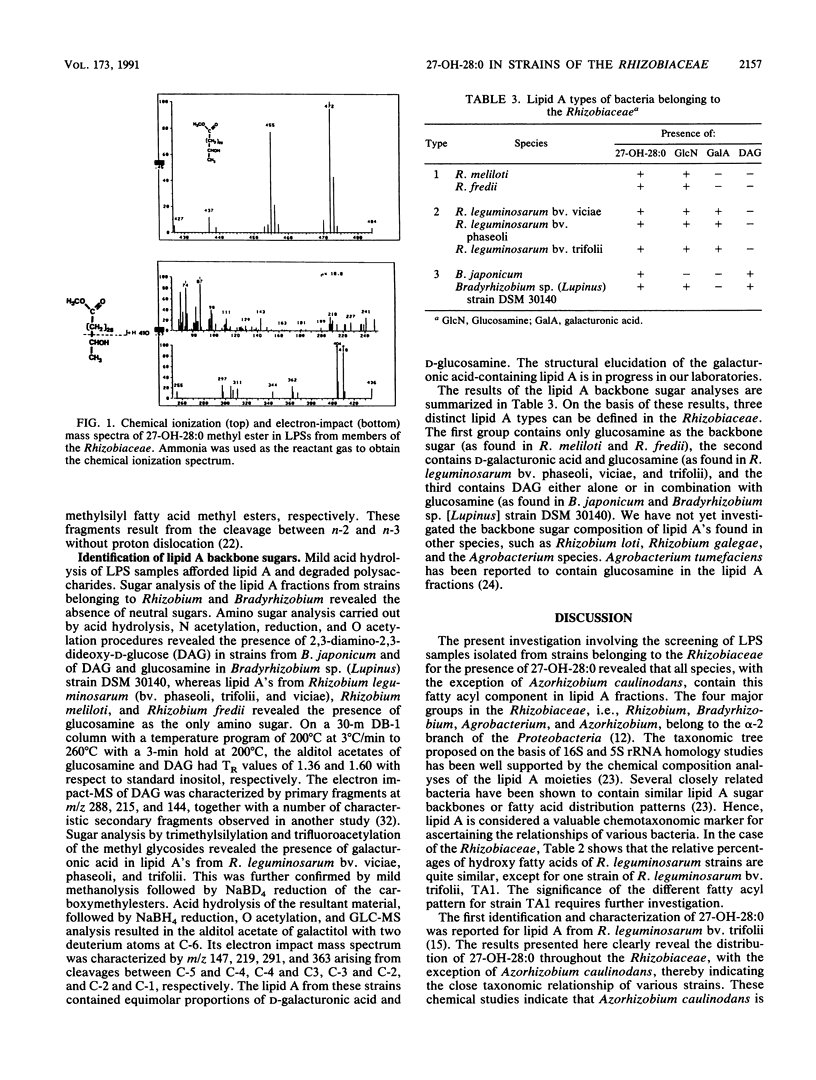
Abstract
Lipopolysaccharides (LPSs) isolated from several strains of Rhizobium, Bradyrhizobium, Agrobacterium, and Azorhizobium were screened for the presence of 27-hydroxyoctacosanoic acid. The LPSs from all strains, with the exception of Azorhizobium caulinodans, contained various amounts of this long-chain hydroxy fatty acid in the lipid A fractions. Analysis of the lipid A sugars revealed three types of backbones: those containing glucosamine (as found in Rhizobium meliloti and Rhizobium fredii), those containing glucosamine and galacturonic acid (as found in Rhizobium leguminosarum bv. phaseoli, trifolii, and viciae), and those containing 2,3-diamino-2,3-dideoxyglucose either alone or in combination with glucosamine (as found in Bradyrhizobium japonicum and Bradyrhizobium sp. [Lupinus] strain DSM 30140). The distribution of 27-hydroxyoctacosanoic acid as well as analysis of lipid A backbone sugars revealed the taxonomic relatedness of various strains of the Rhizobiaceae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlson R. W., Garci F., Noel D., Hollingsworth R. The structures of the lipopolysaccharide core components from Rhizobium leguminosarum biovar phaseoli CE3 and two of its symbiotic mutants, CE109 and CE309. Carbohydr Res. 1989 Dec 21;195(1):101–110. doi: 10.1016/0008-6215(89)85092-x. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Hollingsworth R. L., Dazzo F. B. A core oligosaccharide component from the lipopolysaccharide of Rhizobium trifolii ANU843. Carbohydr Res. 1988 May 1;176(1):127–135. doi: 10.1016/0008-6215(88)84064-3. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Kalembasa S., Turowski D., Pachori P., Noel K. D. Characterization of the lipopolysaccharide from a Rhizobium phaseoli mutant that is defective in infection thread development. J Bacteriol. 1987 Nov;169(11):4923–4928. doi: 10.1128/jb.169.11.4923-4928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Shatters R., Duh J. L., Turnbull E., Hanley B., Rolfe B. G., Djordjevic M. A. The Isolation and Partial Characterization of the Lipopolysaccharides from Several Rhizobium trifolii Mutants Affected in Root Hair Infection. Plant Physiol. 1987 Jun;84(2):421–427. doi: 10.1104/pp.84.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion M., Bhat U. R., Reuhs B., Carlson R. W. Isolation and characterization of the lipopolysaccharides from Bradyrhizobium japonicum. J Bacteriol. 1990 Apr;172(4):1725–1731. doi: 10.1128/jb.172.4.1725-1731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth R. I., Carlson R. W. 27-Hydroxyoctacosanoic acid is a major structural fatty acyl component of the lipopolysaccharide of Rhizobium trifolii ANU 843. J Biol Chem. 1989 Jun 5;264(16):9300–9303. [PubMed] [Google Scholar]
- Hollingsworth R. I., Carlson R. W., Garcia F., Gage D. A. A new core tetrasaccharide component from the lipopolysaccharide of Rhizobium trifolii ANU 843. J Biol Chem. 1989 Jun 5;264(16):9294–9299. [PubMed] [Google Scholar]
- Hollingsworth R. I., Lill-Elghanian D. A. Isolation and characterization of the unusual lipopolysaccharide component, 2-amino-2-deoxy-2-N-(27-hydroxyoctacosanoyl)-3-O-(3-hydroxy- tetradecanoyl)-gluco-hexuronic acid, and its de-O-acylation product from the free lipid A of Rhizobium trifolii ANU843. J Biol Chem. 1989 Aug 25;264(24):14039–14042. [PubMed] [Google Scholar]
- Kersters K., De Ley J. Identification and grouping of bacteria by numerical analysis of their electrophoretic protein patterns. J Gen Microbiol. 1975 Apr;87(2):333–342. doi: 10.1099/00221287-87-2-333. [DOI] [PubMed] [Google Scholar]
- MacKenzie S. L., Lapp M. S., Child J. J. Fatty acid composition of Rhizobium spp. Can J Microbiol. 1979 Jan;25(1):68–74. doi: 10.1139/m79-011. [DOI] [PubMed] [Google Scholar]
- Mayberry W. R. Hydroxy fatty acids in Bacteroides species: D-(--)-3-hydroxy-15-methylhexadecanoate and its homologs. J Bacteriol. 1980 Aug;143(2):582–587. doi: 10.1128/jb.143.2.582-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer H., Krauss J. H., Urbanik-Sypniewska T., Puvanesarajah V., Stacey G., Auling G. Lipid A with 2,3-diamino-2,3-dideoxy-glucose in lipopolysaccharides from slow-growing members of Rhizobiaceae and from "Pseudomonas carboxydovorans". Arch Microbiol. 1989;151(2):111–116. doi: 10.1007/BF00414423. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Robertsen B. K., Aman P., Darvill A. G., McNeil M., Albersheim P. Host-Symbiont Interactions : V. THE STRUCTURE OF ACIDIC EXTRACELLULAR POLYSACCHARIDES SECRETED BY RHIZOBIUM LEGUMINOSARUM AND RHIZOBIUM TRIFOLII. Plant Physiol. 1981 Mar;67(3):389–400. doi: 10.1104/pp.67.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]


