Abstract
Patients having neuropathy associated with Sjögren's syndrome may present with pain and superficial sensory involvement in the absence of sensory ataxia. Treatment for this form of associated neuropathy has not been established. The case of a patient with painful sensory neuropathy associated with Sjögren's syndrome, whose symptoms, particularly pain, responded well to intravenous immunoglobulin both at onset and in a relapse, is reported. Other patients with painful sensory neuropathy associated with Sjögren's syndrome may also be candidates for intravenous Ig treatment.
Ataxic sensory neuropathy associated with Sjögren's syndrome is well recognised.1,2,3 Pathologically, the underlying lesion is a sensory ganglionopathy affecting predominantly large neurones and their axons.2,4 Intravenous immunoglobulin (Ig) treatment is reported to be effective in sensory ataxic neuropathy occurring in people with Sjögren's syndrome.5,6,7,8 Recently, another type of neuropathy associated with Sjögren's syndrome was reported to affect small sensory axons in patients presenting with pain and superficial sensory involvement as opposed to sensory ataxia.1,4,9,10,11 The treatment for this type of neuropathy remains uncertain. We describe a patient with painful sensory neuropathy associated with Sjögren's syndrome, in whom intravenous Ig treatment dramatically reduced painful symptoms.
Case report
A 67‐year‐old man first noted eye and mouth dryness in 1988. Diagnosed with Sjögren's syndrome, he was found to have chronic renal dysfunction, and was treated with oral prednisolone at a daily dose of 10–20 mg. In 1990, a renal biopsy specimen showed membranoproliferative glomerulonephritis, and steroid treatment was continued. In May 2004, he experienced a subacute onset of severe pain and dysaesthesia in his fingers, which subsequently extended to all four extremities. The pain in his hands was so intense that he could not extend his fingers or touch objects. The pain in his feet nearly precluded ambulation. He also had abdominal pain accompanied by severe constipation and was admitted to the Nagoya University Graduate School of Medicine, Nagoya, Japan.
Neurological examination showed reductions in light touch and pinprick perception and temperature sensation; painful dysaesthesias were elicited over the trunk and the four extremities. Dynamic allodynia was not elicited. The pain in his hands and feet was reported to be extremely distressing. In the trunk, painful dysaesthesias were distributed segmentally, predominantly in the Th7–Th10 segments. Perception of vibration and joint position was relatively well preserved. Neither sensory ataxia nor Romberg's sign was observed. Muscle strength and volume were normal. Deep‐tendon reflexes were decreased distally in the extremities. Babinski's sign was absent. Sweating was impaired and complaints of severe constipation continued. Orthostatic hypotension was evident as a decrease of 24 mm Hg in systolic pressure on standing. Thermography showed abnormal skin temperature gradient. Skin blood flow that was monitored at the palm and sole by laser Doppler decreased after stimulation by sound, deep breathing and mental calculations. Sympathetic skin response at the palms was well defined.
Routine haematological laboratory results were normal. Serum biochemical screening indicated a total protein concentration of 5.3 g/dl (normal 6.5–8.0), and urea nitrogen was slightly raised at 21 mg/dl (normal 8.0–20.0). Anti‐Sjögren's syndrome‐A antibodies were positive 35.6 (normal<10), whereas anti‐Sjögren's syndrome‐B antibodies were negative 3.4 (normal<15). Dipstick analysis of urine was positive for protein. In the cerebrospinal fluid (CSF), protein content was moderately raised at 104 mg/dl, whereas the cell count was normal. Schirmer's test and staining with rose bengal were positive. A lip biopsy specimen showed periductal lymphoid cell infiltration and acinar destruction. Examination of motor conduction in the right median nerve showed well‐preserved compound muscle action potential amplitude (5.86 mV) and conduction velocity (56 m/s). Amplitude and conduction velocity also showed well‐preserved motor nerve function in the right ulnar nerve (6.42 mV and 52 m/s, respectively) and right tibial nerve (7.46 mV and 42 m/s, respectively). Sensory conduction examination showed a right median nerve amplitude and conduction velocity of 5.20 μV and 48 m/s, respectively, and a right sural nerve amplitude and conduction velocity of 6.43 μV and 42 m/s, respectively, presenting relatively well‐preserved sensory conduction. Somatosensory evoked potentials were recorded using median nerve stimulation at the wrist. Cortical (N19), cervical (N13) and Erb's point (N9) peaks were at 22.3, 15.5 and 12.0 ms, respectively, representing a slightly delayed N19 latency.
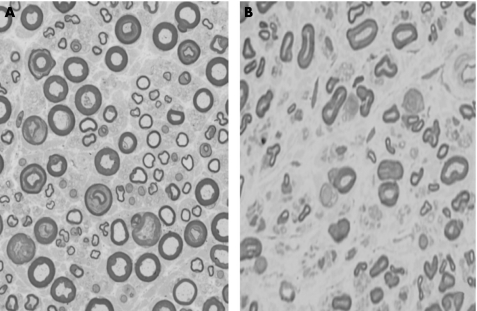
A sural nerve biopsy specimen disclosed myelinated fibre loss, predominantly in the small‐diameter fibres, with axonal degeneration. Large myelinated fibres were relatively well preserved. Vasculitis was not seen. Sprouting was rare (fig 1). Cardiac uptake of meta‐iodobenzylguanidine labelled with iodine‐123 was considerably reduced. The low levels of plasma protein and high levels of urea nitrogen reflected renal dysfunction, most likely resulting from Sjögren's syndrome. Findings from the Schirmer's test, rose bengal staining, lip biopsy specimen examination and anti‐Sjögren's syndrome‐A antibodies fulfilled the revised Japanese and EU–American Diagnostic Criteria for Sjögren's syndrome.12
Figure 1 Sural nerve pathology. (A) Transverse section of a sural nerve specimen from a control. (B) A specimen from the patient. Small‐diameter myelinated fibres are more prominently affected. No axonal sprouts are seen.
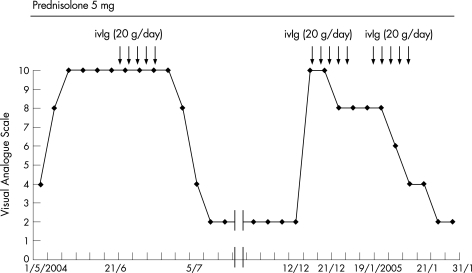
The patient was treated with intravenous Ig (0.4 g/kg for five consecutive days) in June 2004. Two weeks later, he showed a remarkable clinical improvement, and his severe pain in the four extremities had decreased from 10 to 2 according to the Visual Analogue Scale (VAS).13 Light touch, pinprick perception and temperature sensation were slightly improved (VAS 10; fig 2). He was able to extend his fingers and walk without a cane. But abnormal skin temperature gradient on thermography did not improve.
Figure 2 Clinical course of the patient in terms of pain rating. A 10‐cm Visual Analogue Scale was anchored by the two extremes of pain (left, no pain; right, the worst pain imaginable) and assigned the pain rating subjectively according to the manner described previously.13 After the initiation of intravenous Ig (ivIg), his symptoms were improved markedly.
Three months later, however, the patient again experienced severe pain and dysaethesias in the four extremities. By December 2004 (VAS 10; fig 2), he had returned to the state of severe pain that had preceded intravenous Ig treatment. He was readmitted to the Nagoya University Graduate School of Medicine. A second course of intravenous Ig was given (0.4 g/kg for five consecutive days), but pain and dysaesthesia persisted (VAS 6–8; fig 2). Intravenous Ig therefore was given again in January 2005. A week later, he showed clinical improvement comparable to his response in June (VAS 2; fig 2). Although the VAS score improved remarkably after this most recent course of intravenous Ig, protein level and the serum anti‐Sjögren's syndrome‐A titre in the CSF showed little change.
Discussion
The patient described here had Sjögren's syndrome for more than 14 years. Severe pain and painful dysaesthsias developed in the limbs and trunk, with abnormalities predominantly affecting superficial sensory modalities. Sensory ataxia and muscle wasting were absent. Moderately severe autonomic symptoms such as constipation, orthostatic hypotension, decreased sweating and impaired cardiac uptake of meta‐iodobenzylguanidine labelled with iodine‐123 were evident. Pathologically, in the sural nerve, predominantly small myelinated fibres were affected, without pronounced nerve sprouting. Relatively well‐preserved sensory nerve action potentials, sensory evoked potentials and deep‐tendon reflexes were found. These characteristic clinical and laboratory features were highly consistent with those of painful sensory neuropathy without sensory ataxia associated with Sjögren's syndrome, which has recently been described as a distinct form associated with Sjögren's syndrome by our group of clinical investigators as well as by others.1,4,12
The most striking observation in this patient was the remarkable beneficial effect of intravenous Ig treatment on painful symptoms. Pain rated on the VAS was reduced dramatically from 10 to 2 after the first course of treatment. After the second of two courses given after relapse, the patient's ability to carry out activities of daily living was also greatly improved. Furthermore, superficial sensory impairment was less evident on examination after intravenous Ig treatment. To our knowledge, this is the first report showing the therapeutic effect of intravenous Ig on painful sensory neuropathy associated with Sjögren's syndrome. Intravenous Ig has been described by several anecdotal reports as being effective to some extent in the sensory ataxic form of neuropathy associated with Sjögren's syndrome.6,7,8,14 In the sensory ataxic form, the lesion responsible is considered to be a sensory ganglionopathy caused by T cell infiltration of the dorsal root ganglion.2 Therefore, therapeutic response to intravenous Ig may involve immunomodulation, lessening the toxic effects of infiltrating cells. In painful sensory neuropathy, we suspect that the lesion responsible is also within the sensory ganglion, with cell inflammation affecting small neurones rather than large ones as in sensory ataxic form.4 The mechanism underlying benefit from intravenous Ig requires further study, but could parallel the mechanism proposed in the sensory ataxic form—that is, actions against inflammatory cells and their products in the dorsal root ganglion.
Sjögren's syndrome may show a wide range of neuropathic manifestations, including painful sensory neuropathy, sensory ataxic neuropathy and other forms. As this report as well as those concerning ataxic neuropathy indicated responsiveness to intravenous Ig, a randomised prospective trial of intravenous treatment in neuropathies associated with Sjögren's syndrome is warranted.
Abbreviations
VAS - Visual Analogue Scale
Footnotes
Competing interests: None declared.
Informed consent was obtained from the patient described in this study.
References
- 1.Lafitte C, Amoura Z, Cacoub P.et al Neurological complications of primary Sjögren's syndrome. J Neurol 2001248577–584. [DOI] [PubMed] [Google Scholar]
- 2.Griffin J W, Cornblath D R, Alexander E.et al Ataxic sensory neuropathy and dorsal root ganglionitis associated with Sjögren's syndrome. Ann Neurol 199027304–305. [DOI] [PubMed] [Google Scholar]
- 3.Front J, Ramos‐Casals M, de la Red G.et al Pure sensory neuropathy in primary Sjögren's syndrome. Long‐term prospective follow‐up and review of the literature. J Rheumatol 2003301552–1557. [PubMed] [Google Scholar]
- 4.Mori K, Iijima M, Sugiura M.et al Sjögren's syndrome associated painful sensory neuropathy with sensory ataxia. J Neurol Neurosurg Psychiatry 2003741320–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascual J, Cid C, Berciano J. High dose intravenous immunoglobulin for peripheral neuropathy associated with Sjögren's syndrome. Neurology 199851650–651. [DOI] [PubMed] [Google Scholar]
- 6.Molina J A, Benito‐Leon J, Bermejo F.et al Intravenous immunoglobulin therapy in sensory neuropathy associated Sjögren's syndrome. J Neurol Neurosurg Psychiatry 199660699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahasi Y, Takata T, Hosino M.et al Benefit of IVIg for long‐standing ataxic sensory neuropathy with Sjögren's syndrome. Neurology 200360503–505. [DOI] [PubMed] [Google Scholar]
- 8.Donofrio P D. Immunotherapy of idiopathic inflammatory neuropathies. Muscle Nerve 200328273–292. [DOI] [PubMed] [Google Scholar]
- 9.Jerry G, Richard R, Elizabeth R.et al Peripheral neuropathy in Sjögren's syndrome. Muscle Nerve 199013570–579. [DOI] [PubMed] [Google Scholar]
- 10.Mori K, Iijima M, Koike H.et al The wide spectrum of clinical manifestations in Sjogren's syndrome‐associated neuropathy. Brain 20051282518–2534. [DOI] [PubMed] [Google Scholar]
- 11.Chai J, Herrmann D N, Stanton M.et al Painful small‐fiber neuropathy in Sjögren syndrome. Neurology 200565925–927. [DOI] [PubMed] [Google Scholar]
- 12.Vitali C, Bombrdieri S, Jonsson R.et al Classification criteria for Sjögren's syndrome: a revised version of European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 200261554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly A M. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J 200118205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy Y, Uziel Y, Zandman G G.et al Intravenous immunoglobulins in peripheral neuropathy associated with vasculitis. Ann Rheum Dis 2003621221–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]